Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
2020-12-02WilliamRodemerJianliHuMichaelSelzerMichaelShifman
William Rodemer, Jianli Hu, Michael E. Selzer, , Michael I. Shifman,
1 Shriners Hospitals Pediatric Research Center (Center for Neural Repair and Rehabilitation), the Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA
2 Department of Neurology, the Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA
Abstract Some neurons, especially in mammalian peripheral nervous system or in lower vertebrate or in vertebrate central nervous system (CNS) regenerate after axotomy, while most mammalian CNS neurons fail to regenerate. There is an emerging consensus that neurons have different intrinsic regenerative capabilities,which theoretically could be manipulated therapeutically to improve regeneration. Population-based comparisons between “good regenerating” and “bad regenerating” neurons in the CNS and peripheral nervous system of most vertebrates yield results that are inconclusive or difficult to interpret. At least in part, this reflects the great diversity of cells in the mammalian CNS. Using mammalian nervous system imposes several methodical limitations. First, the small sizes and large numbers of neurons in the CNS make it very difficult to distinguish regenerating neurons from non-regenerating ones. Second, the lack of identifiable neurons makes it impossible to correlate biochemical changes in a neuron with axonal damage of the same neuron, and therefore, to dissect the molecular mechanisms of regeneration on the level of single neurons.This review will survey the reported responses to axon injury and the determinants of axon regeneration,emphasizing non-mammalian model organisms, which are often under-utilized, but in which the data are especially easy to interpret.
Key Words: axonal regeneration; identifiable neurons; intrinsic factors; lamprey; Mauthner cell; Müller cell;neuronal death; non-mammalian model organisms; spinal cord injury; zebrafish
Introduction
Spinal cord injury (SCI) results in persistent functional impairments because axons in the central nervous system (CNS)fail to regenerate. This regeneration failure is not observed uniformly among neurons, but varies depending upon intrinsic and extrinsic molecular determinants, which are governed by phylogenetic, developmental stage, and cell subtype influences.
The largest and most unambiguous division of regenerative ability is between the CNS and peripheral nervous system (PNS). Although far from perfect, compared to the CNS, axons in the PNS regenerate robustly after injury. In part, this can be attributed to the more growth-permissive extracellular environment of the PNS because after axon injury, Schwann cells dedifferentiate, secrete trophic factors and rapidly degrade myelin debris [reviewed in Kim et al.(2014)]. However, in recent years, much attention has shifted to the neuron-intrinsic mechanisms that govern axon regeneration. Although these mechanisms are only beginning to be elucidated, evidence suggests that transcriptional changes involving the upregulation of immediate early genes (e.g.,c-Jun) preceded by the expression of regeneration-associated genes (e.g., growth associated protein 43 (GAP-43)) are a key component of the PNS regeneration program (Denny, 2006).Indeed, one of the most important contrasts between the injury response in the CNS and that in PNS may result from the differences in molecular programs initiated in the axotomized neuronal cell bodies (Tetzlaff et al., 1991, 1994; Rossi et al., 2007; Liu et al., 2011).
Not surprisingly, an emerging approach to identifying the similarities and differences in the intracellular molecular responses of lesioned axons is to investigate the gene expression patterns of their perikarya after axotomy. However,given the great diversity of cells in the mammalian CNS,population-based approaches may be difficult to interpret.In one example, investigators compared the gene expression profiles of facial nucleus neurons (i.e., PNS), whose axons can regenerate, with those of red nucleus and Clarke’s nucleus neurons (i.e., CNS), whose axons do not regenerate, using differential display polymerase chain reaction (Schmitt et al., 2003). The authors identified 135 differentially expressed genes for transcription factors, homeobox-proteins, receptors, cytoskeletal proteins, and proteins involved in metabolism and signalling pathways. With such a large number of differentially expressed genes, it is difficult to know which are key to the mechanisms of axon regeneration and which represent elements downstream of regeneration, i.e., those whose expressions are caused by axon regeneration or its functional consequences. More recently, deep sequencing approaches (RNA-seq) are being applied, to distinguish RNA expression patterns of neurons whose axons are regenerating from those whose axons are not regenerating (Gong et al.,2016; Hu et al., 2016).
While mammalian nervous systems have obvious advantages in the search for therapies for human SCI, models of axon injury using early-evolved species such as Caenorhabditis elegans (round worms), Petromyzon marinus (sea lampreys) and Danio rerio (zebrafish), have experimental advantages associated not only with their increased regenerative capacities, but also with their greater anatomical simplicity,smaller neuron numbers, clearly defined neuron subtypes,and in some cases, individually identified neurons.
This review will survey the reported responses to axon injury and the determinants of axon regeneration, emphasizing these early-evolved species, which are often under-utilized,but in which the data are especially easy to interpret. Why do neurons lose much of their regenerative ability during maturation, and why are some axons in the mature mammalian PNS, or in the CNS of lower vertebrates, able to regenerate,while axons in the mammalian CNS do not? An emerging consensus, based in part on findings that individual neurons differ in their abilities to regenerate axons through the same in vivo terrain, is that neurons differ in their intrinsic regenerative capabilities and that manipulation of these neuron-intrinsic mechanisms may allow us to intervene in conditions such as SCI, where therapeutic potential was considered limited by the inabilities of axons to regenerate after injury.
