Adult neurogenesis from reprogrammed astrocytes
2020-12-02BrianGriffithsAnveeBhutaniCreedStary
Brian B. Griffiths, Anvee Bhutani, Creed M. Stary
Department of Anesthesiology, Pain & Perioperative Medicine, Stanford University School of Medicine, Stanford, CA, USA
Abstract The details of adult neurogenesis, including environmental triggers, region specificity, and species homology remain an area of intense investigation. Slowing or halting age-related cognitive dysfunction, or restoring neurons lost to disease or injury represent just a fraction of potential therapeutic applications.New neurons can derive from stem cells, pluripotent neural progenitor cells, or non-neuronal glial cells,such as astrocytes. Astrocytes must be epigenetically “reprogrammed” to become neurons, which can occur both naturally in vivo, and via artificial exogenous treatments. While neural progenitor cells are localized to a few neurogenic zones in the adult brain, astrocytes populate almost every brain structure. In this review,we will summarize recent research into neurogenesis that arises from conversion of post-mitotic astrocytes,detail the genetic and epigenetic pathways that regulate this process, and discuss the possible clinical relevance in supplementing stem-cell neurogenic therapies.
Key Words: astrocyte; brain; dedifferentiation; development; disease; glia; injury; neurogenesis
Introduction
Neurogenesis is the birth of new neurons in the brain and includes the assumption that some of the newly born neurons will successfully integrate into functional synaptic networks, while those that is not will be recycled. The brain undergoes a high degree of neurogenesis during initial development, which precipitously drops during adulthood but does not disappear completely. As a relatively newly discovered phenomenon, much remains unknown about the underlying causes or the potential clinical relevance of adult neurogenesis. Initial evidence suggests that the function inclusion of new neurons helps maintain normal brain functioning, and can help reverse cognitive losses due to aging, disease, or injury. New neurons can derive from stem cells and pluripotent neural progenitor cells, though both of these populations decline in an aging brain. Neurogenesis from non-neuronal glial cells, such as astrocytes, has recently gained interest because while neural progenitor cells are limited to a few neurogenic zones, astrocytes are common in almost every area of the adult brain. Astrocyte-derived neurogenesis requires epigenetic “reprogramming,” which happens both naturally after injury and in response to exogeneous stimuli. Unraveling the genetic pathways within astrocytes that can be manipulated to induce a phenotypical change is vital to using astrocytes as a therapeutic source of new neurons. This review will summarize recent research in astrocyte-derived neurogenesis, the known mechanisms and signaling pathways, and potential therapeutic applications.
Classic neurogenesis background
Neurogenesis is robust during early brain development,with neural stem cells giving rise to radial glial cells, which serve as progenitors for neurons and astrocytes. Historically adult brains were thought to contain a finite number of neurons, with neuronal “plasticity” limited to neuronal loss from aging, disease, or injury. In the early 1990s, researchers reported that new neurons could be cultured in vitro from adult brain tissue of rodents (Richards et al., 1992), followed shortly by in vivo observations of these same processes in rodents (Palmer et al., 1997). The interspecies homology of neurogenesis was established by the discovery that non-human primates and human adults also exhibit in vivo neurogenesis (Eriksson et al., 1998). The regions that retain neurogenic abilities remain an area of controversy, but it is generally agreed that in the adult mammalian brain new neurons are plentiful in the subventricular zone (including the olfactory bulb), and the subgranular zone of the dentate gyrus in the hippocampus (Gage, 2002). However, new neurons have been reported in many other brain areas, such as the striatum (Ernst et al., 2014), cortex (Magavi et al., 2000),and hypothalamus (Kokoeva et al., 2005), and others, and it has been hypothesized that adult neural progenitors “may not be as restricted as implied by their normal location and function” (Palmer et al., 1997). The source of these new neurons outside of canonical neurogenic regions remains a topic of active investigation. Since its first discovery, many facets of adult neurogenesis have been uncovered [for recent reviews, see Augusto-Oliveira et al. (2019); Lei et al. (2019)].
Recent controversy in adult neurogenesis
The natural ability of adult mammalian brains to create new neurons has experienced rekindled controversy with a recent study by Sorrells et al. (2018) that posits hippocampal neurogenesis begins to decrease in early childhood and is not at all present in adult humans or adult non-human primates.The study was widely disseminated and disputes the foundational work for neurogenesis with the hypothesis that human brains may be fundamentally different than those of other species. Paredes et al. (2016) expanded on this argument,describing a possible negative correlation between brain size and potential for neurogenesis. In contrast, Boldrini et al.(2018) concluded that in non-diseased adult hippocampal cells, neurogenesis continues to occur despite aging. They were unable to compare their results directly as the previous studies used thin sections (5 μm) without stereology, and subjected the tissue to low temperatures/pH. More recently,Moreno-Jiménez et al. (2019) corroborated neurogenesis in adult humans, describing hippocampal neurogenesis in neurologically healthy subjects using similar immunohistochemical techniques as Sorrells et al. (2018). They attributed the lack of neurogenesis markers in the original study to methodological problems related to a delayed timing and over-fixation of brain tissue, and that a major marker of new neurons—doublecortin—loses antigenicity after 12 hours of fixation time. In our lab, we have also seen a reduction of doublecortin antigenicity and much higher background staining with fixation time, with increased fluorescent signal after ~12 hour fixations (Figure 1A) compared to ~3 day fixations (Figure 1B). Others have called into question the relevance of adult neurogenesis in inbred laboratory rodents to real world behavior (Oppenheim, 2019). These debates raise interesting methodological questions in how best to quantify new neurons and what exactly constitutes proof of adult neurogenesis, challenging a comprehensive body of literature that details timing, environmental conditions, genetic contributions, and epigenetic regulation of adult neurogenesis in humans. Future studies delineating technical approaches with increased specificity and sensitivity to quantify new neurons in the adult human brain are therefore required to advance the therapeutic potential of neurogenesis.
Neurogenesis as a therapeutic endpoint
Neurogenesis has been a long-standing goal to restore brain function for a host of illnesses and diseases with varying levels of success. In hypoxic-ischemic injuries, such as stroke, the subventricular zone of the brain has been shown to regenerate neocortical neurons in neonatal rodents, suggesting this could be an effective therapy (Yang et al., 2007).In Alzheimer’s disease, doublecortin expression decreases,a phenomenon that may directly contribute to the loss of memory that is commonly associated with the disease, and which may be slowed or reversed by promoting neurogenesis(Moreno-Jiménez et al., 2019). This hypothesis is supported by prior work utilizing the neurosteroid alloprognanalone to promote neurogenesis in in vitro and in vivo rodent Alzheimer’s models, as well as in human cell samples (Brinton and Wang, 2006). Increasing neurogenesis has been shown to work as an therapy to restore cognitive function in rodents subjected to traumatic brain injury, especially when given in conjunction with the neuroprotective drug erythropoietin(Lu et al., 2005). Studies in non-human primates have suggested neurogenesis is essential for effective usage of antidepressant medication, with new hippocampal neurons serving as a marker for treatment efficacy (Perera et al., 2011). In rodent models of Down syndrome, an altered generation of neurons was shown to have been a successful treatment to render mice with normal cognitive function as compared to mice without such treatment (Nakano-Kobayashi et al.,2017). There are sex differences in many diseases and illnesses, and growing evidence that these differences extend to neurogenesis, both as a phenomenon and a therapeutic endpoint, as well (Yagi and Galea, 2019). Finally, there are several active clinical trials assessing the effect of neurogenesis on disorders and diseases including schizophrenia, stroke, and traumatic brain injury (ClinicalTrials.gov, National Library of Medicine (U.S.), 2019).
Neurogenesis is heavily regulated by astrocytes
Astrocytes are non-neuronal glial cells that have been increasingly recognized as important regulators of brain function and disease (Liddelow and Sofroniew, 2019). Adult neural stem cells (aNSCs) in the canonical neurogenic zones are both positively and negatively regulated by contact with astrocytes (Cassé et al., 2018). These astrocytes influence whether aNSCs mature into neurons through membrane-membrane and extracellular signaling (Lim and Alvarez-Buylla, 1999). Migration and forming of synaptic connections of new neurons can also be also influenced by astrocytes. Signaling from astrocytes drastically increases both the proliferation of aNSCs and their commitment to a neuronal fate (Song et al., 2002). However, the degree to which astrocytes promote neurogenesis is modulated by the body’s response to environmental conditions. For example,inflammation can result in lower numbers of new neurons through altered astrocytic production of interleukin-6 production in adult mice (Vallières et al., 2002). Astrocytes become “reactive” during injury, which results in a drastic change in their cellular programming, and may affect their ability to regulate aNSCs (Cassé et al., 2018). The level of astrocyte control over neurogenesis also extends beyond neural stem cells; oligodendrocyte precursors cells are common in the brain and serve to replenish the stock of myelin-contributing glial cells (Simon et al., 2011), but can be diverted from an oligodendrocyte fate by astrocyte signals (Gaughwin et al., 2006). Most interestingly, this level of control over the cell fate of non-neuronal cells even extends to astrocytes themselves.
