Effects of nine saponins of Aralia echinocaulis root on the viability of bone marrow-derived macrophages
2020-11-16FanXueMengyunYanJinchengLiJingzhuSongWenbingZhouLingpengPei
Fan Xue, Mengyun Yan, Jincheng Li, Jingzhu Song, Wenbing Zhou,2*, Lingpeng Pei,2*
1 School of Pharmacy, MINZU University of China, Beijing 100081, China ;
2 Key Laboratory of Ethnomedicine, MINZU University of China, Ministry of Education, Beijing 100081, China
Fan Xue and Mengyun Yan are the first authors who contributed equally to this article.
Abstract
Key words: Aralia echinocaulis Hand.-Mazz.; saponins; bone marrow-derived macrophages; viability
Introduction
Osteoporosis is a systemic disease of abnormal bone metabolism. It is believed that osteoporosis is caused by the down-regulation of osteoblast function and the up-regulation of osteoclast activity[1]. Osteoclasts from precursors in the monocyte/macrophage lineage are multinucleated cells[2], and multinuclear cells induced by macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor-κB ligand (RANKL)are found 5 days after tartrate-resistant acid phosphatase(TRAP) positive staining[3], the increasing number of osteoclasts and excessive absorption of bone can induce bone loss[4-5].
TheAralia echinocaulisHand.-Mazz. from China, is an important ethnomedicinal herb. The root bark ofAralia echinocaulisHand.-Mazz. is used to treat osteoporosis,diabetes, rheumatism and gastrohelcosis in Chinese medicine historically[6-7]. Previous phytochemical investigations of thisAralia echinocaulisHand.-Mazz.include saponins, flavonoids and so on. While our previous study has found that total saponins ofAralia echinocauIisHand.-Mazz. had restrain effects on bone loss in rats with osteoporosis induced by prednisone[8]. Recently, we firstly extracted nine saponins, Tarasaponin IV, Stipuleanoside R2and Araloside C fromAralia echinocaulisHand.-Mazz..Some studies have reported that triterpenoid saponins could protect myocardial injury[9-10]. Here, we aim to investigate the inhibitory effect of the isolated compounds on the viability of M-CSF-induced BMMs.
Materials and Methods
General procedures
The NMR spectra were recorded with Varian VNMRS 600 (600 MHz for1H-NMR and 150 MHz for13C-NMR)and the chemical shifts were given in δ (ppm) scaled with tetramethylsilane as an internal standard. LCMSIT-TOF was recorded on a CII system (Shimadzu, Kyoto,Japan). HPLC was performed on a Shimadzu 20AT system(quaternary pump; Shimadzu, Kyoto, Japan) with a X Aqua C18(4.6 mm i.d. × 250 mm, and 10 mm i.d. × 250 mm, X Aqua, 5 μm; Huapu Xinchuang Technology Co.,Ltd., Zhejiang, China); detectors were SPD (UV detector)and ELSD (evaporative light scattering detector) (Alltech 3300 Evaporative Light Scattering Detector, temperature:70°C, gas flow: 1.6 liters/min). Macroporous resin MCIGEL (Mitsubishi chemical, Tokyo, Japan), silica gel (SiO2;Qingdao Haiyang Chemical Co. Ltd, Qingdao, China),YMC-Pack ODS-A Column (250 × 20.0 mml .D.S-5 μm; YMC, Kyoto, Japan) and YMC*GEL® ODS-AQ-HG C18(12 nm i.d., 50 μm; YMC, Kyoto, Japan) were used for column chromatography. HPLC grade acetonitrile(CH3CN) and methanol (MeOH) were purchased from Fisher Scientific (Fair Lawn, NJ, U.S.A.). Deionized water(H2O) was purified by Milli-Q system (Bedford, MA,U.S.A.). Other reagents for purification, such as methanol(MeOH), chloroform (CHCl3), dichloromethane (CH2Cl2),and n-butanol (n-BuOH), were of analytical grade bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,China).
Plant material
The root barks ofAralia echinocaulisHand.-Mazz.were purchased with Hubei Enshitang Pharmaceutical Company, China (OP 091101) and identified by Professor Yaojun Yang at Beijing University of Chinese medicine. It has been deposited at pharmacognosy laboratory in the school of pharmacy, MINZU University of China.