The references to articles used in this review were retrieved by search of the PubMed and Medline databases for literature describing animal models of SCI from 1946 to 2019. Search was performed using the following conditions:SCI (MeSH Terms) AND (Models, Animal (MeSH Terms).
In addition, search of the PubMed and Medline databases with the following search criteria: spinal cord injury (SCI),“animal models”, “molecular guidance pathway”, “axonal regeneration”, “scar formation”, “in vitro models of spinal cord injury”, “non-mammalian model organisms”, “spinal cord transections”, apoptosis; axonal guidance molecules; netrins;RGM; mRNAs; neogenin; UNC5; DCC was completed. The results were further screened by title and abstract to exclude non-SCI experiments.
Differences between Central Nervous System Neurons that Regenerate Their Axons Well and Those that Do Not
Mammalian nervous systems
For the most part, the neuron-intrinsic factors involved in axon regeneration were identified by changes in expression post-axotomy, and those were further evaluated as possible determinants of axon-growth capability by one of two strategies; either the investigators determined changes in expression during development, while neurons were undergoing loss of regenerative capacity, or they manipulated the expression of the candidate molecules genetically and observed the effect on axon growth. In some cases, regenerative responses to axotomy were compared between neurons of CNS and PNS. However, additional insights into the neuron-intrinsic mechanisms determining the ability of neurons to regenerate their axons can be obtained by comparing the regenerative abilities of similar neurons whose axons project in the same paths, so that environmental differences can be ruled out.The most extensive evidence in this regard is derived from work in lampreys (see below). However, recent evidence in adult mammals supports the concept. Transection or crush of the optic nerve is followed by massive apoptotic death of retinal ganglion cells (RGCs) (90% in rat) and complete failure of axons to regenerate into the brain, unless they are provided a supportive environment, such as a peripheral nerve graft (Aguayo et al., 1991), or their intrinsic regenerative capacity is enhanced by knockdown of growth-inhibitory signaling molecules, such as phosphatase and tensin homolog (PTEN) (Park et al., 2008) or suppressor of cytokine signaling 3 (Park et al., 2009). Although all RGCs display many similar characteristics, in the mouse, they can be divided into more than 30 distinct subtypes (Sanes and Masland,2015), and these vary dramatically in their ability to survive after injury of their axon by optic nerve crush. Unlike most RGCs, α-RGCs are resistant to axotomy, and in PTEN-deficient mice, it is the α-RGCs whose axons regenerate past the crush site (Duan et al., 2015) (the α-RGCs are among the largest RGCs, an interesting exception to the general impression that small caliber axons regenerate more readily than large caliber axons (Scott et al., 1997; Tuszynski and Steward,2012; Zhang et al., 2018). The authors discovered several neuron-intrinsic factors in α-RGCs that might account for their regenerative ability: 1) They have high endogenous levels of mammalian target of rapamycin (mTOR) activity,which turns on protein synthesis and cell proliferation, and normally is inhibited by PTEN; 2) they selectively express a secreted phosphoprotein, osteopontin (OPN), which is capable of stimulating mTOR activity; and 3) they selectively express receptors for insulin-like growth factor 1. Ectopic expression of OPN in combination with insulin-like growth factor 1 promoted regeneration of α-RGC axons as effectively as PTEN inhibition (Duan et al., 2015). That the regenerative ability is related to mTOR activity is suggested by the failure of M1-RGCs to regenerate their axons in response to PTEN knockdown, even though these neurons survive axotomy. Unlike α-RGCs, M1-RGCs did not show high levels of endogenous mTOR activity. But mTOR alone is insufficient explain the regenerative ability of α-RGCs because exogenous OPN failed to induce axon regeneration in M1-RGCs,even though OPN increased mTOR activity in these and most other RGCs (Duan et al., 2015).
Similarly, Purkinje cells in the adult rat cerebellum are known for their poor regenerative capabilities, even when presented with otherwise growth-permissive environments.By comparison, neurons of the inferior olive, lateral reticular nucleus, and deep cerebellar nuclei vigorously regenerate axons into growth-permissive transplants. When their response to axotomy is compared with that of Purkinje cells,“good regenerator” neurons all upregulate the transcription factors c-Jun, GAP-43, and nicotinamide adenine dinucleotide phosphate diaphorase, but most axotomized Purkinje cells do not (Dusart et al., 2005). The Janus kinase (JAK) and signal transducers and activators of transcription (STAT)signaling pathways are crucial mediators of cytokine effects on neurons, transmitting information from outside the cell into the nucleus. After peripheral axotomy, JAK and STAT mRNAs increased in rat facial and hypoglossal neurons,whose axons regenerate after injury. By contrast, axotomized Clarke’s nucleus neurons, whose axons do not regenerate,showed no increase in JAK/STAT expression (Schwaiger et al., 2000). Sciatic nerve transection results in phosphorylation and activation of STAT in dorsal root ganglion neurons.Sustained perineural infusion of the JAK2 inhibitor AG490 in the proximal nerve stump after sciatic nerve transection blocked STAT3 phosphorylation and resulted in compromised neurite outgrowth in vitro (Qiu et al., 2005). Thus JAK/STAT3 might be involved in the switch from physiological patterns of gene expression to a regeneration program that is activated only after nerve injury. Unfortunately,Clarke’s nucleus neurons project entirely within the CNS,and thus it is not possible to determine whether their lack of regeneration is due to an intrinsic inability to upregulate JAK/STAT, or this inability is a consequence of other inhibitory factors imposed by the CNS environment.
