Remote ischemic conditioning approach for the treatment of ischemic stroke
2020-12-02SeyedMohammadSeyedsaadat,DavidF.Kallmes,WaleedBrinjikji
Stroke is the leading cause of disability and death in North America. There has been growing interest in identifying neuroprotective strategies to reduce ischemic burden in patients with acute ischemic stroke. However, despite extensive clinical trials, no neuroprotective agent has been found for prevention of ischemic damage.Remote ischemic preconditioning (RIC) is a promising non-invasive strategy that has been proven to provide renal and cardioprotection and has recently found to have a potential broad application in the treatment of neurovascular disease, which has bee linked to its possible effects on the release and activation of endogenous neuroprotective substances against the ischemia/reperfusion injuries in experimental studies. This endogenous neuroprotection might vaccinate neural tissues against effects of acute IR following primary infarction insult. Regardless of the method of RIC administration,through manual or automated blood pressure cuff, RIC procedure is inexpensive and easy to use. Based on the experimental and clinical data, application of RIC avoids possible adverse effects and interactions associated with chemical pharmacological agents. In previous clinical studies RIC was safe and associated with only minor transient adverse effects in few cases, including petechia and minor limb pain, which were mostly resolved shortly after completing the treatment. RIC involves between three to five cycles of 5 minutes blood pressure cuff inflation and 5 minutes of deflation on the upper or lower extremity. RIC can be applied before (Pre-RIC),during (Per-RIC) and after (Post-RIC) infarction and can be safely continued for a prolonged period of time in human.
The neuroprotective mechanism of RIC includes inhibition of pathophysiological cascades which begin following primary ischemic insult. RIC has been found to work through anti-apoptosis,anti-inflammatory, anti-oxidative, and mitochondria modulatory mechanisms, as well as through endogenous release of vascular protective mediators, and transcriptional upregulation of protective pathways (Hess et al., 2015; Zhou et al., 2018). Although multiple preclinical and clinical studies have demonstrated the efficacy of RIC in reducing infarction size in acute and elective settings for acute ischemic stroke (AIS), the ability of RIC in mitigation of ischemic damage needs to be proven in future studies to translate this treatment model into the bedside. So far, few clinical studies have investigated the underlying mechanism of RIC and optimal RIC protocol in patients with cerebrovascular diseases (CVD) based on the advanced molecular and neuroimaging surrogate biomarkers.This perspective seeks to update the current evidence on the effects and molecular mechanisms of action of RIC on neuroimaging biomarkers and in the treatment of CVD based on animal and clinical studies and discuss the perspectives for the future studies.
RIC in animal models: timing, duration and outcome:In previous experimental models of AIS, per-conditioning (Per-RIC) use has been consistently associated with significant improvement of short- and long-term outcome in rat and mice animal models (Hahn et al., 2011; Hoda et al., 2014; Ma et al., 2017; Kitagawa et al., 2018).Ma et al. (2017) and Kitagawa et al. (2018) suggested that Per-RIC significantly reduces early ischemic injury through preventing collateral collapse and therefore facilitating the delivery of neuroprotective agents to the salvageable tissue in penumbra. In another study, Hahn et al. (2011) showed that both Pre-RIC and Per-RIC reduce infarct size; however, Per-RIC was associated with superior efficacy over Pre-RIC. Hoda et al. (2014) showed significant neuroprotective benefits of Per-RIP therapy. While most studies have focused on the single dose short-lasting neuroprotective benefits of Pre- and Per-RIC, recently, the ability of repeated chronic daily Post-RIC to reduce neural damage has been supported by several preclinical animal studies and its neurorestorative effects have been further supported by findings from recent clinical trials in ischemic stroke patients. Ren et al. (2015) showed that continued repeated administration of RIC [i.e., post-conditioning (post-RIC)]for 14 days after reperfusion was associated with even stronger neuroprotection against cerebral ischemia/reperfusion injury. The neurorestorative effects of Post-RIC have been demonstrated in previous experimental studies.Figure 1demonstrates the schematic representation of suggested underlying mechanism by which Per-RIC and continued Post-RIC treatment can benefit to mitigate ischemic damages. As shown in the illustration, in early phase of ischemic damage, Per-RIC can reduce neurological and behavioral deficits by decreasing infarct size, brain edema, blood-brain barrier permeability, and oxidative stress. While, continued Post-RIC administration can even further enhance plasticity and neurorecovery through increasing cerebral blood flow (CBF), collateral circulation, and neural grow factors which leads to improved astrogliosis,vasculogenesis and neurogenesis.RIC in human studies:timing, duration and outcome:Previous clinical studies have indicated the safety and feasibility of RIC in patients with AIS, aneurysmal subarachnoid hemorrhage and following interventional procedures including mechanical thrombectomy, elective carotid endarterectomy, and endovascular intracranial aneurysm repair (Gasparovic et al., 2017; Mohammad Seyedsaadat et al., 2019). Recent studies have also demonstrated the efficacy of RIC in aSAH, intracranial atherosclerotic stenosis and in those who undergo cardiac surgeries, and carotid artery stenting.Similar to animal stroke models, RIC administration in patients with CVD was associated with reduced infarct size and volume, improved neurological and functional outcomes as well as decreased future ischemic events.