Extraction and isolation
The root barks ofAralia echinocaulisHand.-Mazz. (2 kg)were extracted thrice with 70% ethanol under reflux for 2 h and the solvent was evaporated under reduced pressure to give a brown extractum (400 ml). After the residual extract was isolated on macroporous resin MCI and eluted with a gradient mixture of EtOH:H2O (10:90; 30:70; 50:50;70:30 and 90:10) to give 9 fractions (Fr. 1-9)[11-13].
Fr. 4 (23.17 g) was subjected to silica gel column chromatography eluting with gradient mixture of CHCl2:MeOH:H2O (90:30:5; 70:25:5; 70:30:6; 75:33:6 and 70:33:6, the underlayer) to yield 8 fractions (Fr. A1-8). Fr. A6 (7.00 g) amount was separated by repeated chromatography over YMC*GEL® ODS-AQ-HG C18(12 nm i.d., 50 μm), eluting with CH3CN:water (contains 0.01% formic acid) (28:72; 30:70; 32:68, the underlayer) to give 7 fractions (Fr. D1-7). The Fr. D1 was the Compound I(0.979 g). The Fr. D2 (200 mg) and Fr. D5 (243 mg) were dissolved in CH3CN:water (containing 0.01% formic acid)= 32:68 solution. Then, the solution was filtered, and the filtrate was prepared by preparative liquid over YMC-Pack ODS-A Column (250 × 20.0 mml .D.S-5 μm), eluting with CH3CN:water (containing 0.01% formic acid) = 32:68 solution. Last, Compound II (14 mg) and Compound III(67 mg) were separately obtained.
Fr. 5 (13.72 g) was subjected to silica gel column chromatography eluting with gradient mixture of CHCl2:MeOH:H2O (75:25:5; 70:22:5; 75:25:5; 70:26:5;65:25:5, 70:43:10, the underlayer) to yield 12 fractions (Fr.B1-12). Fr. B10 (1.60 g) amount was separated by repeated chromatography over YMC*GEL® ODS-AQ-HG C18 (12 nm i.d., 50 μm), eluting with CH3CN:water (containing 0.01% formic acid) (28:72, the underlayer) to give 3 fractions (Fr. G1-3). The Fr. D1 was the Compound I (0.979 g). The Fr. F12 (194 mg) was dissolved in CH3CN:water(containing 0.01% formic acid) = 32:68 solution, the solution was filtered, and the filtrate was prepared by preparative liquid over YMC-Pack ODS-A Column (250 ×20.0 mml .D.S-5 μm), eluting with both same proportion of solution on CH3CN:water (containing 0.01 formic acid),in which Compound IV (8 mg) was obtained. After the Fr.G2 (200 mg) was dissolved in CH3CN:water (containing 0.01% formic acid) = 32:68 solution and the Fr. F5 (217 mg) was dissolved in CH3CN:water (containing 0.01% formic acid) = 35:65 solution, the solution was filtered, and the filtrate was prepared by preparative liquid over YMCPack ODS-A Column (250 × 20.0 mml .D.S-5 μm), eluting with both same proportion of solution on CH3CN:water(containing 0.01 formic acid), in which Compound V (8 mg) and Compound VI (12 mg) were separately obtained.
Fr. 6 (4.00 g) amount was separated by repeated chromatography over YMC*GEL® ODS-AQ-HG C18(12 nm i.d., 50 μm), eluting with CH3CN:water (containing 0.01% formic acid) (35:65, the underlayer) to give 8 fractions (Fr. M1-8). The Fr. M4 (190 mg) and Fr. M7 was dissolved in CH3CN:water (containing 0.01% formic acid)= 35:65 solution. Then, the solution was filtered, and the filtrate were prepared by preparative liquid over YMCPack ODS-A Column (250 × 20.0 mml .D.S-5 μm), eluting with CH3CN:water (containing 0.01 formic acid) = 35:65,in which Compound VII (44 mg) was obtained.
