Abscisic acid, a promising therapeutic molecule to prevent Alzheimer’s and neurodegenerative diseases
2020-12-02AnaMaría,Sánchez-Pérez
Neuroinflammatory processes induce neuronal damage and underlie the onset of neurodegenerative pathologies, including Alzheimer’s disease (AD) (Calsolaro and Edison, 2016). Several conditions, ranging from emotional stress to metabolic syndrome, including traumatic brain injury, chronic infections or gut microbiota disruption can cause or aggravate neuroinflammation. Moreover, aging is the major factor associated with neurodegenerative diseases.
Suffering metabolic syndrome (obesity, insulin resistance,chronic inflammation) during a lifetime is associated with a higher risk of cognitive decline in the elderly (Yuan et al., 2001).On the contrary, physical exercise reduces neuroinflammation and improves memory, facilitating synaptic plasticity and neurogenesis (van Praag et al., 1999). Unfortunately, the incidence of metabolic syndrome is continuously increasing in most countries. This fact, together with a longer life expectancy, forecast a future with a growing occurrence of dementia and neurodegenerative pathologies. Thus, it is a great challenge of modern societies to devise efficient strategies to prevent and treat neurodegeneration in the elderly.
In our most recent study, we proposed that the regular intake of the phytohormone abscisic acid (ABA) (Figure 1A)can effectively prevent memory loss in a murine model of AD and skews microglia to their resting anti-inflammatory status.Moreover, we have proved that early intervention significantly augments the probability of preventing cognitive impairment.
ABA is a natural product present in fruit and vegetables (avocado, apricot, citruses, etc.). It was only known a few years ago for its peripheral anti-glycemic and anti-inflammatory properties (Zocchi et al., 2017). Interestingly, ABA is also synthesized in mammals upon different stimuli. In fact, in response to a glucose load, ABA can be detected in plasma from healthy humans but not in plasma from diabetic patients (Ameri et al., 2015).
Our group has studied the phytohormone ABA’s capability to prevent memory loss in a rodent model of metabolic syndrome(Sánchez-Sarasúa et al., 2016). In this model, chronic ABA administration reduces brain proinflammatory cytokine expression and improves hippocampal neurogenesis (Ribes-Navarro et al., 2019).
Because these effects could be attributed to ABA’s peripheral insulin-sensitizing properties, we sought to study a neuroinflammation model with no peripheral comorbidities, therefore we chose the triple transgenic murine model of AD. If our hypothesis was correct, ABA could be considered as a new therapeutic molecule preventing neurodegenerative diseases.
The triple transgenic mice are homozygous for three mutant transgenes (presenilin1; amyloid precursor proteinSwe and tau-P301L). Mutations in amyloid precursor protein and presenilin1 are human mutations identified in familial AD, which account for < 10% of all cases. The Tau mutation (tauP301L) is not a typical human AD mutation, but the switch Proline-Leucine facilitates deposition of intracellular Tau misfolded protein, and that mimics the microfibrillar tangles that are characteristics of human AD. An important aspect is that these mutations induce the AD pro-inflammatory microglia state, presumably due to the accumulation of toxic misfolded proteins and/or toxic beta-amyloid oligomers (Thei et al., 2018). Therefore, this murine model of AD provides a useful tool to study potential therapeutic strategies against this disease.
We set out an experimental model where ABA was chronically administered to wild type and transgenic animals, and compared its effects to vehicle-treated subjects. Two administration patterns were considered. The long treatment group of mice started ABA or vehicle administration at young ages (mice were 3 months of age and they had no disease manifestations) and received ABA for 5 months (5M group). The short treatment group started ABA administration at 5 months of age (at this time point, triple transgenic mice begin to show the first neurological symptoms) and received ABA for 3 months (3M group).Finally, all cohorts performed behavioral paradigms to evaluate hippocampal-dependent memory at 8 months of age. Subsequent post-mortem analysis was carried out to study microglia status.
We found that the behavioral performance was significantly improved in the transgenic ABA 5M group compared to untreated transgenic mice. ABA 5M transgenic mice behaved equally to wild type controls. Only a few transgenic animals from the ABA 3M cohort showed improved cognitive function compared to untreated transgenic, suggesting a small ABA effect. We believe that this finding is still positive for human patients. Besides, ABA did not seem to affect wild type controls,indicating that it is a safe procedure (Espinosa-Fernandez et al.,2019).
