Therapeutic effect of regulating autophagy in spinal cord injury: a network meta-analysis of direct and indirect comparisons
2020-12-02DuoZhangDiZhuFangWangJiChaoZhuXuZhaiYuanYuanChenXiLi
Duo Zhang , Di Zhu , Fang Wang, Ji-Chao Zhu Xu Zhai, Yuan Yuan, Chen-Xi Li
1 Department of Orthopedics, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
2 Department of Orthopedics, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
3 Department of Emergency, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
4 Department of Spinal Cord Injury Rehabilitation, China Rehabilitation Research Center, Beijing, China
Abstract Objective: An increasing number of studies indicate that autophagy plays an important role in the pathogenesis of spinal cord injury, and that regulating autophagy can enhance recovery from spinal cord injury.However, the effect of regulating autophagy and whether autophagy is detrimental or beneficial after spinal cord injury remain unclear. Therefore, in this study we evaluated the effects of autophagy regulation on spinal cord injury in rats by direct and indirect comparison, in an effort to provide a basis for further research.Data source: Relevant literature published from inception to February 1, 2018 were included by searching Wanfang, CNKI, Web of Science, MEDLINE (OvidSP), PubMed and Google Scholar in English and Chinese. The keywords included “autophagy”, “spinal cord injury”, and “rat”.Data selection: The literature included in vivo experimental studies on autophagy regulation in the treatment of spinal cord injury (including intervention pre- and post-spinal cord injury). Meta-analyses were conducted at different time points to compare the therapeutic effects of promoting or inhibiting autophagy,and subgroup analyses were also conducted.Outcome measure: Basso, Beattie, and Bresnahan scores.Results: Of the 622 studies, 33 studies of median quality were included in the analyses. Basso, Beattie, and Bresnahan scores were higher at 1 day (MD = 1.80, 95% CI: 0.81-2.79, P = 0.0004), 3 days (MD = 0.92, 95%CI: 0.72-1.13, P < 0.00001), 1 week (MD = 2.39, 95% CI: 1.85-2.92, P < 0.00001), 2 weeks (MD = 3.26, 95%CI: 2.40-4.13, P < 0.00001), 3 weeks (MD = 3.13, 95% CI: 2.51-3.75, P < 0.00001) and 4 weeks (MD = 3.18,95% CI: 2.43-3.92, P < 0.00001) after spinal cord injury with upregulation of autophagy compared with the control group (drug solvent control, such as saline group). Basso, Beattie, and Bresnahan scores were higher at 1 day (MD = 6.48, 95% CI: 5.83-7.13, P < 0.00001), 2 weeks (MD = 2.43, 95% CI: 0.79-4.07, P =0.004), 3 weeks (MD = 2.96, 95% CI: 0.09-5.84, P = 0.04) and 4 weeks (MD = 4.41, 95% CI: 1.08-7.75, P =0.01) after spinal cord injury with downregulation of autophagy compared with the control group. Indirect comparison of upregulation and downregulation of autophagy showed no differences in Basso, Beattie, and Bresnahan scores at 1 day (MD = -4.68, 95% CI: -5.840 to -3.496, P = 0.94644), 3 days (MD = -0.28, 95%CI: -2.231-1.671, P = 0.99448), 1 week (MD = 1.83, 95% CI: 0.0076-3.584, P = 0.94588), 2 weeks (MD = 0.81,95% CI: -0.850-2.470, P = 0.93055), 3 weeks (MD = 0.17, 95% CI: -2.771-3.111, P = 0.99546) or 4 weeks(MD = -1.23, 95% CI: -4.647-2.187, P = 0.98264) compared with the control group.Conclusion: Regulation of autophagy improves neurological function, whether it is upregulated or downregulated. There was no difference between upregulation and downregulation of autophagy in the treatment of spinal cord injury. The variability in results among the studies may be associated with differences in research methods, the lack of clearly defined autophagy characteristics after spinal cord injury, and the limited autophagy monitoring techniques. Thus, methods should be standardized, and the dynamic regulation of autophagy should be examined in future studies.
Key Words: autophagy; Basso, Beattie, and Bresnahan scores; indirect comparison; meta-analysis; nerve regeneration; neural regeneration; neurological function; rat models; regulation; spinal cord injury; strategy analysis
Introduction
Spinal cord injury (SCI) is a debilitating traumatic disease that is a huge burden for families and society, with no effective treatment (Nowrouzi et al., 2017; Yi et al., 2017).The pathophysiologic mechanisms of SCI remain unclear,despite numerous studies (Huang et al., 2018, 2019; Sharma et al., 2019; Wei et al., 2019). Autophagy (mainly referring to macroautophagy) is a generic term for the cellular processes that deliver cytoplasmic materials to the lysosome to degrade and recycle cell contents (Goldshmit et al., 2015; Li et al., 2019).
Autophagy regulates cell death in central nervous system diseases and improves neurological recovery (Galluzzi et al.,2016; Liu et al., 2017c). Although accumulating evidence shows that autophagy plays a critical role in SCI, several controversial views and debates still exist, especially on the overall effect of regulating autophagy and whether autophagy should be induced or inhibited. Some researchers have reported that upregulating autophagy promotes neuronal survival and the recovery of neurological function (Chen et al.,2013; Tang et al., 2014). However, other studies have shown that downregulating autophagy produces better therapeutic results (Fang et al., 2016; Xie et al., 2017a). Therefore, it is necessary to clarify whether upregulation or downregulation of autophagy is the best strategy, to lay the foundation for future studies. Although many tools have been used for evaluating SCI recovery, the Basso, Beattie, and Bresnahan (BBB)score is still the most widely used for neurological functional assessment. A number of studies compared upregulation or downregulation of autophagy with vehicle control, but only a few compared upregulation directly with downregulation.In this study, we performed direct and indirect network meta-analyses of rat models of SCI in which autophagy was upregulated or downregulated by comparing BBB scores to identify the best strategy. Additional systematic review was conducted to help guide future studies of autophagy in SCI.
Data and Methods
Literature search
The following electronic databases were searched from their inception to February 1, 2018: Web of Science, MEDLINE(OvidSP), PubMed, Wanfang, China National Knowledge Infrastructure (CNKI) and Google Scholar. The search was designed and executed by an experienced doctor (Duo Zhang). The search strategy consisted of two main components: spinal cord injury and autophagy, and the results were limited to rat studies. The keywords included “autophagy”,“spinal cord injury”, “rat”, with various combinations of the operators “AND” and “OR” during the literature retrieval.
Study selection, inclusion and exclusion criteria
Two independent reviewers performed the literature selection and included studies if the following criteria were met:(1) the study assessed the therapeutic effect of regulating autophagy in SCI with autophagy level evaluation; (2) the study was performed in rats in vivo; (3) the study contained groups treated with vehicle; (4) the study reported BBB scores.
The exclusion criteria for the title and abstract screen phase included the following: (1) not a primary study; (2)not an intervention of interest (regulation of autophagy); (3)data were not obtained or data were incorrect; (4) did not evaluate autophagy level after intervention.
Study characteristics and data extraction
Data were extracted from the full-text publications. The following items were extracted: author, year, language, species/strain, animal gender, number of animals in control and experimental groups, the method for the establishment of animal models, therapeutic agents, dosage, timing, duration,regulation of autophagy, and BBB scores. Higher BBB scores indicate better recovery of neurological function. BBB scores were extracted as mean ± standard deviation (SD) for the different time points. If BBB scores were not available in the text, but only presented in graph form, the data were obtained by measuring the graphs (Liu et al., 2017a). In brief,the graph was imported into Photoshop software, and we obtained the coordinate value of the scale interval of the Y-axis and the scatter plot or histogram with the SD line. The mean and SD were calculated by converting the coordinate values and scale interval.
Quality assessment of included studies
The SYRCLE’s Risk of Bias tool, based on the Cochrane Risk of Bias tool, was used to assess the risk of bias of all included studies, and was performed by two independent investigators (Hooijmans et al., 2014). SYRCLE’s Risk of Bias tool contains 10 entries that are related to selection bias, performance bias, detection bias, reporting bias and other biases.Disagreements were resolved by discussion.
Outcome measure
Locomotor function, evaluated with the BBB scale, was the main evaluation index. Normal function is rated as 21 points; lower scores reflect greater locomotor functional impairment.
Data management and statistical analysis
If studies contained multiple independent paired groups,they were treated as separate experiments; if studies contained multiple experimental groups with the same trends of autophagy regulation and a single control group, they were consolidated; if studies contained a single control group, but multiple experimental groups with different types of autophagy regulation, the control group was split (Higgins, 2011).Data were analyzed using RevMan 5.3 software (Cochrane Library, https://community.cochrane.org/help/tools-andsoftware/revman-5/revman-5-download), ITC software(Canadian Agency for Drugs and Technologies in Health,CADTH, https://www.cadth.ca/resources/itc-user-guide/download-software-win-xp), and mean difference (MD) and corresponding 95% confidence interval (CI) were used as the effect measures. Heterogeneity was quantified using the
χ2test, and the effect measure was I2(0% ≤ I2< 25% indicates no heterogeneity, 25% ≤ I2< 50% indicates low heterogeneity,50% ≤ I2< 75% indicates median heterogeneity, and 75% ≤I2indicates high heterogeneity). The fixed effects model was used if I2< 50%, otherwise, the random effects model was used. Subgroup analysis was performed based on the type of SCI model, and was used to analyze the source of heterogeneity. The BBB scores were analyzed according to the time after SCI, from 1 day to 4 weeks. P < 0.05 was considered statistically significant. Our procedures accorded with the PRISMA guidelines for reporting systematic review/meta-analysis (Moher et al., 2010).
Results
Description of the included studies
The comprehensive search strategy on the effect of autophagic regulation in SCI rat models resulted in 622 records (227 from the Web of Science, 45 from Medline (OvidSP), 83 from PubMed, 58 from WanFang data, 64 from CNKI and 145 from Google Scholar). After duplicates were removed,486 studies were left. After title and abstract screening, 85 studies were screened full-text. Ultimately, 33 studies were included in the meta-analysis shown with the PRISMA flow diagram (Chen et al., 2013, 2014; Hao et al., 2013; Tang et al.,2014; Gao et al., 2015, 2016; Li et al., 2015b, 2016b, 2018a, b;Seo et al., 2015; Yue et al., 2015; Fang et al., 2016, 2017; Sun et al., 2016; Wang et al., 2016, 2017a, b, 2018a, b; Zhou et al.,2016a, b; Bai et al., 2017; Liu et al., 2017b; Xie et al., 2017a,b; Zhang et al., 2017a, b; Zhao et al., 2017a, b; Miao et al.,2018; Tong et al., 2018) (Figure 1). The characteristics of all included studies are described in Table 1.
Risk of bias and quality of the included studies
Based on risk of bias assessment, 21 studies (64%) stated that the allocation was randomized, while no studies described the method of randomization or whether the allocation was adequately concealed. It was probably because the background of the animals was homogenous (Figure 2). Furthermore, many items were scored as “unclear”, as were items 3,5, 6, 8 and 9, indicating that these animal studies could have been better.
Overall and subgroup meta-analyses of the effects of upregulation of autophagy on BBB scores
Because assessments of BBB scores were performed at different time points, meta-analyses were also performed at the following different time points: 1 day, 3 days, 1 week, 2 weeks ± 1 day, 3 weeks ± 1 day, and 4 weeks ± 1 day after SCI (termed 1 day, 3 days, 1 week, 2 weeks, 3 weeks and 4 weeks).
Eight of the 33 studies examining upregulation of autophagy reported BBB scores at 1 day after SCI (Chen et al.,2014; Li et al., 2015b; Gao et al., 2016; Sun et al., 2016; Fang et al., 2017; Wang et al., 2017a; Zhao et al., 2017a; Miao et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly elevated BBB scores using the random effects model (MD = 1.80, 95% CI: 0.81-2.79, P = 0.0004). However, heterogeneity was quite high (I2= 97%). These studies were separated into three subgroups according to the type of SCI model. As shown in Figure 3,upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 1.43, 95% CI: 0.28-2.58,P = 0.01) and the compression injury group (MD = 0.80,95% CI: 0.15-1.45, P = 0.02) with high inter-subgroup heterogeneity (I2= 91.4%; Figure 3). The sensitivity analysis showed no heterogeneity (I2= 0%) between the contusion injury and compression injury groups.
Ten of the 33 studies examining upregulation of autophagy reported BBB scores at 3 days after SCI (Tang et al., 2014;Gao et al., 2015, 2016; Yue et al., 2015; Li et al., 2016b; Wang et al., 2016, 2017b; Zhou et al., 2016a; Zhang et al., 2017a;Zhao et al., 2017a). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 0.92,95% CI: 0.72-1.13, P < 0.00001). However, heterogeneity was moderate (I2= 68%). These studies were separated into three subgroups according to the SCI model. As shown in Figure 4,upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 0.98, 95% CI: 0.73-1.23,P < 0.00001), with low subgroup heterogeneity (I2= 38%)and high inter-subgroup heterogeneity (I2= 87.9%; Figure 4).Sensitivity analysis showed similar heterogeneity levels.
Of the 33 studies examining upregulation of autophagy, 17 reported BBB scores at 1 week after SCI (Tang et al., 2014;Gao et al., 2015, 2016; Fang et al., 2016; Li et al., 2016a, b,2018a; Sun et al., 2016; Wang et al., 2016, 2017a, b, 2018a;Zhou et al., 2016a; Bai et al., 2017; Zhang et al., 2017a, b;Zhao et al., 2017a). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 2.39,95% CI: 1.85-2.92, P < 0.00001). However, heterogeneity was quite high (I2= 84%). Based on the type of SCI model,these experiments were separated into four subgroups. As shown in Figure 5, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD =2.21, 95% CI: 1.55-2.86, P < 0.00001), with high subgroup heterogeneity (I2= 84%) and high inter-subgroup heterogeneity (I2= 75.4%; Figure 5). Sensitivity analysis showed similar heterogeneity levels.
Similarly, 17 of the 33 studies that examined upregulation of autophagy reported BBB scores at 2 weeks after SCI (Chen et al., 2013, 2014; Gao et al., 2015, 2016; Li et al., 2016b,2018a; Sun et al., 2016; Wang et al., 2016, 2018b; Zhou et al.,2016a, b; Bai et al., 2017; Wang et al., 2017a; Zhang et al.,2017a, b; Zhao et al., 2017a; Tong et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 3.26, 95% CI: 2.40-4.13, P < 0.00001).However, heterogeneity was quite high (I2= 90%). Based on the type of SCI model, the experiments were separated into two subgroups. As shown in Figure 6, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 2.71, 95% CI: 1.99-3.44, P < 0.00001),with medium subgroup heterogeneity (I2= 74%), and in the compression injury group (MD = 4.06, 95% CI: 2.48-5.65,P < 0.00001), with high subgroup heterogeneity (I2= 94%).The inter-subgroup heterogeneity was moderate (I2= 56.4%;Figure 6). After two studies (Zhou et al., 2016b; Li et al.,2018a) were excluded, the heterogeneity in the compression injury subgroup and subgroup difference diminished to 0,and the overall heterogeneity decreased from high (I2= 90%)to moderate (I2= 68%).
Sixteen of the 33 studies that examined upregulation of autophagy reported BBB scores at 3 weeks after SCI (Chen et al., 2013, 2014; Tang et al., 2014; Gao et al., 2015, 2016; Li et al., 2016a; Wang et al., 2016, 2018a, b; Zhou et al., 2016a;Bai et al., 2017; Liu et al., 2017b; Zhang et al., 2017a, b; Zhao et al., 2017a; Tong et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly improved BBB scores using the random effects model (MD
= 3.13, 95% CI: 2.51-3.75, P < 0.00001). However, heterogeneity was quite high (I2= 85%). Based on the type of SCI model, the experiments were separated into four subgroups.As shown in Figure 7, upregulation of autophagy significantly improved BBB scores in the contusion injury group(MD = 2.90, 95% CI: 2.00-3.81, P < 0.00001), with high subgroup heterogeneity (I2= 87%), and in the compression injury group (MD = 3.71, 95% CI: 2.99-4.42, P < 0.00001), with low subgroup heterogeneity (I2= 0%). The inter-subgroup heterogeneity was low (I2= 49.4%; Figure 7). Subgroup heterogeneity decreased (I2= 6.2%) after the transection injury(TI) subgroup was excluded.