Non-mammalian models
Comparisons between “good-regenerating” and “bad-regenerating” neurons in the CNS and PNS of most vertebrates yield results that are inconclusive or difficult to interpret. At least in part, this reflects heterogeneity of neuronal populations. The mammalian nervous system in particular imposes several methodological limitations. 1) The high bar imposed by numerous neuron-intrinsic and environmental factors makes it difficult to achieve axon regeneration in the CNS,so that it is difficult to know for certain how to compare the regenerative abilities of neurons other than by their responses to specific therapeutic manipulations. 2) In the most commonly studied parts of the nervous system, there is a need to use partial injuries (spinal cord) or crush injuries(optic nerve), which results in uncertainty about whether a particular neuron has been axotomized. 3) The difficulties are compounded by the small sizes and large numbers of neurons in the CNS. 4) While retrograde tracing can often identify those neurons whose axons span the level of an injury, it often is difficult to determine whether this represents regeneration of injured axons or collateral sprouting by spared axons. 5) Because neurons are not individually identifiable, it is not possible to know with certainty what the normal molecular features of a neuron are, so that it is not certain whether damage to its axon has resulted in molecular changes. If the identity of a particular neuron is imprecise,then knowledge about the molecules it expresses also is imprecise, beyond the specific markers being assayed by in situ hybridization, immunohistochemistry or other techniques.The new technologies, including laser capture microdissection and fluorescent activated cell sorting are potentially very useful approaches to collect or identify subpopulation of cells based on shared characteristics. As a selection criterion becomes more stringent, it is conceivable that these subpopulations may resemble collections of individually identified cells. However, as typically applied today, in vivo selection criteria are too imprecise to be assured of homogeneity.
In order to get around these limitations, non-mammalian species, in which at least some neurons can be individually identified, and whose axonal projections are known, have been used to distinguish “good-regenerating” from “bad-regenerating” neurons. While many of invertebrate organisms have identified neurons (for example, leech Hirudo medicinalis), sometimes very large size (molluscs Aplysia californica or Lymnaea stagnalis), invertebrate nerve regeneration have been review extensively in the past (Moffet, 2012). Moreover,while some authors described differences in regenerating abilities between identified neurons in Aplysia (Freedman and Nutz, 1988; Hamilton and Fredman, 1998), mechanisms responsible for such differences were not described or discussed. Therefore, in our current review we are focusing mainly on the experimental models that include the nervous systems of the nematode C. elegans, and the spinal-projecting systems of the zebrafish D. rerio and sea lamprey P. marinus.
Nematodes
C. Elegans is one of the most studied model organisms in biology. Its nervous system contains only 302 neurons, and its pattern of neuronal connectivity has been completely mapped (White et al., 1986). Using laser axotomy, it was possible to demonstrate regeneration of axons in this species (Yanik et al., 2004). The extent of regeneration varied depending on the identity of the neuron, the stage of animal development, and the location of the axotomy along the axon length. The inhibitory γ-aminobutyric acidergic motor neurons and the mechanosensory neurons (PLM, ALM,AVM) showed robust regeneration and have been studied in several laboratories (Wu et al., 2007; Gabel et al., 2008).In contrast, several axons of sensory neurons in the animal’s head (ASH, AWC) displayed a lack of regeneration after axotomy. For example, AFD chemosensory neurons are unable to regrow their sensory dendrites and axons (Chung et al.,2006). However, recent data suggest that ASH actually could re-establish very short but functional connections.
The precise mechanisms that distinguish “good-” and“bad-regenerating” neurons have not yet been elucidated.For example, while activation of the delta like non-canonical notch ligand 1 mitogen-activated protein kinase pathway is required for regeneration of “good regenerating” motor neurons (Hammarlund et al., 2009) and PLM sensory neurons(Ghosh-Roy et al., 2010) after axotomy, no similar data exist for the “bad-regenerating” ASH or AWC neurons. Similarly,while regeneration of axons belonging to mechanosensory neurons (PLM, ALM) show more robust and accurate regrowth in animals lacking the VAB-1 Eph receptor tyrosine kinase (Wu et al., 2007), the “bad-regenerating” ASH and AWC neurons were not investigated. Recent experiments employed a large-scale mutation-based screen of the“good-regenerating” mechanosensory neurons PLM to discover genes that may play a role in regeneration of adult axons (Chen et al., 2011). Among 654 conserved genes, the authors found several functional gene clusters that promote or repress axon growth, including genes involved in axon guidance, membrane excitability, neurotransmission, and synaptic vesicle endocytosis. Interestingly, many of those genes are not required for axon outgrowth during development and were not previously implicated in axon regeneration. The authors identified several neuron-intrinsic and neuron-extrinsic pathways that inhibited regeneration of PLM axons in wild-type C. elegans. However, because their studies were focused on “good-regenerating” neurons, questions remained whether activation of these inhibitory pathways are responsible for the poor regeneration of the “bad-regenerating”neurons (Wu et al., 2007; Gabel et al., 2008).