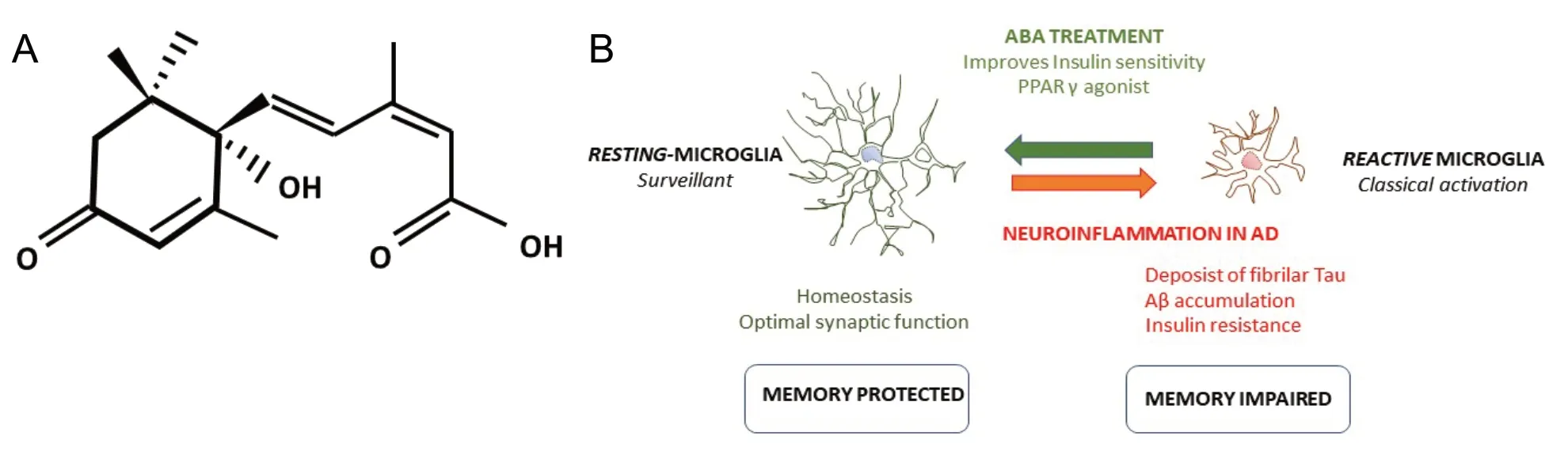
Figure 1 Abscisic acid (ABA) treatment.
Microglia is the brain resident macrophage cell and represents the first line of the innate immune response against toxic stimuli. Microglia morphology reveals their activation state(Savage et al., 2019). Hence, in “normal” or healthy situations,microglia presents many ramifications, the resting microglia.In this status, microglial cells are actively surveillant the brain.When there is a toxic stimulus, such as injury, infections, dead cells or misfolded proteins, microglia cells suffer a stepwise loss of branches, becoming more rounded with fewer and shorter divisions. In this morphological state, microglia are known as activated or reactive. Also, both microglia phenotypes display a different pattern of secreted cytokines. Ideally, reactive microglia slowly return to the resting state after the initial toxic insult is cleared up. But in conditions of chronic insults (e.g., metabolic syndrome) or continuous production of misfolded proteins (e.g.,the murine AD model) microglia remains in their proinflammatory polarized status, secreting more proinflammatory cytokines, which in turn reduces insulin signaling in neurons, which generates neuronal stress and hence entering in a degenerative spiral. Even though microglia activation is a very complex process, specific and simple morphological parameters are widely used as a reliable marker to evaluate neuroinflammation (Davies et al., 2017).
There is extensive and solid evidence supporting the role of microglia activity in AD (Hemonnot et al., 2019). Thus, we examined the microglia morphology in our model. We analyzed two brain areas involved in memory (the prefrontal cortex and the hippocampus). Three morphological parameters were measured; perimeter, area, and fractal (branching) in all groups at 8 months of age. We confirmed that untreated AD transgenic mice displayed the activated microglia phenotype, compared to wild type controls that displayed the resting phenotype. Interestingly, in both transgenic mice treated with ABA (3M and 5M), microglia displayed the resting phenotype (higher perimeter, higher fractal, and higher area) indistinguishable from wild type controls. This suggests that ABA may protect cognitive function by preventing excess of proinflammatory microglia(Figure 1B). Interestingly, despite the microglia resting phenotype, the 3M ABA cohort did not perform as well in the memory test. These findings suggest that microglia state is not enough in itself to guarantee optimal neuronal performance, but it likely precedes it. It remains unknown whether ABA has a direct effect on microglia polarization or whether ABA reduces the toxic burden in neurons (e.g., sensitizing to insulin signaling), and therefore diminishing toxic indications to microglia. Further experiments will be required to decipher these questions.
In conclusion, early intervention, and longer ABA treatment prevented cognitive deterioration, very likely by favoring microglia resting state (thus reducing neuroinflammation), still, it is plausible that later interventions, starting when neuropathological symptoms have already appeared, may be effective if ABA is administered for longer periods. If that were the case, it would mean that neurodegeneration can also be treated once it is diagnosed and represents a hopeful perspective for human patients. However, further research is required to ascertain whether neurodegeneration can be effectively treated. Considering current knowledge, controlling neuroinflammation at early ages is required as a neuroprotective factor to preserve cognitive function in the elderly.
This work was supported by Plan Propi, No. UJI-B2018-01, to AMSP.
Ana María Sánchez-Pérez*
Department of Medicine, Universitat Jaume I, Castellón de la Plana,Spain
*Correspondence to:Ana María Sánchez-Pérez, PhD,sanchean@uji.es.
orcid:0000-0002-5811-0005 (Ana María Sánchez-Pérez)
Received:August 29, 2019
Peer review started:September 2, 2019
Accepted:October 12, 2019
Published online:December 13, 2019
doi:10.4103/1673-5374.270307
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy