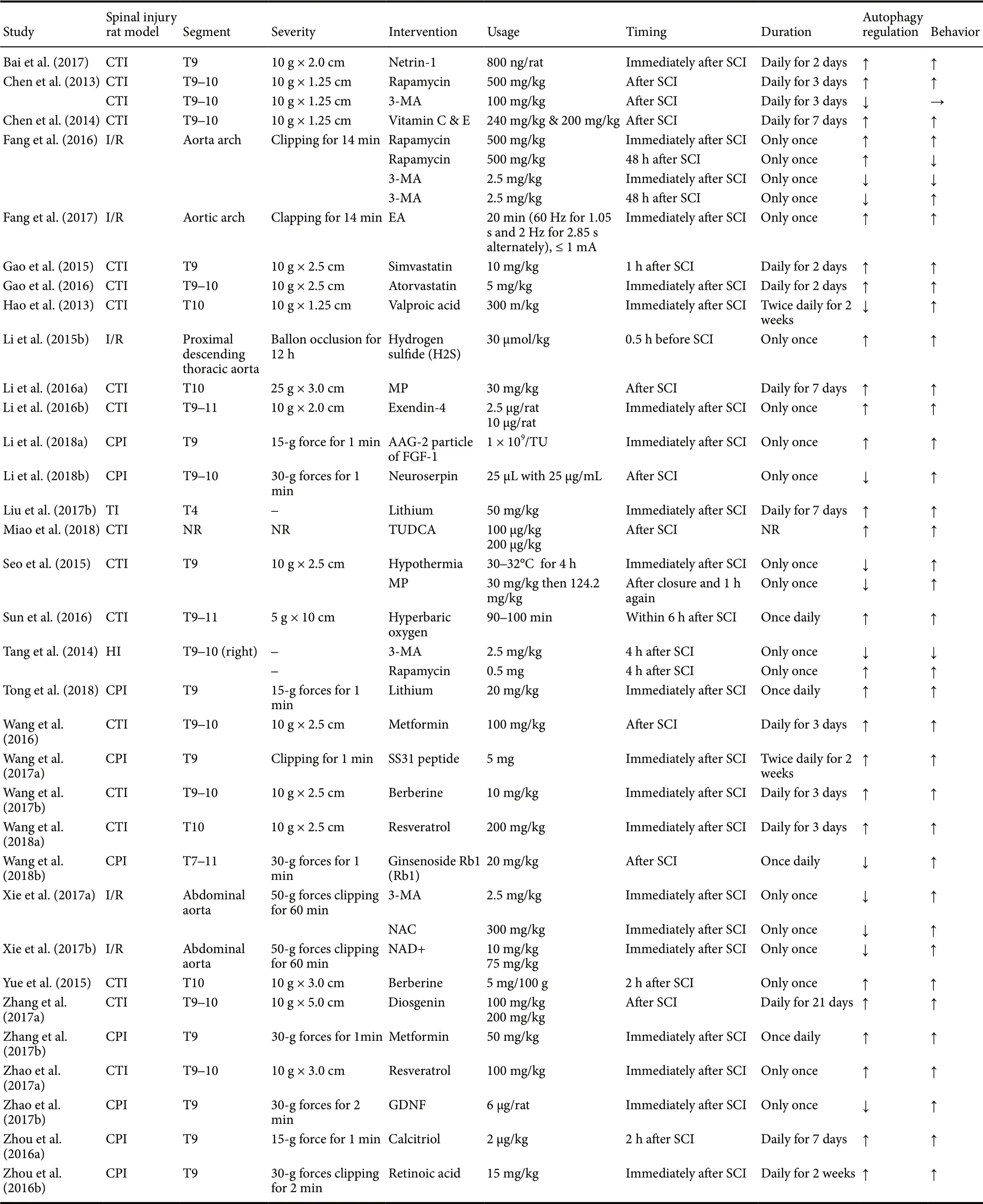
Table 1 Characteristics of the included animal studies
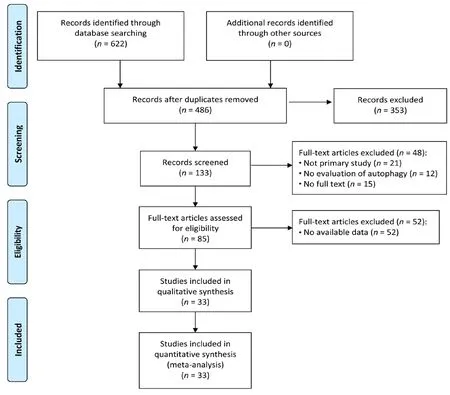
Figure 1 Flow diagram of the literature search and selection results.
Ten of the 33 studies that examined upregulation of autophagy reported BBB scores at 4 weeks after SCI (Chen et al.,2013, 2014; Gao et al., 2015, 2016; Wang et al., 2016, 2018 a; Zhou et al., 2016a; Zhang et al., 2017b; Zhao et al., 2017a;Tong et al., 2018). Meta-analysis revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 3.18, 95% CI: 2.43-3.92, P <0.00001). However, heterogeneity was moderate (I2= 74%).The experiments were separated into two subgroups according to the type of SCI model used. As shown in Figure 8,upregulating autophagy significantly improved BBB scores in the contusion injury group (MD = 2.93, 95% CI: 2.02-3.84,P < 0.00001), with high subgroup heterogeneity (I2= 79%),and in the compression injury group (MD = 3.84, 95% CI:2.97-4.71, P < 0.00001), with low subgroup heterogeneity (I2= 0%). The inter-subgroup heterogeneity was moderate (I2=50.1%; Figure 8). The subgroup heterogeneity decreased (I2= 0.3%) after one contusion injury study was excluded (Wang et al., 2016).
Overall and subgroup analyses of the effects of downregulation of autophagy on BBB scores
Downregulation of autophagy increased BBB scores at 1 day, and 2, 3 and 4 weeks after SCI, but not at 3 days (MD =1.20, 95% CI: -0.74-3.14, P = 0.23) or 1 week (MD = 0.56,95% CI: -1.11-2.23, P = 0.51). Subgroup analysis revealed that downregulating autophagy did not significantly improve BBB scores in the contusion injury group at 1, 3 or 4 weeks,but did so at 2 weeks (MD = 0.88, 95% CI: 0.29-1.48, P =0.004). In the compression injury group, downregulation of autophagy significantly ameliorated BBB scores at 1, 2, 3 and 4 weeks (Figures 9-14).
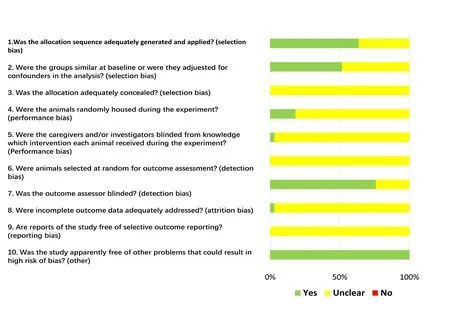
Figure 2 Graph of the risk of bias for the included studies.
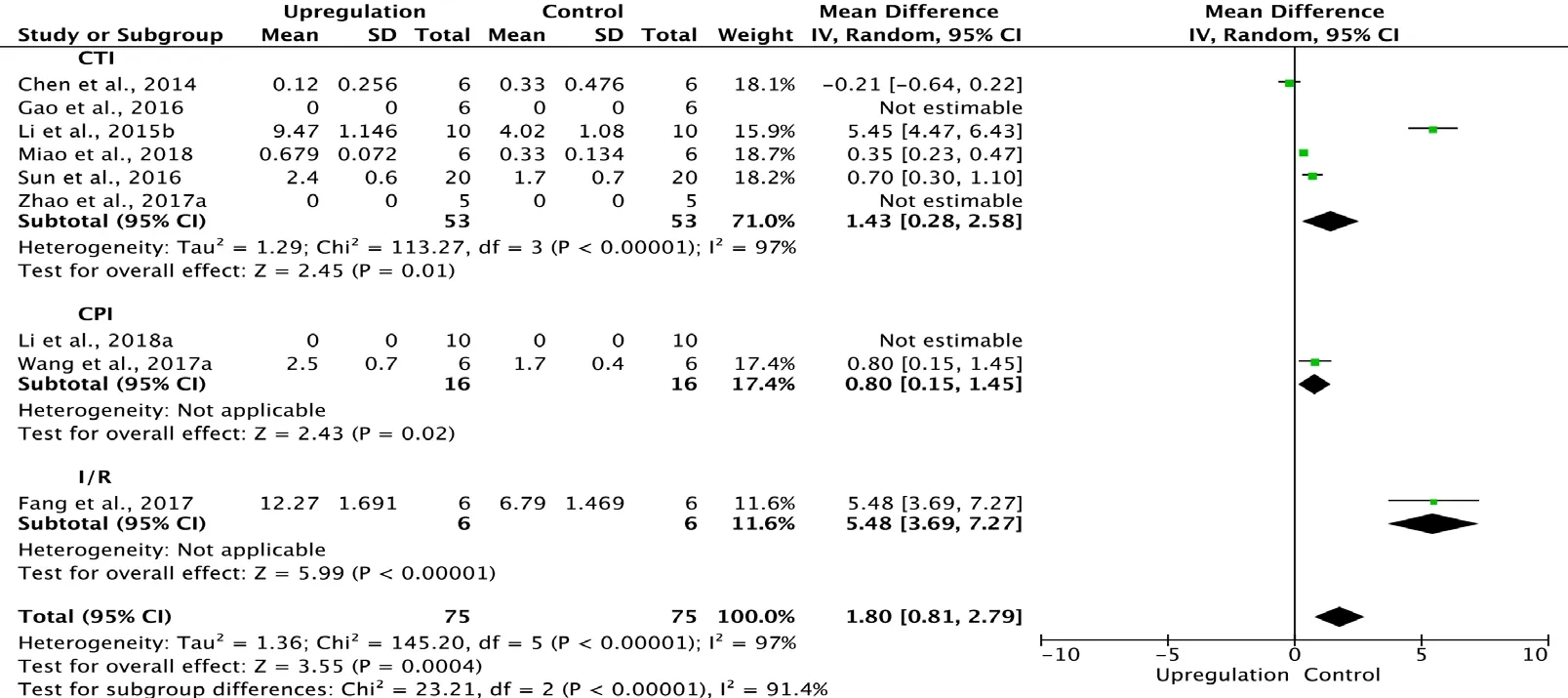
Figure 3 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 1 day after spinal cord injury.