Fr. 8 was dissolved in CH3CN:water (containing 0.01% formic acid) = 47:53 solution. Then, the solution was filtered, and the filtrate were prepared by preparative liquid over YMC-Pack ODS-A Column (250 × 20.0 mml .D.S-5 μm), eluting with CH3CN:water (containing 0.01 formic acid) = 47:53 solution, to give five fractions (Fr. O1-5). The Fr. O3 (100 mg) was dissolved in CH3OH. The solution was filtered, and the filtrate were prepared by preparative liquid over YMC-Pack ODS-A Column (250 × 20.0 mml.D.S-5 μm), eluting with CH3CN:water (containing 0.01 formic acid) = 47:53 solution, the Compound VIII (21 mg)and Compound IX (11 mg) were obtained separately.
Compound I was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C53H84O23; HR-MS:m/z1087.5308[M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.78,δ0.83,δ0.90,δ0.92,δ0.93,δ1.06,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported (Tables 1-2). Based on previous literature[14],Compound I is Tarasaponin IV (Figure 1).
Compound II was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C53H84O23; HR-MS:m/z1087.5290[M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.78,δ0.84,δ0.90,δ0.92,δ0.93,δ1.06,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported (Tables 1-2). Based on previous literature[15],Compound II is Stipuleanoside R2 (Figure 1).
Compound III was white amorphous powder.Liebermann-Burchard reaction and Molish reaction were both positive. Molecular formula was C53H84O23; HR-MS:m/z1087.5310 [M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.78,δ0.83,δ0.90,δ0.92,δ0.93,δ1.06,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz)data are reported (Tables 1-2). Based on previous literature[16],Compound III is Araloside C (Figure 1).
Compound IV was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C54H86O24; HR-MS:m/z1117.5408[M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.79,δ0.85,δ0.90,δ0.92,δ0.94,δ1.05,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported(Tables 1-2). Based on previous literature[17], Compound IV is Decaisneanaside (Figure 1).
Compound V was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C48H76O19; HR-MS:m/z955.4891[M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.79,δ0.84,δ0.89,δ0.92,δ0.94,δ1.06,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported (Tables 1-2). Based on previous literature[18],Compound V is Chikuselsusaponin V (Figure 1).
Compound VI was white amorphous powder.Liebermann-Burchard reaction and Molish reaction were both positive. Molecular formula was C47H74O18; HR-MS:m/z925.4758 [M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.82,δ0.88,δ0.91,δ0.95,δ1.06,δ1.26 were six methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported (Tables 1-2).Based on previous literature[19], Compound VI is Araloside A (Figure 1).
Compound VII was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C42H66O14; HR-MS:m/z793.4395 [MH]¯;1H-NMR (CD3OD, 600 MHz)δ0.78,δ0.83,δ0.89,δ0.92,δ0.94,δ1.03,δ1.14 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported (Tables 1-2). Based on previous literature[20], Compound VII is Chikuselsusaponin IVa (Figure 1).
Compound VIII was white amorphous powder.Liebermann-Burchard reaction and Molish reaction were both positive. Molecular formula was C41H64O13; HR-MS:m/z763.4300 [M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.92,δ1.08,δ1.09,δ1.10,δ1.13,δ1.42,δ1.44 were seven methylprotons signatures.13C-NMR (CD3OD, 150 HMz) data are reported(Tables 1-2). Based on previous literature[20], Compound VIII is Narcissiflorine (Figure 1).
Compound IX was white amorphous powder. Liebermann-Burchard reaction and Molish reaction were both positive.Molecular formula was C36H56O9; HR-MS:m/z631.3877[M-H]¯;1H-NMR (CD3OD, 600 MHz)δ0.89,δ1.05,δ1.07,δ1.10,δ1.41 were five methylprotons signatures.13C-NMR(CD3OD, 150 HMz) data are reported (Tables 1-2). Based on previous literature[20], Compound IX is Ealenduloside E (Figure 1).