Non-mammalian vertebrates
A confounding problem in many invertebrate models (and in some vertebrates as well) is the short distances covered by the regenerative axon growth. At short range, this growth may represent developmental mechanisms by which, guided by the actin-myosin molecular motors of growth cone filopodia,axons select their longer growth pathways (Miller and Suter,2018). In the mature nervous system, these developmental guidance mechanisms may be represented by the short-range collateral sprouting of spared axons to innervate postsynaptic targets denervated by injury of their neighbors (Murray and Goldberger, 1974; Benowitz et al., 1999). But the mechanisms may be different from the mechanisms of longer-distance regeneration of the injured axons themselves (Lurie et al.,1994; Blesch and Tuszynski, 2009; Jin et al., 2009). For this reason, some laboratories have looked to larger animals, including non-mammalian vertebrates, where it has been easier to determine when an axon has been interrupted, and thus to distinguish between the different modes of axon growth, and to confirm the heterogeneity among neurons in their ability to regenerate their injured axons.
Perhaps the best models for studying mechanisms underlying heterogeneity among neurons in their abilities to regenerate their axons are the sea lamprey and zebrafish, because they combine the advantages of phylogenetic relation to mammals, partial regenerative capacity, and the presence of identified spinal-projecting neurons. In this section, we will compare regeneration in lamprey spinal axons with that in zebrafish and other teleosts. The zebrafish has the advantage that there is a large and growing transgenic methodology that allows site-directed mutagenesis and other modern genetics techniques to be used. During early larval development, several pairs of reticulospinal (RS) neurons can be individually identified, and because the animal is translucent at this stage,these neurons can be seen in the intact animal (Hanneman et al., 1988). However, this translucency lasts only a few days after hatching, while the larval animal still has a yolk sac. Thus it is difficult to know whether axon growth observed after spinal cord transection reflects mechanisms of regeneration or development. At later stages, except for the Mauthner cell,most of the large identified neurons have disappeared or are no longer distinct from their surrounding neurons, so they no longer are individually identifiable.
Lampreys
Lampreys are jawless fish (class Agnatha), which retain a notochord and do not have a boney spine. Together with their class relatives the hagfishes, lampreys represent the earliest-evolved vertebrates (approximately 500 million years ago). The sea lamprey has a very long life cycle, with a larval filter-feeding stage lasting 5 years or more, followed by a 2-3 year parasitic adult stage (Potter et al., 1982). This makes the lamprey unsuitable for transgenic studies, even though its genome has been sequenced (Smith et al., 2013). During the larval phase, the animal is in a relatively stable state of neurological development, with several pairs of large identified RS neurons, whose axons project almost the entire length of the animal (Rovainen, 1979). These and the approximately 2500 smaller RS neurons (determined in Ichtheomyzon unicuspis, not P. marinus), many of which terminate in the rostral spinal cord, constitute the main descending system transmitting commands from the brain to the spinal cord in lampreys (Bussieres et al., 1999). Even in 5-year-old larvae and adults, the sea lamprey recovers behaviorally following SCI and RS axons regenerate across a complete transection(Rovainen, 1976; Selzer, 1978; Wood and Cohen, 1979; Yin and Selzer, 1983; Cohen et al., 1988, 1989; Lurie and Selzer,1991c; Davis and McClellan, 1994a) (Figure 1). RS and other axons of lampreys regenerate selectively in their correct paths (Yin et al., 1984; Mackler et al., 1986; Lurie and Selzer,1991c) and within the limited distance of their growth, they form synapses selectively with appropriate target neurons distal to the transection (Mackler and Selzer, 1985, 1987).
This impressive degree of axon regeneration is not shared equally by all spinal-projecting neurons. The RS system in the lamprey includes four bilateral reticular nuclei of the brainstem: the mesencephalic reticular nucleus and the anterior, middle, and posterior rhombencephalic reticular nuclei (ARRN, MRRN, and PRRN, respectively). These nuclear groups include 36 large identified RS neurons, among them the giant Müller cells and a pair of Mauthner neurons(Whiting, 1957; Rovainen, 1967, 1979; Nieuwenhuys, 1972;Swain et al., 1993, 1995). The neuronal map of the lamprey brain is shown in Figure 1B. The RS neurons in lamprey display great heterogeneity in their regeneration abilities- some neurons are good regenerators (regeneration frequency > 50%, e.g., M4, I3, B2) and others rarely regenerate(regeneration frequency < 30%, e.g., I1, Mauthner) (Jacobs et al., 1997). Because neurons whose axons regenerate well are located adjacent to neurons whose axons regenerate poorly,and because axons of both “good-regenerating” and “bad-regenerating” neurons project in the same spinal tracts proximal to the transection (Selzer, 1978; Lurie and Selzer, 1991a,b) and grow through the same glial scar tissue, neither the location of the perikaryon, nor the projection path of the injured axon determines the probability of axon regeneration.Thus heterogeneity in regeneration probably reflects neuron-intrinsic differences among the neurons.