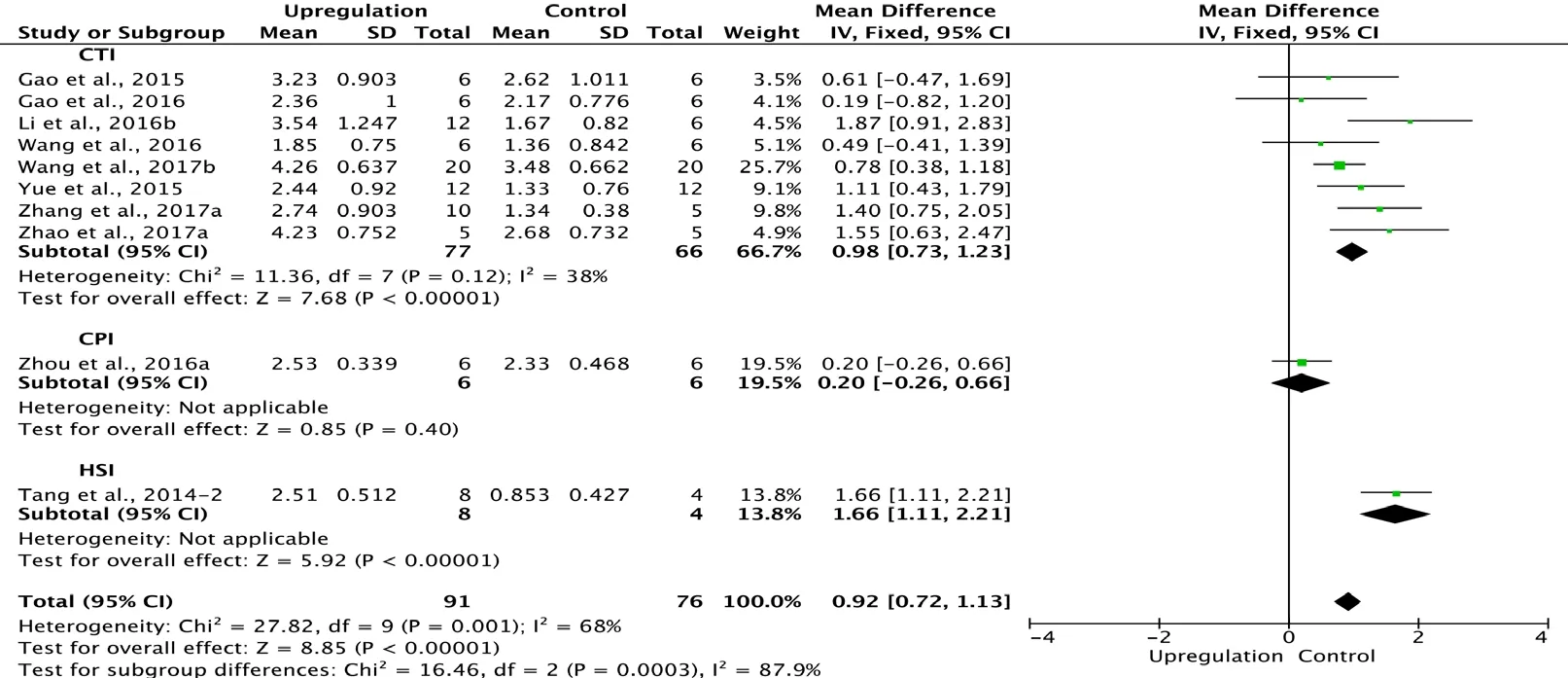
Figure 4 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 3 days after spinal cord injury.
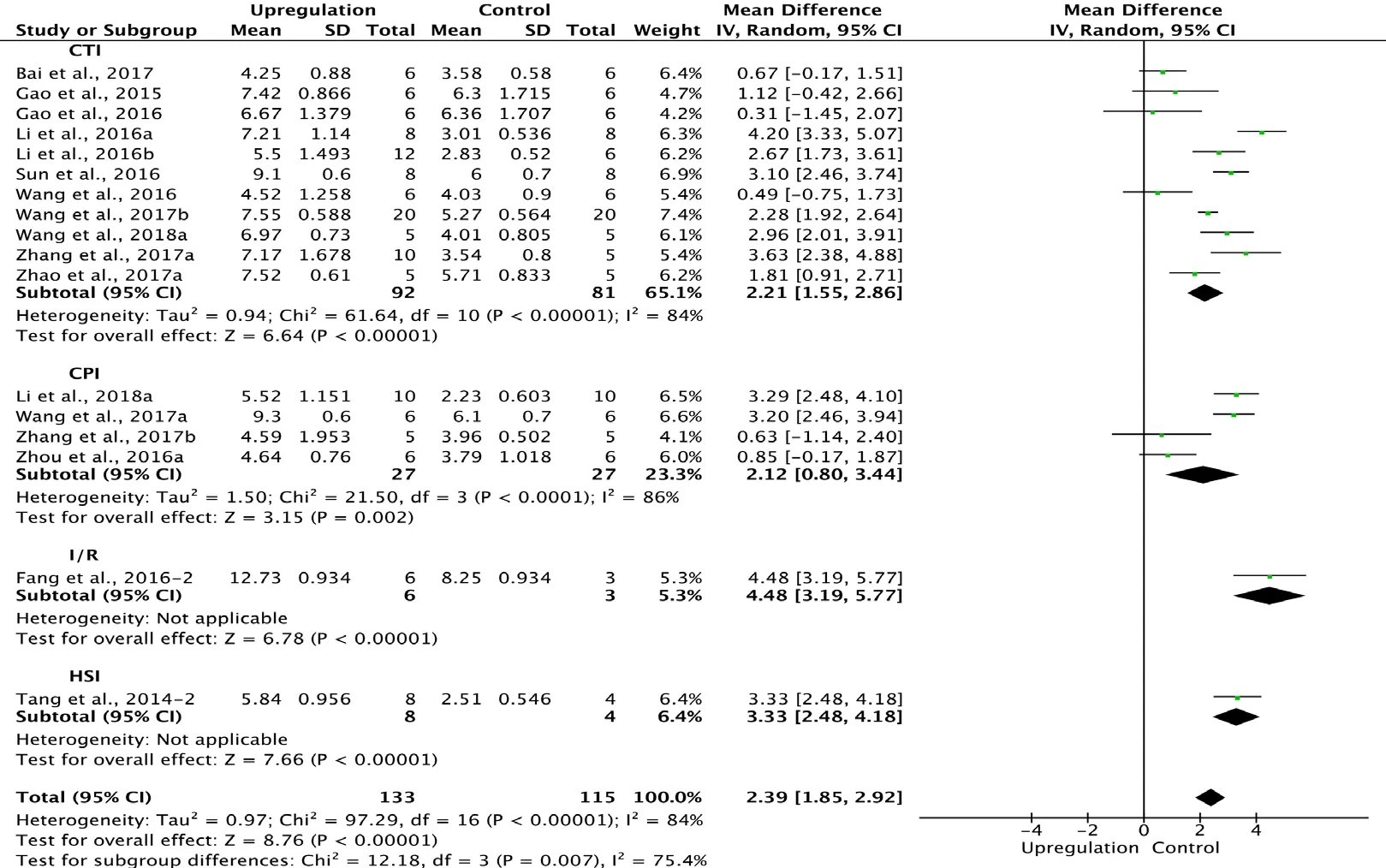
Figure 5 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 1 week after spinal cord injury.
Three of the 34 studies that examined downregulation of autophagy reported BBB scores at 1 day after SCI (Xie et al., 2017a, b; Zhao et al., 2017b). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores, as assessed with the fixed effects model (MD = 6.48, 95% CI: 5.83-7.13, P < 0.00001).Heterogeneity was quite low (I2= 0%; Figure 9).
Two of the 34 studies that examined downregulation of autophagy reported BBB scores at 3 days after SCI (Tang et al., 2014; Zhao et al., 2017b). Meta-analysis of these experiments revealed that downregulation of autophagy did not significantly elevate BBB scores using the random effects model (MD = 1.20, 95% CI: -0.74-3.14, P = 0.23). However,heterogeneity was quite high (I2= 99%; Figure 10). Sensitivity analysis showed similar heterogeneity.
Six of the 33 studies that examined downregulation of autophagy reported BBB scores at 1 week after SCI (Hao et al., 2013; Tang et al., 2014; Seo et al., 2015; Fang et al., 2016;Zhao et al., 2017b; Li et al., 2018b). Meta-analysis of these experiments revealed that downregulation of autophagy did not significantly increase BBB scores using the random effects model (MD = 0.56, 95% CI: -1.11-2.3, P = 0.51). However, heterogeneity was quite high (I2= 97%). Based on the type of SCI model used, the experiments were separated into four subgroups. As shown in Figure 11, downregulation of autophagy did not improve BBB scores in the contusion injury group (MD = 0.39, 95% CI: -0.12-0.90, P = 0.13), with low heterogeneity (I2= 0%). In contrast, downregulation of autophagy improved BBB scores in the compression injury group (MD = 2.64, 95% CI: 1.03-4.24, P = 0.001), with high heterogeneity (I2= 95%). The inter-subgroup heterogeneity was also high (I2= 94.8%; Figure 11). The heterogeneity was similar when any single study was excluded.
Six of the 33 studies examining downregulation of autophagy reported BBB scores at 2 weeks after SCI (Chen et al.,2013; Hao et al., 2013; Seo et al., 2015; Zhao et al., 2017b;Li et al., 2018b; Wang et al., 2018a). Meta-analysis of these experiments revealed that downregulation of autophagy significantly enhanced BBB scores, as assessed with the random effects model (MD = 2.43, 95% CI: 0.79-4.07, P = 0.004).However, heterogeneity was quite high (I2= 96%). The experiments were separated into two subgroups according to the SCI model. As shown in Figure 13, downregulation of autophagy significantly improved BBB scores in the contusion injury group (MD = 0.88, 95% CI: 0.29-1.48, P = 0.004),with low heterogeneity (I2= 2%). Downregulation of autophagy also increased BBB scores in the compression injury group (MD = 3.64, 95% CI: 2.04-5.23, P < 0.00001), but with high heterogeneity (I2= 93%). The inter-subgroup heterogeneity was high (I2= 90.0%; Figure 12). The heterogeneity was similar when any single study was excluded.