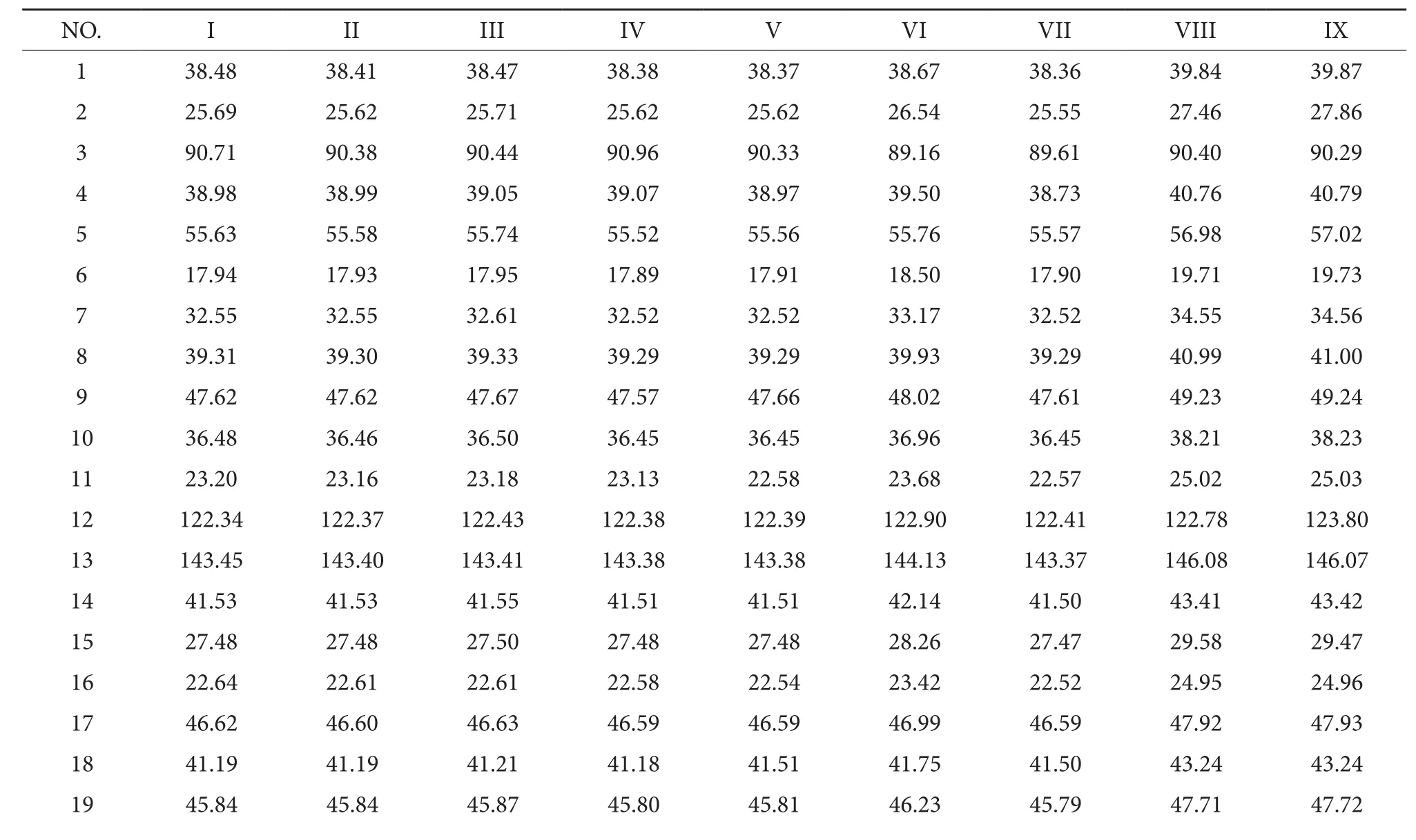
Table 1 The δc 13C-NMR data of Compound I-Compound IX

20 30.15 30.14 30.15 30.10 30.10 30.80 30.09 32.23 32.24 21 33.51 33.50 33.52 33.47 33.47 34.04 33.47 35.49 35.50 22 32.18 32.14 32.14 32.06 32.52 33.17 32.52 34.47 34.48 23 27.13 27.10 26.85 26.85 27.48 28.18 27.47 29.43 29.47 24 15.61 15.52 15.19 15.41 15.47 16.94 15.52 18.21 18.24 25 14.71 14.63 14.67 14.56 14.57 15.54 14.57 16.69 16.71 26 16.38 16.35 16.36 16.31 16.31 17.46 16.30 18.64 18.65 27 25.04 25.00 25.04 24.89 24.88 26.13 24.85 27.82 27.86 28 176.62 176.61 176.64 176.60 176.60 176.36 176.61 181.42 181.42 29 31.73 31.72 31.74 31.71 32.07 33.17 32.05 34.43 34.45 30 22.64 22.61 22.61 22.58 23.13 23.78 23.12 24.95 24.96

Table 2 13C-NMR data of Compound I-Compound IX

6 61.71 3-Gal 1 103.22 103.17 2 70.06 71.09 3 71.59 74.71 4 69.03 76.95 5 75.81 76.79 6 61.14 61.11 4-Wyl 1 103.13 2 75.81 3 76.89 4 70.51 5 65.50 4-Gal 1 101.84 2 71.09 3 75.81 4 69.02 5 76.88 6 62.18images/BZ_9_581_1645_1899_2235.pngR1 R2 R3 R4images/BZ_9_638_2630_987_2891.pngimages/BZ_9_1632_2616_1651_2637.pngimages/BZ_9_2007_2617_2027_2637.pngimages/BZ_9_1943_2617_2244_2670.pngCompound I H images/BZ_9_1464_2633_1717_2808.pngimages/BZ_9_1503_2754_1819_2894.pngimages/BZ_9_1843_2627_2191_2888.pngimages/BZ_9_1081_2949_1400_3224.pngimages/BZ_9_1526_2972_1691_3194.pngimages/BZ_9_1944_2953_2242_3006.pngCompound II H images/BZ_9_1944_3086_2091_3130.pngimages/BZ_9_1740_3138_1757_3156.png