Search Strategy and Selection Criteria
Studies included in this review were found on the Google Scholar and PubMed databases, between March 2019 and August 2019, using the search terms: dedifferentiated astrocytes, reprogrammed astrocytes, adult neurogenesis, dedifferentiation, and various combinations of the above phrases.
Neurogenesis from DedifferentiatedAstrocytes
Unlike neurons, glial cells readily proliferate to maintain stable numbers in the brain. Before reaching full maturity, astrocyte progenitors maintain their ability to change their cell fate, and the conversion of radial glia to astrocytes appears to be bidirectional (Hunter and Hatten, 1995). The process of regressing to a previous, less-specialized cell fate is known as “dedifferentiation”. These cells can then differentiate back into a specialized cell, though not necessarily the same cell fate they previously occupied (Figure 2). In adults, astrocytes can still enter periods of lability after injury, during the period of “reactive gliosis”. In a stab wound model, up to 50% of astrocytes reentered the cell cycle between three days and a week after injury, a portion of which proliferated to create more astrocytes around the site of injury (Simon et al.,2011). Within the brain, these labile cells are usually pushed back to an astrocyte cell fate (Buffo et al., 2008); however,several in vitro studies have demonstrated that a subset of astrocytes that have reentered the cell cycle can dedifferentiate and reprogram into neurons in response to injury (Buffo et al., 2008; Yang et al., 2009; Robel et al., 2011; Magnusson et al., 2014) or environmental stressors (Yu et al., 2006). In neurogenic regions of the brain, some neurons are born from astrocytes even under normal conditions (Seri et al., 2001).In addition to astrocytes, the capacity to dedifferentiate has been demonstrated in microglia, oligodendrocytes and radial glia (Grinspan et al., 1996; Yokoyama et al., 2004; Mori et al., 2005), suggesting the deprograming of these cells may share similar developmental origins and be an important property of a normally functioning brain. The transcriptional profiles of neural stem cells and reactive astrocytes share many similarities (Götz et al., 2015), but several different pathways have been found to play a role in the conversion of astrocytes to neurons.
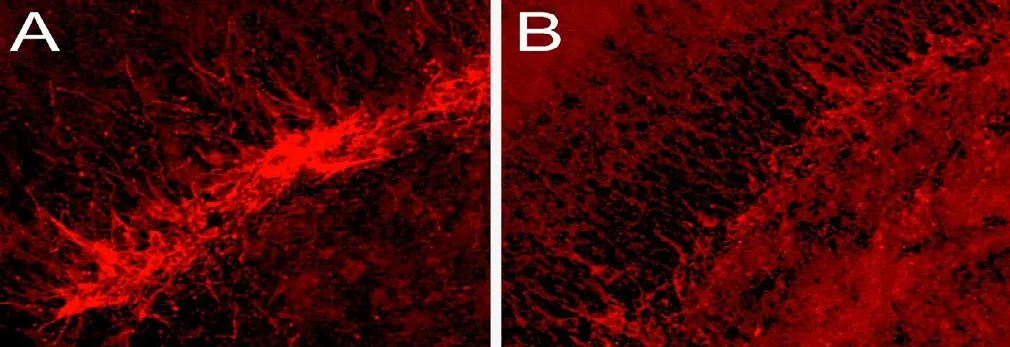
Figure 1 Doublecortin expression in the dentate gyrus.