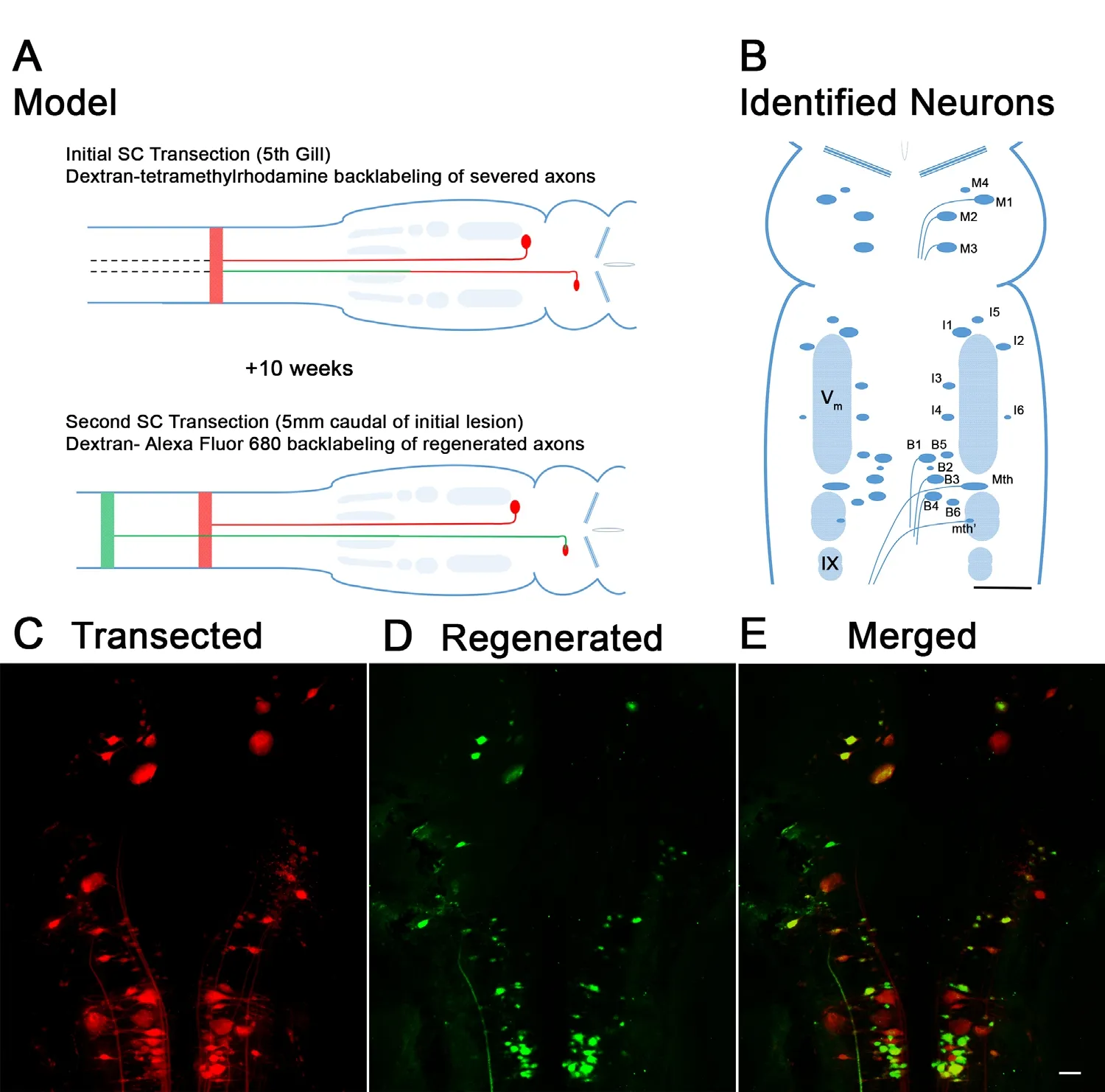
Figure 1 Retrograde labeling of regenerating reticulospinal neurons after spinal cord transection.
Studies on lampreys (and zebrafish, see below) have provided some clues to the intraneuronal mechanisms that might make for a good or bad regenerating neuron, but we have only scratched the surface. In lampreys, the regenerative capacity of RS neurons correlated with their ability to resume expression of mRNA for the neurofilament protein neurofilament-180, after an initial axotomy-induced downregulation (Jacobs et al., 1997). Because neurofilament-180 reexpression occurred at the time when most RS neurites first appear in the scar - 4 to 6 weeks post-transection (Yin and Selzer, 1983), it has been proposed that transport of neurofilaments or their assembly in the growing axon tip may contribute to the mechanism of axon regeneration (Lurie et al., 1994; Hall et al., 1997; Jacobs et al., 1997; Jin et al., 2009).
Although it is unlikely that differences in the extracellular environment can explain the large differences in regenerative ability among CNS neurons, this does not mean that the extracellular environment does not play an important role.Growing axons receive signals from a plethora of chemo-attractive and -repulsive cues that are recognized by multiple receptors. It is possible that differing neuronal abilities to respond to these environmental cues represent neuron-intrinsic mechanisms of influencing the regenerative abilities of axons. Five major families of axon guidance molecules- netrins, semaphorins, slits, repulsive guidance molecule(RGM) and ephrins - guide axons to specific sites by providing attractive or repulsive cues during the formation of neural networks. These factors are similar in invertebrates and vertebrates (Goodman, 1996; Tessier-Lavigne and Goodman,1996). Morphogens, including members of the Wnt family,bone morphogenetic proteins, and Sonic Hedgehog (Shh)may also function in axon guidance (Schnorrer and Dickson,2004; Zou and Lyuksyutova, 2007). The effects of the various guidance molecules and their receptors have been studied primarily in vitro or in the context of embryonic development. Whether they play a role in regeneration in the injured adult CNS is much less clear. Several guidance molecules are expressed in the adult CNS and their expression is upregulated following injury. Since many of adult CNS neurons continue to express guidance receptors, neurons in the CNS are likely to remain responsive to guidance cues throughout life, and this could be a cause of regeneration failure. The extracellular environment of the mature mammalian CNS is not very supportive of axon regeneration, and this has been ascribed to the need for stability in established neural circuitry (Manitt et al., 2001; Manitt and Kennedy, 2002; de Wit and Verhaagen, 2003).
Therefore, the inability of “bad-regenerating” neurons to regenerate their axons in the adult CNS may be attributable to persistent expression or reexpression of repulsive guidance cues and their receptors. The “bad-regenerating” large RS neurons in lampreys, e.g., the Mauthner, I1, B1, and B3neurons, expressed UNC-5, Neogenin, PlexinA and EphB.
These are receptors that mediate the chemorepulsive actions of guidance molecules. On the other hand, neurons that are known to regenerate well, e.g., I3, I4, B2and B6, almost never expressed those receptors (Shifman and Selzer, 2000b, 2007;Shifman et al., 2009; Barreiro-Iglesias et al., 2012). Obviously, a neuron’s ability to respond to environmental inhibitory cues requires that it express surface receptors for those cues,which might explain why “good-regenerating” neurons in lamprey brain can regenerate axons through a spinal cord environment that has many extracellular inhibitory molecules (Shifman and Selzer, 2000a, 2007; Shifman et al., 2009).
Using in situ hybridization we reported that “bad-regenerating” RS neurons preferentially express Neogenin mRNA and also RGM (Neogenin ligand) in spinal cord. Because Neogenin transmits the chemorepulsive effects of RGM, we hypothesized that inhibition of Neogenin expression would block the repulsive action of RGM and enable the axons of Neogenin expressing RS neurons to regenerate. Indeed,blocking expression of Neogenin mRNA in RS neurons by in vivo delivery of morpholino antisense oligonucleotides increased the numbers of regenerated “bad-regenerating” RS neurons, reflecting enhanced regeneration of RS axons after SCI (Chen and Shifman, 2019). These results strongly suggest involvement of the Neogenin—RGM axis in restricting RS axon regeneration.
Mauthner neurons are the best-known example of“bad-regenerating” neurons. In our studies of large larval sea lampreys, less than 10% of Mauthner cells regenerated their axon across a transection site (Jacobs et al., 1997); others reported slightly higher regeneration rates (Davis and Mc-Clellan, 1994b). In those that did not regenerate, their axon retracted up to 2 mm, and occasionally more, and eventually they undergo apoptosis (Shifman et al., 2008). Similarly poor regeneration was seen in many RS neurons of adult zebrafish(Becker et al., 1997) and goldfish (Sharma et al., 1993). Even in zebrafish embryos, 65% of Mauthner cells did not regenerate at all (Bhatt et al., 2004). Moreover, Mauthner neurons that showed some regenerative sprouting, their axons did not cross the lesion site. Axons grew aberrantly, either out of the spinal cord through ventral roots into muscle, or turned rostralward within the spinal cord (Bhatt et al., 2004).
During postnatal development in mammals, the central-projecting axons of dorsal root ganglion cells lose their ability to regenerate, and this was ascribed to a developmental reduction in intraneuronal levels of cyclic adenosine monophosphate (cAMP) (Cai et al., 2001). Evidence of a role for cAMP as an intraneuronal determinant of regenerative ability was obtained by studying the regenerative capacity of the Mauthner cell after SCI in larval zebrafish (Bhatt et al., 2004). Injecting cell-permeable cAMP analog dibutyryl-cAMP onto the neuronal perikaryon produced robust,directionally correct Mauthner axon regeneration, which was not observed in response to injection of vehicle-only or dibutyryl-cyclic guanosine monophosphate. Because the experiments were done at an early developmental stage, when the zebrafish still has a yolk sac, it could be argued that this regeneration really represented an effect on axon development, and not on regeneration of mature injured axons. In similar experiments on lamprey RS axons after SCI, cAMP reduced initial retraction, accelerated subsequent regeneration up to 11-fold (Jin et al., 2009), and promoted survival of their perikarya in vivo (Lau et al., 2013).
What cellular mechanisms could be responsible for the effects of manipulating cAMP levels in Mauthner neurons on their regeneration abilities? As we mentioned early,our in situ hybridization experiments demonstrate that all Mauthner neurons co-expressed the multiple axon guidance receptors: Deleted in Colorectal Cancer (DCC), Uncoordinated-5 (UNC-5), Neogenin and PlexinA - receptors that mediate repulsive actions of netrins, RGM, and semaphorins guidance molecules (Shifman and Selzer, 2000b,2007; Shifman et al., 2009; Chen et al., 2017). Moreover,after SCI, message for UNC-5 was upregulated primarily in the Mauthner cell (Shifman and Selzer, 1999). One potential mechanism for modulating netrin and semaphorin signaling involves regulation of cyclic nucleotide levels within growth cones. In Xenopus spinal neurons in vitro, decreased cAMP levels converted DCC-mediated netrin attraction into repulsion, whereas increased cAMP levels resulted in attraction (Ming et al., 1997; Nishiyama et al., 2003).Multiple in vitro studies demonstrate that protein kinase A activation modulates netrin signaling and that treatments that affect protein kinase A activity can modulate netrin guidance. Thus several guidance molecules use the cyclic nucleotides as common signaling pathways, with increased levels of cAMP resulting in chemoattraction, whereas reducing cyclic nucleotide levels produces chemorepulsion or inhibition of growth cone movement. If persistent or reexpression of repulsive guidance cues and their receptors produces net chemorepulsion on regenerating axons, we might expect that increasing cAMP levels will convert the repulsion to chemoattraction and enhance regenerating ability of “bad-regenerating” neurons. Elevation of cAMP activity in cultured Xenopus spinal neurons converted the chemorepulsive responses of their axons upon contact with myelin-associated glycoprotein (MAG) to chemo-attraction(Song et al., 1997, 1998).