Long-term (> 180 days) Post-RIC therapy has been strongly associated with improved outcome in many prior clinical studies. In all of these 5 phase-2 clinical trials, long-term Post-RIC was associated with decreased stroke reoccurrence in symptomatic intracranial stenosis, decreased new brain lesions in patients with carotid artery stenosis undergoing carotid artery stenting, and decreased cognitive impairment and white matter hyper-intensity in patients with cerebral small vessel disease. The efficacy of long-term Post-RIC use in improvement of the clinical outcome of CVD patients is consistent with the results of animal studies, which might indicate the similar ability and potential of long-term Post-RIC in humans to improve the outcome through enhancing the collateral circulation, CBF and neurogenesis. However, to the best of our knowledge, no study has evaluated the effects of Per-RIC in combination with long-term Post-RIC on the clinical outcome of AIS patients, and prevention of thromboembolic events following endovascular aneurysm repair.Therefore, a large multi-center clinical trial warrants to study the effects of Per-RIC plus long-term Post-RIC on short- and long term outcome and complications in patients with AIS who undergo endovascular thrombectomy and evaluate the effects of this regimen on molecular neurovascular remodeling, collateral circulation, CBF,and functional connectivity using biochemical blood analysis and advanced imaging techniques such as diffusion-weighted imaging/perfusion weighted imaging mismatch/region, Arterial spin labeling,functional, and resting-state functional MRI. Therefore, we speculate that these results can fill a crucial niche in better understanding the underlying mechanism of RIC and prevention and treatment of ischemic brain injury by providing continued neuroprotection.
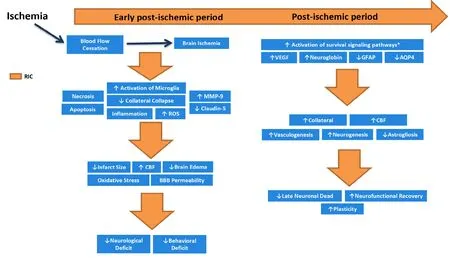
Figure 1 Schematic representation of suggested underlying mechanism by which Per-RIC and continued Post-RIC treatment can benefit to mitigate ischemic damages.
In conclusion, based on the current data, a combined Per-RIC plus long-term Post-RIC regimen might maximize the neuroprotective benefits of RIC in the treatment of patients with ischemic brain injuries or those who are at high risk for ischemic brain damages. Further research is required to validate the therapeutic efficiency of this combination regimen in the treatment of CVD.
We would like to thank Amanda, Munoz Casabella, MD for her help in literature search and designing the table.
SMS received American Heart Association fellowship award, No.19POST34381067.
Seyed Mohammad Seyedsaadat*, David F. Kallmes,Waleed Brinjikji
Department of Radiology, Mayo Clinic, 200 First St SW, Rochester,MN, USA
*Correspondence to:Seyed Mohammad Seyedsaadat, MD,Seyedsaadat.SeyedMohammad@mayo.edu or arman.saadat@gmail.com.
orcid:0000-0001-8188-3455 (Seyed Mohammad Seyedsaadat)
Received:June 19, 2019
Peer review started:June 21, 2019
Accepted:September 29, 2019
Published online:December 13, 2019
doi:10.4103/1673-5374.270303
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy