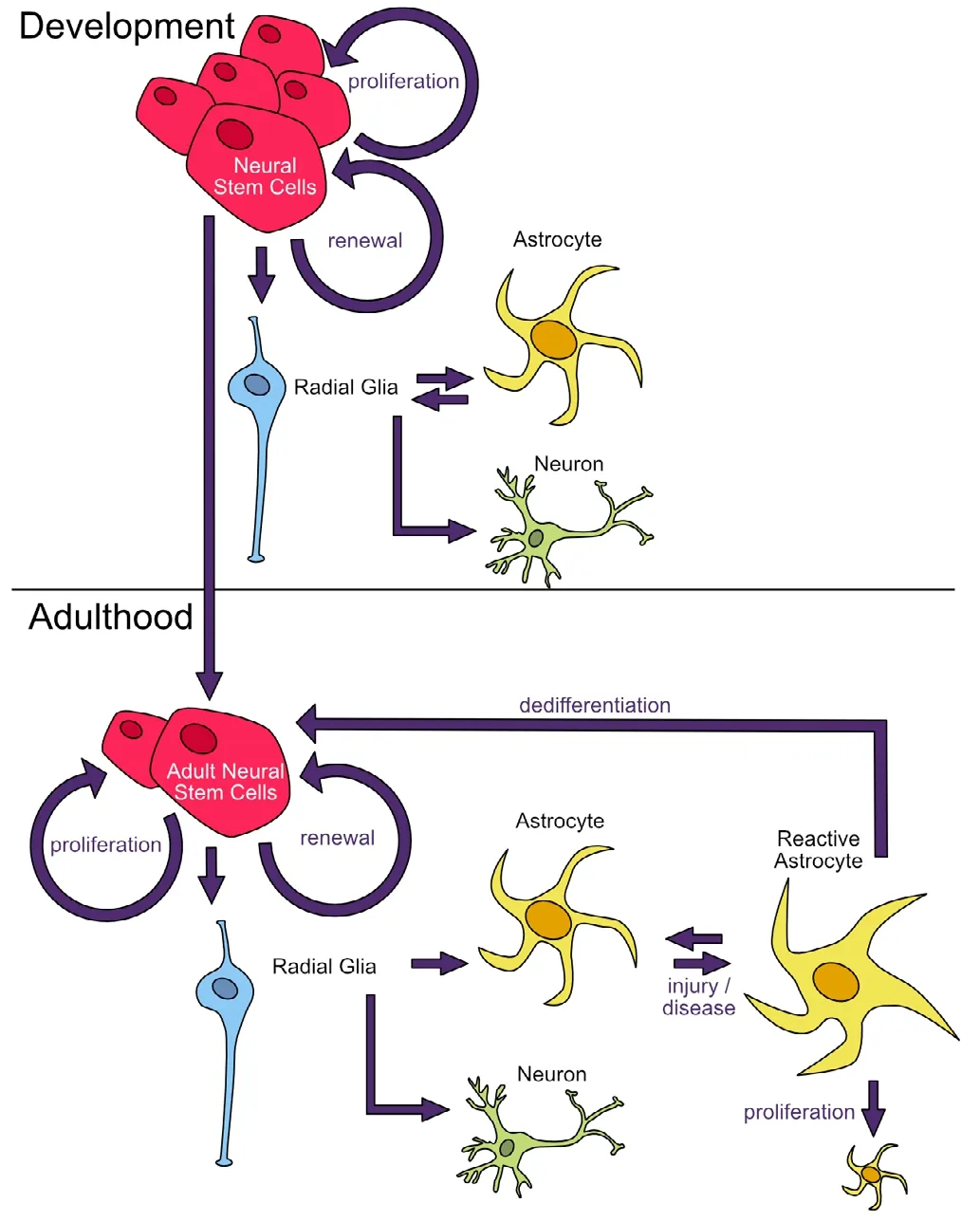
Figure 2 Neuronal and astrocyte development.
Dedifferentiation pathways
Many of the factors involved in differentiation are normally expressed in neural stem cells but cease expression after maturation. Immature astrocytes can be easily reprogramed into neurons through expression of Neurog1, Neurog2 and Mash1, and though these neurons grow more slowly than natural neurons, they still gain functional abilities when cultured with active cortical neurons (Berninger et al., 2007).
Epigenetic changes
Changing the programing of adult astrocytes usually involves the expression of several different pathways, epigenetic silencing of mature astrocyte genes, and removing silencing marks from progenitor genes to restore a pluripotent state (Robel et al., 2011). For example, increased acetylation of H3K9K14 near NeuroG1 and NeuroG2 is observed during astrocyte dedifferentiation in mouse primary neuronal cultures (Hirabayashi et al., 2009). Interestingly, many of the downstream targets of these two transcription factors do not seem to be silenced themselves, and forced expression can directly lead to astrocyte dedifferentiation (Robel et al.,2011). Inhibiting the actions of DNA methyltransferases,which normally silence genes as a cell matures, can keep stem cells from resuming an astrocytic phenotype (Bulstrode et al., 2017). In contrast, increasing expression of Ezh2, a histone methyltransferase that catalyzes H3K27me3 and leads to stable gene repression, is necessary but not sufficient to cause astrocyte dedifferentiation (Sher et al., 2010). By increasing Ezh2, genes necessary for astrocyte maintenance are silenced, and the cell resumes a partial NSC morphology.NeuroD4 was found to reprogram astrocytes to neurons, and blocking NeuroD4 with transcriptional repressors lead to the accumulation of H4K20me3 and astrocytes that did not dedifferentiate (Masserdotti et al., 2015).
Transcription factor pathways
Altering the expression of several other transcription factors and downstream targets has been observed to change astrocyte fate. In particular the transcription factors Nanog Homeobox, POU Class 5 Homeobox 1 (OCT4), Forkhead Box G1 (FOXG1), SRY-Box 2 (SOX2), and Cell Cycle Exit and Neuronal Differentiation 1 (CEND1) all have the capability,either alone or in concert with each other, to dedifferentiate post-mitotic astrocytes (Corti et al., 2012; Niu et al., 2013;Aravantinou-Fatorou et al., 2015; Bulstrode et al., 2017).This effect is further increased when astrocytes are treated with epidermal growth factor (EGF- and fibroblast growth factor-rich media. Forkhead Box O3, a transcription factor regulated by FOXG1/SOX2, is highly expressed in mature astrocytes, and transcription repression by FOXG1/SOX2 is partly responsible for reversion to a aNSC phenotype (Bulstrode et al., 2017). Another necessary-but-not-sufficient factor is fibroblast growth factor 4, a mitogen important for cell proliferation that pushes NSCs toward a neural fate (Feng et al., 2014). Re-expression of the transcriptional repressor(BMI1) in differentiated astrocytes resulted in their reversion to NSC-like cells, and they produced the NSC markers nestin, CD133, and SOX2 (Moon et al., 2008). Furthermore,these cells can then differentiate back into astrocytes or become neurons or oligodendrocytes. Activation of the NF-κB pathway via the introduction of tumor necrosis factor-positive media has been shown to cause GFAP+astrocytes to re-express immature neuronal markers CD44, Musashi-1,and OCT4 (Gabel et al., 2016) in mature astrocyte cultures and in vivo brains. Sonic Hedgehog signaling acts in concert with OCT4 to increase astrocyte reprogramming (Yang et al., 2019). Astrocytes that express bone morphogenetic protein 4 were susceptible to dedifferentiation through noggin exposure (Michelucci et al., 2016). Overexpression of Cyclin-dependent kinase 6 in mature astrocytes resulted in a higher motility and a morphology representative of a more dedifferentiated state (Slomiany et al., 2006). Induction of the growth factor Erb-B2 Receptor Tyrosine Kinase 2 can cause astrocytes to revert to radial glial cells in the cortex,which then proliferate and become neurons (Ghashghaei et al., 2007; Yang et al., 2011). A combination of transcription factors and a microRNA—collective referred to as NeAL218 and consisting of NEUROD1, ASCL1, LMX1A, and the microRNA miR-218—reprogrammed astrocytes to neurons in both cell culture and in vivo experiments (Rivetti di Val Cervo et al., 2017). In addition to OCT4 and SOX2, the transcription factors Kruppel Like Factor 4 and MYC Proto-Oncogene have also been observed to participate in the dedifferentiation of astrocytes (Ruiz et al., 2010).
MicroRNA pathways
Intracellularly, aNSCs mature into neurons through multiple,complex changes in gene expression. MicroRNAs are small non-coding transcripts ~22-23 nucleotides long that inhibit the translation of mRNA and often target many members of the same regulatory pathway, with some having over a thousand putative targets. Change in microRNA expression driven by extracellular signals facilitate the transition of aNSCs to differentiated states (Stappert et al., 2018). Several miRNAs have been found to work in concert to drive this process, and afterwards maintain specialized gene expression patterns through the inhibition of proliferative genes.Let-7b is highly expressed in both differentiated neurons and astrocytes and regulates proliferation and differentiation (Zhao et al., 2010). miR-9 helps drive neural stem cells toward a neural fate (Zhao et al., 2009). Similarly miR-124,the most common microRNA in the brain (Lagos-Quintana et al., 2002), regulates the timing of neurogenesis in the canonical neurogenic zones (Cheng et al., 2009). Blocking miR-124 expression through the transcriptional repressor(REST) is essential to the development of astrocytes (Conaco et al., 2006). Treatment with miR-128, among other factors,was shown to increase astrocyte dedifferentiation (Rivetti di Val Cervo et al., 2017). Two microRNAs, miR-302 and miR-367, in conjunction with the histone deacetylase inhibitor valproic acid, were successful in reprogramming adult astrocytes (Ghasemi-Kasman et al., 2015). Expression of miR-181a influences neural stem cells to an astrocyte fate,whereas inhibition of miR-181a produces more neurons (Xu et al., 2014). After injury, treatment with miR-181a inhibitor increases the number of new neurons in the rat hippocampus through increased neurogenesis, possibly through the increased conversion of astrocytes to neurons (Griffiths et al., 2019). Neurogenic properties of astrocytes are dependent on local environmental factors, including Notch signaling(Imayoshi et al., 2008; Magnusson et al., 2014) and CDON expression. CDON is normally silenced through interactions with MeCP2 in astrocytes (Yasui et al., 2013), however, in a recent study we observed a significant post-injury increase in CDON expression in animals treated with miR-181a inhibitor post-injury (Griffiths et al., 2019). Treatment with microRNA inhibitors or mimics after injury, when astrocytes are reactive, may help drive reactive, dedifferentiated astrocytes toward a neural fate.
Reprogrammed neuronal subtype
Neurons are vastly heterogenous, and the ability to create new neurons in response to injury or disease will rely on the ability to drive differentiation toward relevant neuronal subtypes. Dedifferentiated astrocytes can be directed toward either an excitatory glutamatergic fate with NeuroG2, or an inhibitory GABAergic phenotype with Dlx2 (Heinrich et al.,2010). In another study, the ability to selectively transform astrocytes into glutamatergic neurons did not result in their forming synaptic connections until the transcriptional repressor INSM1 was also expressed (Masserdotti et al., 2015),suggesting potential therapies may need multi-timepoint transcriptional control. Sonic Hedgehog signaling is present in reactive astrocytes that gain aNSC properties in a stab wound injury model, but not in models of stroke (Sirko et al., 2013), though both models have been shown to dedifferentiate astrocytes. This suggests astrocyte dedifferentiation may be a convergent consequent of separate gene regulation responses, depending on the mode of injury. Though many of the factors in this section work on several species tested,some worked exclusively on human or rodent astrocytes(Zhang et al., 2015) (Table 1). Understanding the differences in the pathways for neuron type or species specificity will be important for developing and selecting appropriate treatments in the future.
Astrocyte therapies
Neurogenesis via astrocyte reprogramming has also been investigated for therapy purposes for a variety of conditions.Alzheimer’s disease leads to a drastic increase in reactive astrocytes, but not an increase in neurogenesis (Boekhoorn et al., 2006). In a model of Alzheimer’s disease, reactive astrocytes were easily reprogrammed to functional neurons(Guo et al., 2014). Parkinson’s disease results from the loss of dopaminergic neurons and replacing lost dopaminergicneurons with reprogrammed astrocytes results in improved motor behavior in a mouse model and in human cells (Rivetti di Val Cervo et al., 2017). It is also a promising therapy for stroke patients, as astrocytes are more resilient to the loss of oxygen that results in wide-spread damage to neurons(Chouchane and Costa, 2012). In a stroke model, self-renewal and dedifferentiated astrocytes were seen in the cortical peri-infarct area (Shimada et al., 2012). Additionally, thousands of new neurons appear in the striatum in the weeks following stroke (Arvidsson et al., 2002; Zhang et al., 2009;Li and Clevers, 2010; Magnusson et al., 2014), and one- to two-thirds of the new neurons are estimated to have been generated from local astrocytes. In the presence of transplanted neural stem cells, astrocytes were observed to revert to a developmentally earlier programming and reform into radial glial cells to aid the migration of new neural stem cells(Leavitt et al., 1999). Though this review focuses on astrocyte dedifferentiation in the brain, there is parallel research being done on using astrocyte reprogramming to replace neurons in injured spinal cord (Su et al., 2014).