Six of the 33 studies that examined the effect of downregulating autophagy reported BBB scores at 3 weeks after SCI(Chen et al., 2013; Hao et al., 2013; Tang et al., 2014; Seo et al., 2015; Zhao et al., 2017b; Wang et al., 2018a). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores using the random effects model (MD = 2.96, 95% CI: 0.09-5.84, P = 0.04).However, heterogeneity was quite high (I2= 99%). Based on the type of SCI model used, the experiments were separated into three subgroups. As shown in Figure 14, downregulation of autophagy did not significantly ameliorate BBB scores in the contusion injury group (MD = 1.22, 95% CI:-0.18-2.63, P = 0.09), with high heterogeneity (I2= 88%).In contrast, downregulation of autophagy improved BBB scores in the compression injury group (MD = 6.56, 95% CI:3.03-10.08, P = 0.0003), with high heterogeneity (I2= 96%).The inter-subgroup heterogeneity was high (I2= 95.3%; Figure 13). The heterogeneity was similar when any single study was excluded.
Five of the 33 studies that examined the effect of downregulating autophagy reported BBB scores at 4 weeks after SCI(Chen et al., 2013; Hao et al., 2013; Seo et al., 2015; Zhao et al., 2017b; Wang et al., 2018b). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores using the random effects model(MD = 4.41, 95% CI: 1.08-7.75, P = 0.01). However, heterogeneity was quite high (I2= 98%). Based on the SCI model used, the experiments were separated into two subgroups.As shown in Figure 14, downregulation of autophagy did not significantly increase BBB scores in the contusion injury group (MD = 1.36, 95% CI: -0.30-3.02, P = 0.11), with high heterogeneity (I2= 87%). In comparison, downregulation of autophagy improved BBB scores in the compression injury group (MD = 7.44, 95% CI: 3.00-11.87, P = 0.001), with high heterogeneity (I2= 98%). The inter-subgroup heterogeneity is shown in Figure 14 (I2= 84.2%).
Publication bias
Funnel plots were drawn to evaluate publication bias. The shape of the funnel plots at 3 days of upregulation of autophagy and at 1 day of downregulation of autophagy appeared approximately symmetrical (Figures 15 and 16), suggesting no publication bias. However, publication bias was obvious at other time points.
Discussion
The role of autophagy in neurotrauma remains less clear and whether autophagy is good or bad is under debate in experimental models of brain injury or SCI (Wu and Lipinski, 2019). An increasing number of studies have focused on the effects of autophagy in treating SCI (Zhang et al.,2018). Both upregulation and downregulation of autophagy have been shown to have therapeutic effects in SCI,even in the same SCI rat model (Seo et al., 2015; Gao et al.,2016). However, there is lack of studies directly comparing upregulation with downregulation of autophagy for SCI.Therefore, we performed this meta-analysis with direct and indirect comparisons.
Therapeutic effects of autophagic regulation in SCI
In this comprehensive meta-analysis, we analyzed and described the effects of upregulation and downregulation of autophagy on BBB scores in SCI rat models. The neurological function of rats with SCI improved at almost every time point after the enhancement or inhibition of autophagy, expect for downregulation of autophagy at 3 days and 1 week after SCI. Indirect comparison revealed similar therapeutic effects of upregulation and downregulation of autophagy.The results of our current analyses are consistent with our previous publication, indicating that autophagy plays a very important role in SCI (Zhang et al., 2018).
Impact of the SCI model
The subgroup analysis led to the novel finding that the type of SCI model strongly influences autophagy processes after injury. The hemisection injury model, contusion injury model, compression injury model and the ischemia/reperfusion injury model are the four most widely used traumatic SCI models. These different SCI models produce different autophagic patterns after injury. In the hemisection injury model, autophagy levels increase and peak at 3 days after SCI(Kanno et al., 2009, 2011; Hou et al., 2014; Tang et al., 2014).In the contusion injury model, although enhancement of autophagy was observed at 1, 3, 4 and 7 days after SCI among the various studies, the autophagic patterns appear to differ(Sekiguchi et al., 2012; Walker et al., 2012; Chen et al., 2013,2014; Li et al., 2016b). In some studies, the autophagy level increased and peaked 2 hours after SCI, while in other studies, autophagy peaked at 14 days after SCI (Hao et al., 2013;Wang et al., 2014). Differences in the severity of contusion injury may contribute to the variability. For example, injury in the former study (10 g weight dropped from a height of 25 mm) was more severe than in the latter (8 g weight dropped from a height of 25 mm). In compression injury models,autophagy levels generally increase after SCI. However, few studies have examined the temporal pattern of autophagy(Zhou et al., 2015, 2016b; Zhang et al., 2017b). Wang et al.(2018b) reported that LC-3II/LC3-I increased and peaked at 7 days after SCI. Two peaks of autophagy were seen at 8 and 72 hours after I/R injury with 14-min clipping of the thoracic aortic arch in one study. In another study, the autophagy level increased from 3 hours and peaked at 24 hours after I/R injury with 10-minute balloon occlusion of the thoracic aorta (Fang et al., 2016; Wei et al., 2016).
Our meta-analysis finding that inhibition of autophagy improves BBB scores in rats with compression injury, but not contusion injury, together with previous studies suggests that the type and severity of SCI strongly affects the activation and pattern of autophagy, as well as the response to autophagic regulation (Lipinski et al., 2015). It is difficult to compare severities among the various injury models, given that the mechanisms of primary and secondary injury in the different SCI models are still unclear (Anwar et al., 2016).
Standardizing the SCI model, including the type, severity and injury site, may help clarify the role of autophagy and its regulation in SCI. Contusion injury is the most widely used model in experimental studies, particularly as half of SCI patients have this type of injury (Norenberg et al., 2004).We recommend the contusion injury model produced by the New York University impactor, using a 10-g rod dropped from a height of 25 mm, as the standardized SCI model in rats.
Dual character of autophagy in SCI
An interesting feature of autophagy is its dual character—moderate autophagic activation appears to promote cell survival, while excessive activation promotes cell death (Galluzzi et al., 2016). Autophagic cell death is considered a form of non-apoptotic programmed cell death, but is also considered a type of regulated cell death, indicating that the definition and role of autophagic cell death are still unclear (Clarke,1990; Berry and Baehrecke, 2007). Additionally, it is unclear whether autophagic cell death is an unsuccessful attempt to avoid programmed cell death (Yonekawa and Thorburn,2013). Furthermore, there are complex interactions between autophagic and apoptotic signaling pathways and proteins,including p53, Ser/Thr kinase and other effectors. Recent studies suggest that inducing autophagy reduces apoptosis of neural cells, while autophagic flux blockade enhances apoptosis (Fang et al., 2016; Zhou et al., 2016a; Zhang et al.,2017b; Wang et al., 2018a). However, current concepts do not adequately explain all experimental results (Tang et al.,2014; Chen et al., 2017; Jin et al., 2017; Wang et al., 2018b),suggesting that the mechanisms and regulation of autophagy need further investigation.
Other factors that should be considered
As we summarized in Table 1, different drugs affected autophagy, of which 3-MA and rapamycin are most commonly used for inhibiting and inducing autophagy. For the same intervention, there are several factors that could impact the effects of regulating autophagy in treating SCI in rats, such as the timing, dosage, route of administration, and duration of treatment (Zhang et al., 2013; Tang et al., 2014; Zhou et al., 2015; Fang et al., 2016; Zhao et al., 2017b). Upregulation or downregulation of autophagy may have different, or even opposing, effects at different time points, as reported by Fang et al. (2016). These investigators found that BBB scores were worse when autophagy was inhibited at an early stage(immediately) after SCI, while BBB scores were improved when autophagy was stimulated at an early stage after SCI.However, inhibiting autophagy increased BBB scores at the late stage (48 hours) after SCI, while BBB scores were not improved when autophagy was stimulated at this stage. We posit that autophagy is a dynamic process that should be divided into several stages, and that it plays positive or negative effects in the different stages. Further study is needed to clarify the time-dependency of autophagic processes and their regulation.
Limitations
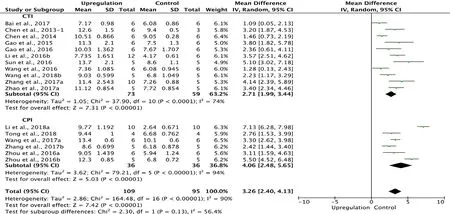
Figure 6 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 2 weeks after spinal cord injury.