Convergence of signaling pathways for neuronal survival and axonal regeneration after axotomy
The inability of “bad-regenerating” neurons to undergo axonal regeneration in the adult CNS may be attributable to another signaling pathway, that involving caspases-mediated apoptosis. In Nissl-stained (Shifman et al., 2008) and neurofilament-immunostained (Hu et al., 2013) brain wholemounts from lampreys surviving 12 or more weeks after spinal cord transection, several identified RS neurons had disappeared. Retrograde fluorescent labeling from the site of transection combined with TUNEL histochemistry suggested that death of several identified RS neurons was initiated as early as 4 weeks post-transection, reaching a peak at 12-16 weeks, although the actual disappearance of the neurons lagged behind the onset of TUNEL positivity. Using fluorescent-labeled inhibitors of caspases activation, the apoptotic process could be detected by two weeks after transection.These observations suggested that some cells were dying by apoptosis. In addition, the same axotomized RS neurons expressed Neogenin mRNA (Shifman et al., 2009). Inhibition of Neogenin by morpholino antisense oligonucleotides prohibited activation of caspases and improved the survival of RS neurons at 10 weeks after SCI. These data provide new evidence in vivo that Neogenin is involved in retrograde neuronal death and failure of axonal regeneration after SCI(Chen and Shifman, 2019).
In mammals, embryonic CNS neurons can regenerate their axons after injury (Kalil and Reh, 1979; Ferretti et al.,2003), but during development, neurons undergo a transcriptionally regulated switch that limits their regenerative capacity (Van Kesteren et al., 2011; Lu et al., 2014). Unfortunately, the nature of the switch is not known. It now seems unlikely that a single gene accounts for it, but epigenetic mechanisms - DNA methylation and histone modifications— are intriguing candidates because they result in changes of chromatin structure, and thereby influence transcription of multiple genes. Acetylation is one of the most widely studied epigenetic modifications. The enzymes responsible for regulating acetylation are lysine acetyltransferases, which add acetyl groups to lysine residues, and histone deacetylases(HDACs), which remove the acetyl groups (Roth et al., 2001;Delcuve et al., 2012). HDACs are evolutionarily highly conserved. Previous work has suggested that histone acetylation appears to play an important role in PNS and optic nerve regeneration (Gaub et al., 2010, 2011; Finelli et al., 2013;Puttagunta et al., 2014).
We have cloned and sequenced several lamprey HDACs and lysine acetyltransferases (Chen et al., 2016), describing their expression in regenerating and non-regenerating RS neurons after SCI. The expression of the KAT2A, KAT5 and P300 and HDAC3 did not change after SCI in either good or bad regenerators. However, the numbers of both good- and bad-regenerating neurons expressing HDAC1 mRNA were decreased 2-4 weeks post-transection (TX), but at 10 weeks only “good regenerators” expressed HDAC1 mRNA. Moreover, HDAC1 was preferentially expressed in regenerated neurons, but not in non-regenerating neurons (Chen et al.,2016). Therefore, SCI causes significant changes in HDAC1 expression, which may modulate survival or regeneration programs.
Zebrafish
Though less extensive, the data on heterogeneity in regenerative ability of spinal-projecting axons in the zebrafish support many of the findings in lamprey (Becker and Becker,2008). The adult zebrafish spinal cord receives descending input from at least 20 different neuronal groups in the brain (Becker et al., 1997). Those neurons are located in the brainstem, mainly in the nucleus of the medial longitudinal fasciculus, the reticular formation, and the octavo-lateralis area. Mauthner neurons are located in the intermediate reticular formation. In addition to the “main” Mauthner neuron, several serial homologues of the Mauthner cell have been individually identified in the brainstem of larval zebrafish. Two of these homologues, MiD2cm and MiD3cm,can also be identified in the adult brainstem (Eaton et al.,2001). Approximately 50% of spinal-projecting neurons of the zebrafish brain regenerate their axons readily after SCI,and these are clustered in specific brain nuclei, including the nucleus of the medial longitudinal fascicle, the intermediate reticular formation and the magnocellular octaval nucleus.However, neurons in other spinal-projecting nuclear groups are bad regenerators, e.g., the red nucleus, the nucleus of the lateral lemniscus and the tangential nucleus (Becker et al., 1997), and only 10—15% of their axons regenerate. As in the lamprey, good and bad-regenerating neurons send their regenerating axons through the same injury scar and project into the same paths (Becker et al., 1998, 2005). Therefore,the heterogeneity in regenerative ability is not a function of the extracellular environment. Instead, “good regenerating”zebrafish neurons express certain regeneration-associated genes after SCI (Becker et al., 1998, 2005). The mRNAs for the cell recognition molecules L1.1, L1.2 and the growth-related gene GAP-43, but not neural cell adhesion molecule was observed in the “good-regenerating” neurons of the medial longitudinal fasciculus, the intermediate reticular formation, and the magnocellular octaval nucleus in the brain.However, the “bad-regenerating” neurons in the red nucleus, the nucleus of the lateral lemniscus, and the tangential nucleus did not show these changes in mRNA expression.As in mammalian spinal cord that has been manipulated to enhance regeneration, monoaminergic neurons seem to be especially able to regenerate their axons. Also as in the lamprey, when axons regenerated, they grew only a few millimeters, and did not reach all of their original targets in the caudal spinal cord. Studies in zebrafish also have shown that“good-” but not “bad-regenerating” neurons increased GAP-43 expression after injury (Becker et al., 1998, 2005). Moreover, after axotomy, rat motoneurons, which regenerate their axons, underwent 10-20-fold increase in GAP-43 mRNA levels (Fernandes et al., 1999).
Axon Diameter and Regenerative Ability
Most of this review focuses on molecular expression patterns to explain why some neurons are better at regenerating their axons than others. However, there may be important and mechanistically simpler proximate influences that might trigger downstream molecular regenerative responses.Across species, small caliber axons seem to regenerate more efficiently than larger axons. Following SCI in mammals,the small serotonergic raphe-spinal axons regenerate much more readily than the large corticospinal axons (Tuszynski and Steward, 2012). In the sea slug, Aplysia californica, axon regeneration following bilateral crush of the cerebral-buccal connectives, small caliber axons regenerate first, followed by medium-sized axons, while large axons fail to regenerate altogether (Scott et al., 1997). Additional caliber-dependent responses have been observed after contusion SCI in cats,and crush SCI or ischemic injury in rats (Blight and Decrescito, 1986; Fehlings et al., 1989; Olby and Blakemore, 1996).A notable exception to this size principle is axon regeneration of the RGCs (Duan et al., 2015). Although crushing the optic nerve results in massive cell death among RGCs, it is the largest RGC-subtype, the αRGCs, that are the best survivors and the most responsive to interventions intended to enhance axon regeneration.
Clearly, there are complexities in the mammalian CNS that make dissection of this question difficult. The lamprey offers a rare opportunity to use its individually identified RA neurons to test the importance of axon caliber in regeneration.In lampreys, perikaryal size is perhaps the best predictor of whether an individual neuron will survive and regenerate(r = -0.92) (Zhang et al., 2018). Lamprey good regenerators(> 50% likelihood of axon regeneration after TX) tend to be small, with cross-sectional areas averaging approximately 6× 102μm2; while bad regenerators tend to be large, averaging 25 × 102μm2. Although the mechanism underlying this observed correlation remains poorly understood, it appears to be related to axolemmal resealing. The large caliber axons of the large bad-regenerator neurons reseal slowly, some axons remaining open to dextran dyes more than 24 hours post-TX. This delay in membrane resealing is associated with activation of caspases in the cell body, which can be reduced by inducing rapid resealing with polyethylene glycol (Zhang et al., 2018). While it is clear that the initial cellular response to injury is an important determinant of survival and regeneration, the mechanisms by which delayed resealing influences neuronal survival and regenerative responses remain to be elucidated.
Summary and Conclusions
The search for ways to treat CNS injuries has unearthed many molecular factors in the environment of mature CNS that are not present in the embryo, nor in the PNS, that inhibit axon growth in vitro, and appear to mediate growth inhibition in the mature CNS in vivo. Yet targeting these growth-inhibiting molecules has not resulted in sufficient axon regeneration to restore function to the injured CNS,as exemplified by SCI. It might be that the number of such inhibitory molecules is so great that only a cocktail of several treatments will suffice. However, it may also be that during maturation, neurons become intrinsically less able to mount a regenerative response or to overcome the effects of environmental inhibitors. In this case, the differential abilities of individually identified neurons of similar type to regenerate their axons through the same environment could allow us to better understand the molecular mechanisms that lead to regenerative failure in the mature human CNS. As summarized above, neurons may differ in their intrinsic ability to respond to growth-inhibiting and growth-promoting environmental factors. Structural and physiological features of neurons are also implicated, such as axon caliber and the ability of neurons to deliver translational machinery to the injured axon tip. Determining the reasons for the differential regenerative abilities among identified neurons and neuron types will allow us to better tease apart the determinants of regeneration and identify therapeutic targets to promote functional recovery after CNS injury. Non-mammalian vertebrate CNS models provide particularly favorable anatomical and molecular windows for this search.
Author contributions:MIS wrote whole review with exception of section Mammalian nervous systems from Differences between Central Nervous System Neurons that Regenerate Their Axons Well and Those that Do Not that was written by MES. Section Axon Diameter and Regenerative Ability was written by WR and JH. They also created Figure 1 for this article.MES edit and proofread entire article.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by 85310-PHI Shriners Research Foundation (to MIS), NIH R01 NS092876 (to MES).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Melissa Renee Andrews, University of St Andrews, UK.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy
- Using our mini-brains: cerebral organoids as an improved cellular model for human prion disease