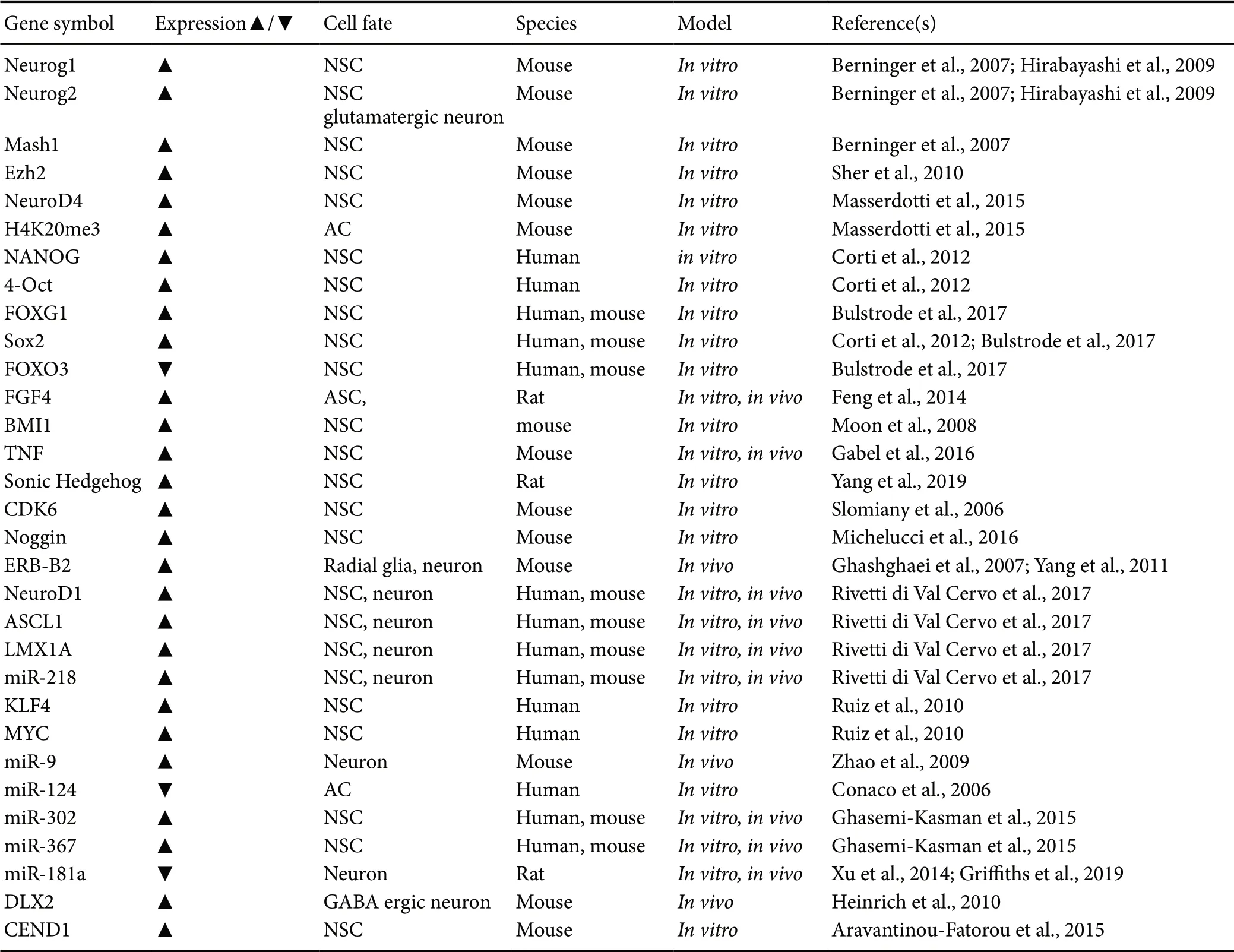
Table 1 Genes and epigenetic changes that alter cell fate
A particularly interesting case is injury sustained to the hippocampal CA1 after global cerebral ischemia (Li and Stary, 2016). After injury, neurons in the CA1 dwindle to almost undetectable levels, but reappear after several weeks of healing. The new CA1 cells have minimal observed BRDU labeling despite vast numbers of new neurons (Sugawara et al., 2000). Because of the absence of proliferating or migrating cells in CA1, these new neurons may be from dedifferentiated astrocytes. Reactive astrocytes after global cerebral ischemia are common in the CA1, while classic adult neurogenesis is not thought to occur in that brain region (Zeisel et al., 2015). Our own research suggests that dedifferentiating astrocytes may contribute to the neurogenesis seen in this injury model. The above cases are only a few of the currently research models, but astrocyte dedifferentiation has the potential to produce revolutionary therapies for a plethora of other disease and injuries.
Dedifferentiation risks
Altering the genetic programming of astrocytes can also lead to negative consequences, such as their ability to develop into glioblastoma multiforme (Friedmann-Morvinski et al.,2012). Many gliomas are thought to be the result of natural astrocyte dedifferentiation gone wrong. Expression of transforming growth factor-α can lead to astrocyte dedifferentiation, but the resulting cells acquire oncogenic properties and become cancerous with even mild environmental stress(Dufour et al., 2009). Platelet-derived growth factor receptor,a protein commonly expressed in cancerous cells, was successful at dedifferentiating astrocytes and neurons, but also lead to cancerous phenotypes (Dufour et al., 2009). The expression of epidermal growth factor in the absence of tumor suppressor genes p16INK4a and p19ARF leads to astrocyte dedifferentiation and cancer (Bachoo et al., 2002). Additionally, it is very likely that forcing changes in expression of a few genes will not fully erase epigenetic regulation of all astrocyte-expressed genes, and they will retain a “memory” of their previous programming and exhibit altered phenotypes from naturally derived neurons (Tian et al., 2011). This may increase the risk of unintended gene expression even after a cell has matured and assumed normal functioning. However,understanding the pathways involved in astrocyte dedifferentiation and reprogramming may serve as a launching point for treating glioblastoma as well. Manipulation of these pathways can cause glioblastoma to stop their malignant proliferation and assume a more stem-cell like state (Dahan et al., 2014).
In addition to cancerous growths, the inclusion of new neurons into existing neuronal networks has the potential to disrupt functional signaling, introducing noise and dysregulation into a stable system. Many conditions that observe an increase in adult neurogenesis also note the phenomenon of “abberent neurogenesis”. For example, uncontrolled neurogenesis has been shown to play a role in the development of epilepsy (Jessberger et al., 2007; Cho et al., 2015). Stroke leads to an increase in adult neurogenesis, but many of the new neurons do not integrate properly and contribute to cognitive issues (Niv Fanny et al., 2012). Traumatic brain injury leads to a large increase in neurogenesis, but results atrophied astrocytes unable to regulate the new neurons(Robinson et al., 2016).
Conclusions
Finely controlled neurogenesis has the potential to benefit many disorders and diseases that result from the loss of healthy neurons. However, endogenous neural stem cell populations are limited to a few areas of the brain. Astrocytes are common throughout the brain, and new astrocytes are regenerated throughout the lifespan. Their natural ability to change their developmental programming back to an earlier state through dedifferentiation, transform into neurons,and integrate to form functional synapses within existing networks represents an exciting target for future researchers and clinicians. Advancing techniques to better quantify new neuron formation in the adult human brain are needed to advance clinical implementation. Notably, many devastating cancers are caused by uncontrolled astrocyte dedifferentiation. While this represents a substantial consideration and off-target effect of clinical neurogenic approaches, astrocyte-based diseases might also provide a model to further delineate astrocyte-mediated neurogenesis.
Author contributions:All authors wrote the manuscript and approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by the American Heart Association, No. 18POST33990395 (to BBG), American Heart Association,No. 14FTF-19970029 (to CMS), and National Institutes of Health, No.NS107445 (to CMS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Tetsuro Ishii, University of Tsukuba, Japan; Hans-Gert Bernstein, University of Magdeburg, Germany; Randall L. Davis,Oklahoma State University, USA; Ivan Fernandez-Vega, Hospital Universitario Central de Asturias, Spain.
杂志排行
中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy
- Using our mini-brains: cerebral organoids as an improved cellular model for human prion disease