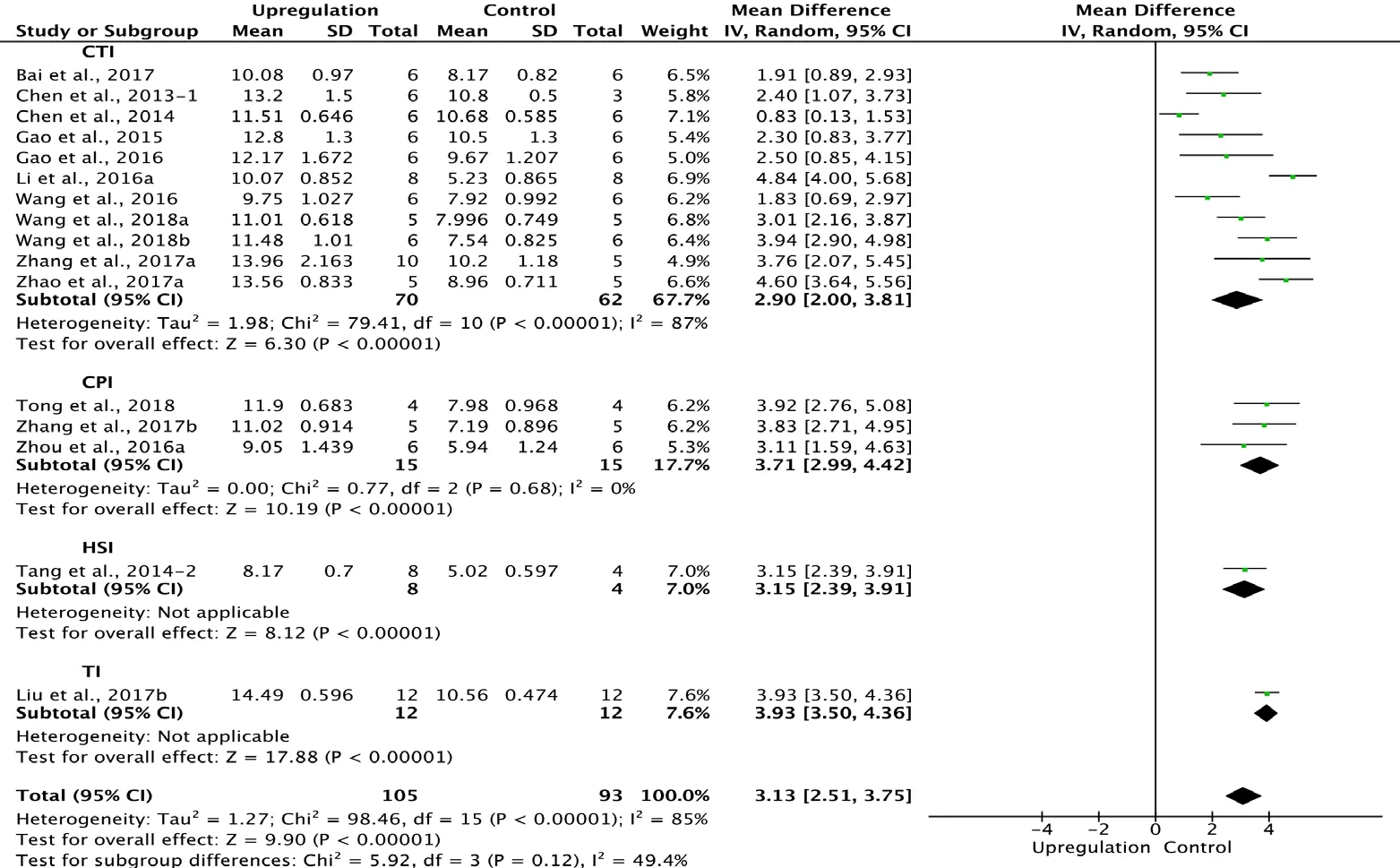
Figure 7 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 3 weeks after spinal cord injury.
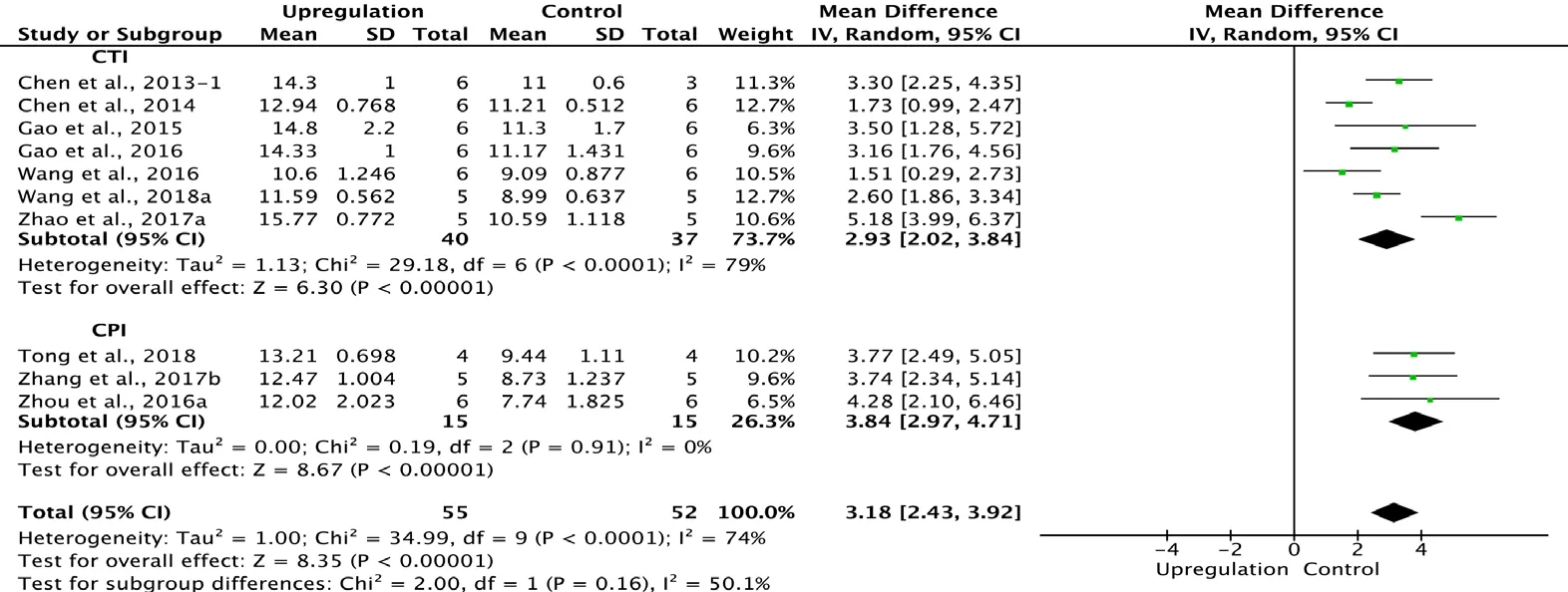
Figure 8 Effect of upregulation of autophagy on Basso, Beattie,and Bresnahan scores at 4 weeks after spinal cord injury.
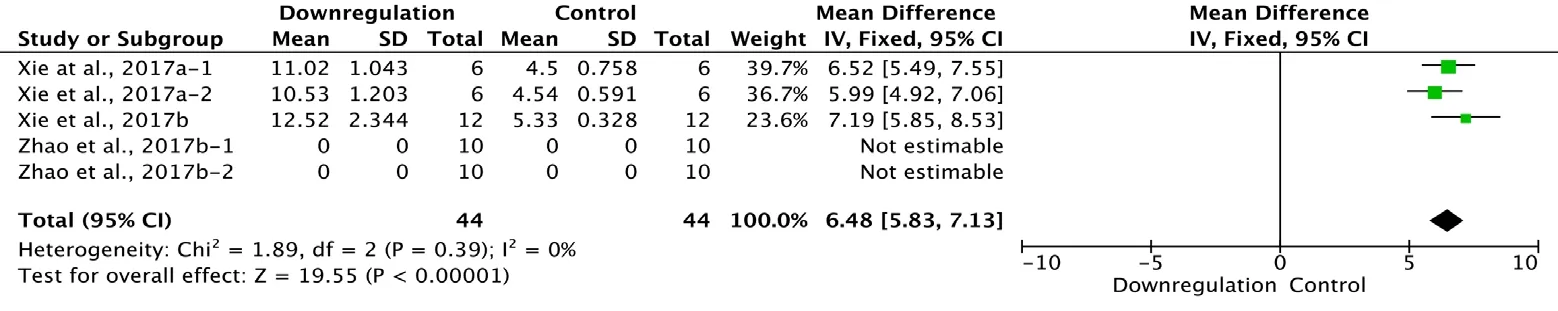
Figure 9 Effect of downregulation of autophagy on Basso, Beattie,and Bresnahan scores at 1 day after spinal cord injury.
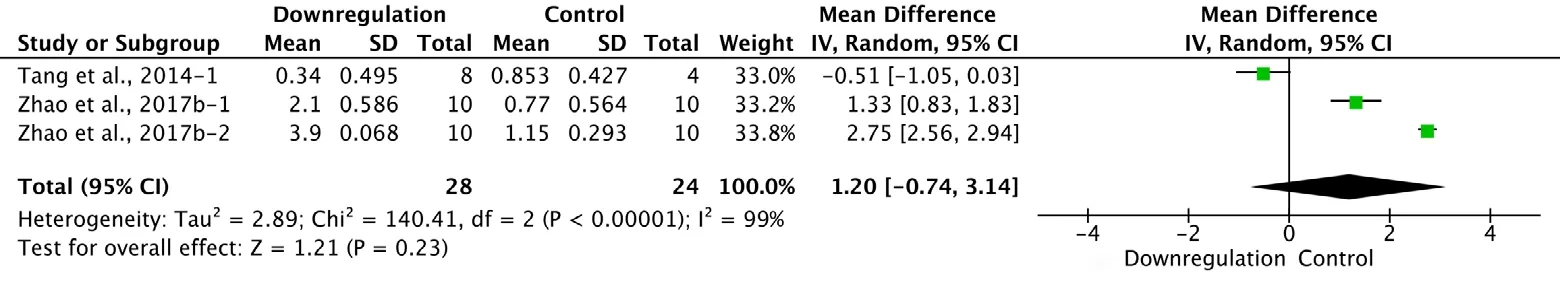
Figure 10 Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 3 days after spinal cord injury.
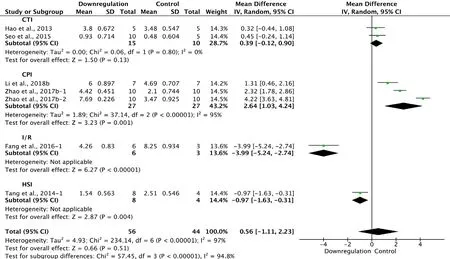
Figure 11 Effect of downregulation of autophagy on Basso, Beattie,and Bresnahan scores at 1 week after spinal cord injury.
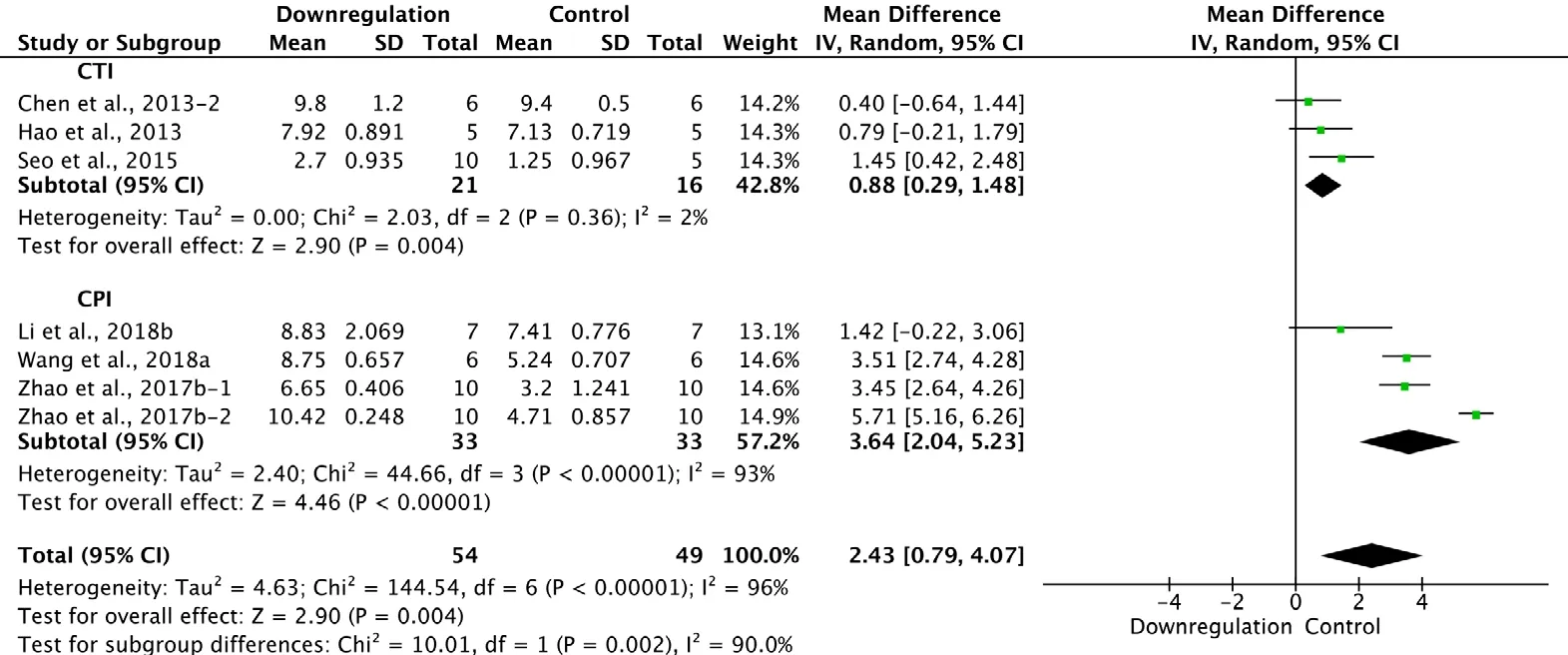
Figure 12 Effect of downregulation of autophagy on Basso, Beattie,and Bresnahan scores at 2 weeks after spinal cord injury.
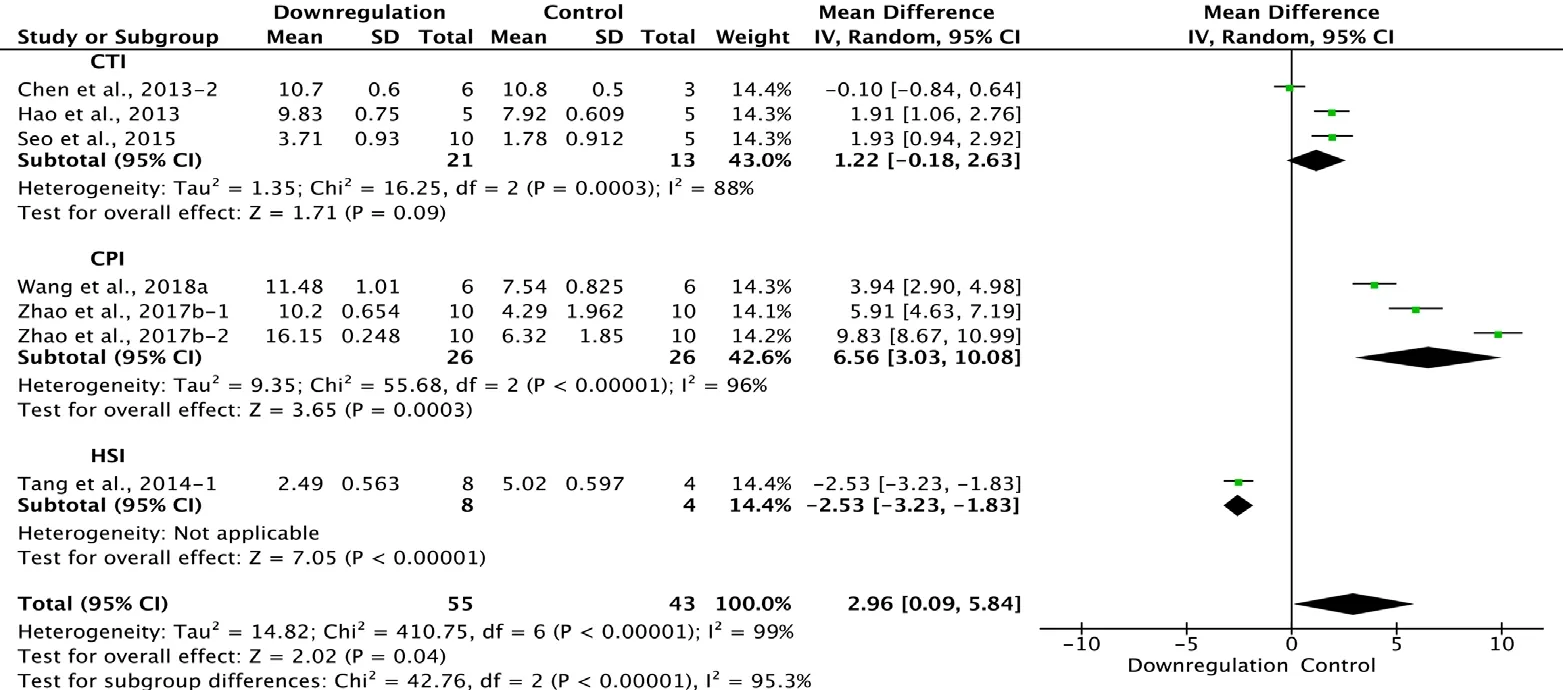
Figure 13 Effect of downregulation of autophagy on Basso, Beattie,and Bresnahan scores at 3 weeks after spinal cord injury.
Our meta-analysis has several limitations. First, the number of included studies was limited, and most of these studies focused on the downregulation of autophagy, which may affect the results. The quality of the included studies was also not high, based on evaluation with SYRCLE’s Risk of Bias tool. Many items were assessed as “unclear”, indicating that the methodological quality was unclear, especially because of inadequate blinding, lack of random selection of animals for outcome assessment, or selective outcome reporting. Thus,selection bias, detection bias, attrition bias and reporting bias may be high in these studies. The SYRCLE tool was established in 2014, but many of the studies were published before this date. Methodological quality should be improved by investigators in future studies. High heterogeneity was a widespread feature of the included studies, although it is common in animal studies, and we performed subgroup analysis to clarify potential influencing factors (Li et al., 2015a). There was no commercial funding for any of the studies. Second,different agents, timings, dosages, administration routes and duration of treatment were not consistent among the included studies, and it was difficult to compare the therapeutic effects at the same level of autophagy. Third, we excluded several studies because of the lack of BBB scores. While the BBB score is not the only method to evaluate neurological function, it is the most widely used. Although some studies evaluated neuronal survival or regeneration, the evaluation was not standardized to enable comparison. Moreover, the most important outcome is functional recovery, rather than improved histological findings. Therefore, BBB scores are still currently the best index for comparison. We await a novel method for objective and accurate evaluation.
Implications for clinical practice
Based on the results of our meta-analysis, both upregulation of autophagy and downregulation of autophagy improved long-term neurological recovery in SCI rats. However, the analysis failed to identify major differences between upregulation and downregulation of autophagy. Therefore, much work remains to be done. Research methods need to be improved, such as application of transgenic animals, establishment of standardized protocols, and the development of new noninvasive monitoring techniques for autophagy. At present, there are no autophagy-modulating agents in clinical use. However, autophagy is a potential therapeutic target worth greater attention.
Conclusions
Regulating autophagy promotes the recovery of neurological function. Indirect comparisons between upregulation and downregulation of autophagy and the systematic review underscore the need for standardized research protocols and for a focus on the dynamic regulation of autophagy. Despite the limitations, this meta-analysis provides new insights to help guide the design of high-quality future animal studies with clinical relevancy.
Author contributions:Study concept and design: DZhang; literature search: YY; study selection, inclusion, exclusion and data extraction:DZhang, JCZ; data analysis: DZhang, FW, XZ; graphs and tables production: CXL; manuscript drafting: DZhang, DZhu; All authors approved the final version of the manuscript.
Conflicts of interest:The authors have no conflicts of interest to declare.
Financial support:This work was supported by the Beijing Excellent Talent Training Funding of China, No. 2017000021469G215 (to DZhang); the Natural Science Foundation of Capital Medical University of China, No. PYZ2018081 (to DZhang); the Youth Science Foundation of Beijing Tiantan Hospital of China, No. 2016-YQN-14 (to DZhang).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Reporting statement:This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Beijing Tiantan Hospital, Capital Medical University, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study areavailable from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Warin Krityakiarana, Mahidol University-Ratchasuda College, Thailand.
Additional file:open peer review report 1.

Figure 14 Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 4 weeks after spinal cord injury.
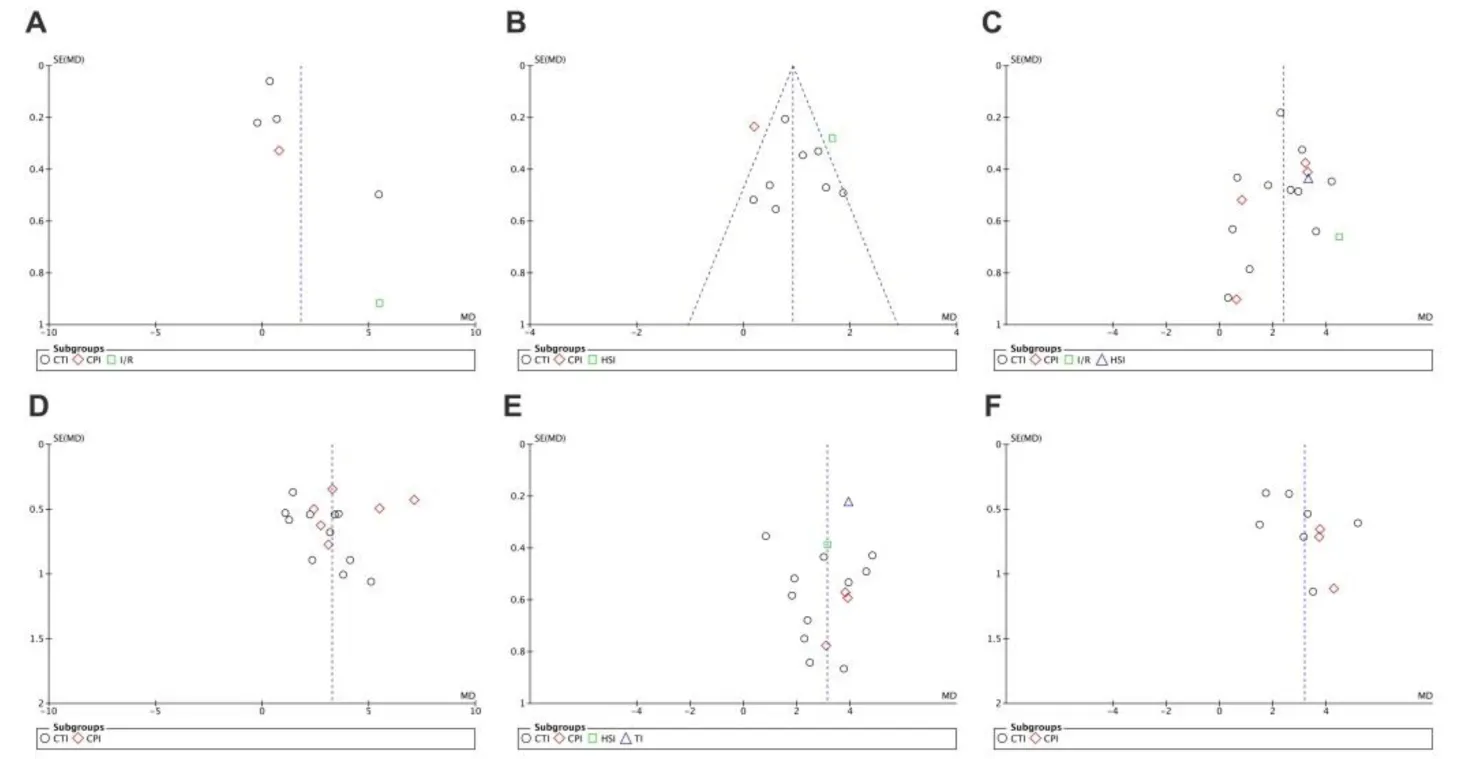
Figure 15 Funnel plot for detection of publication bias in meta-analysis of the upregulation of autophagy at different time points.
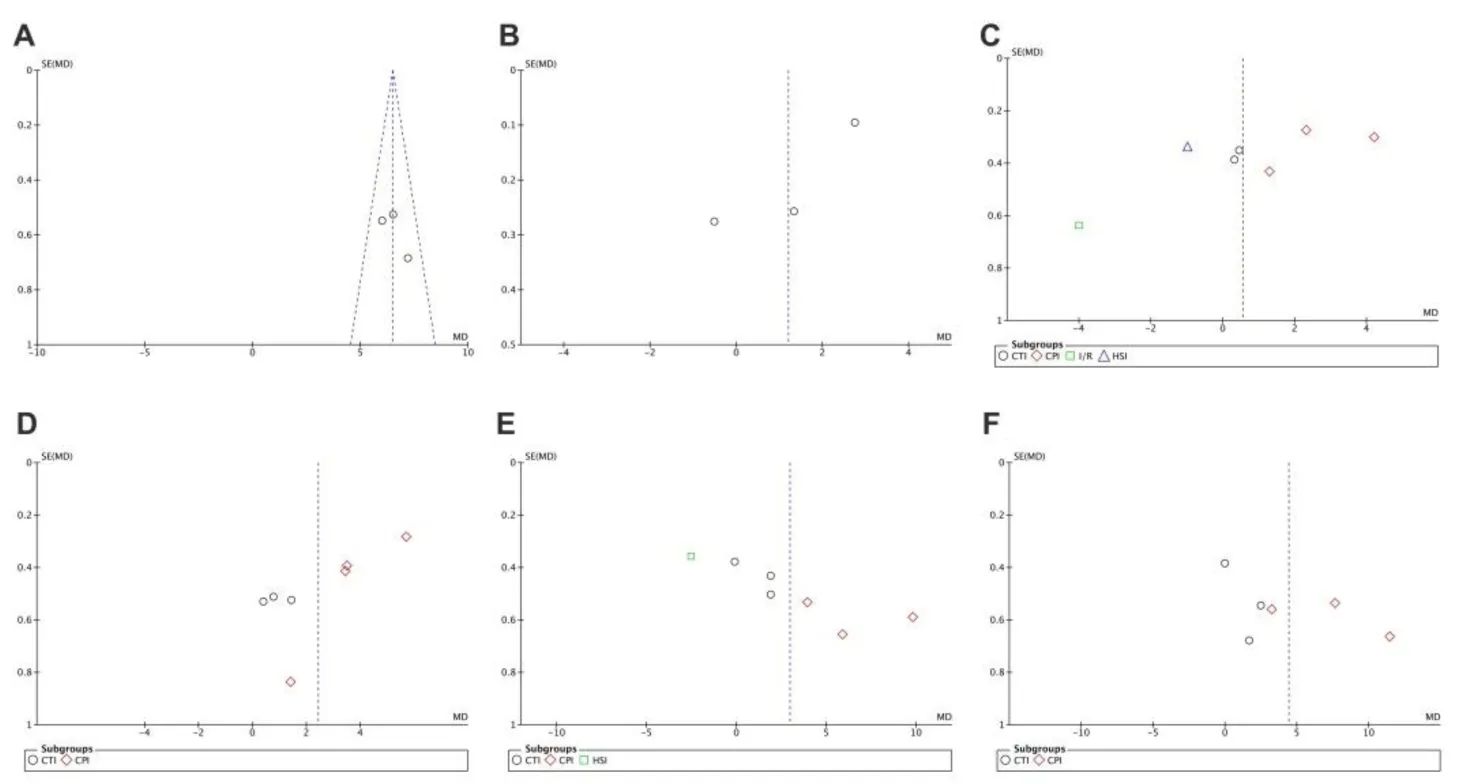
Figure 16 Funnel plot for detection of publication bias in meta-analysis of downregulation of autophagy at different time points.
杂志排行
中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy