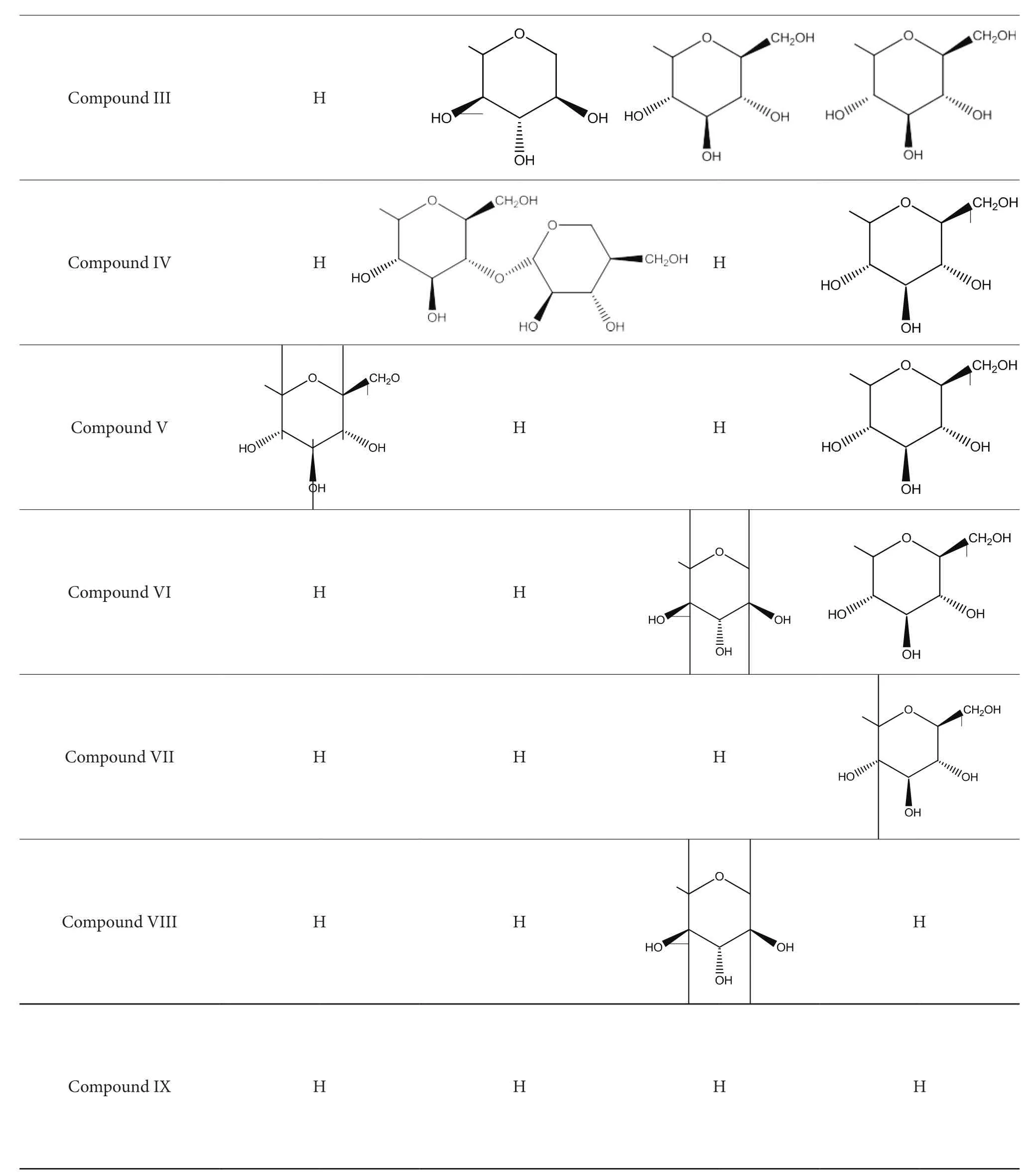
Figure 1 Compound I-Compound IX
Cell Culture and Viability Assay
Primary bone marrow-derived macrophages (BMMs)were isolated from SD rats and cultured in alpha-minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS) and 30 ng/mL recombinant human M-CSF(Peprotech, U.S.A.). BMMs were cultured in a 96-well at a density of 1×104cells per well in 200 μL α-MEM containing 10% FBS and 30 ng/mL M-CSF for 2 days,removed 100 μL medium from the culture and replaced with 100 μL of freshly medium.
After 24 hours, five compounds (Tarasaponin IV, Stipuleanoside R2, Araloside C, Araloside A, Chikuselsusaponin IVa) were added to BMMs incubated for 24 hours at concentration(20, 40, 60, 80 μg/mL), respectively. Nine compounds(Tarasaponin IV, Stipuleanoside R2, Araloside C,Chikuselsusaponin V, Decaisneanaside, Araloside A,Chikuselsusaponin IVa, Narcissiflorine, Ealenduloside E) were added to BMMs incubated for 24 hours at concentration (0.5, 5 μg/mL), respectively. Cell Counting Kit-8 (Dojindo, Japan) assay was used to test cell growth.
Results and Discussion
In this study, these nine compounds were tested for the viability by M-CSF which induced BMMs. As shown in the following tables and figures (Tables 3 and Figure 2), we first evaluated the viability effects of these compounds on BMMs to investigate their cytotoxicity.Compared with the control group, the test exhibited inhibitory activities against BMMs, which suggested that the compounds may have a positive effect on the inhibition of osteoclasts. Accordingly, five compounds(Tarasaponin IV, Stipuleanoside R2, Araloside C, Araloside A, Chikuselsusaponin IVa) significantly inhibited BMMs with an IC50 value of 48.185±1.683 μg/mL, 70.830±1.850 μg/mL, 133.722±2.126 μg/mL, 73.171±1.864 μg/mL and 0.547±0.262 μg/mL, respectively.
In addition, seven compounds (Tarasaponin IV,Stipuleanoside R2, Araloside C, Chikuselsusaponin V,Decaisneanaside, Araloside A, Chikuselsusaponin IVa)in the concentrations of 0.5 and 5 μg/mL exhibited weak cytotoxicity (Table 4 and Figure 3). Two compounds(Narcissiflorine and Ealenduloside E) exhibited promotive effects on the BMMs viability at 0.5 μg/mL concentration and almost eliminated the BMMs at 5 μg/mL concentration in this study.
Mature osteoclasts, the only cell type primarily involved in bone resorption, are multinucleated giant cells formed by proliferation, differentiation, and fusion of mononucleate hematopoetic precursor cells. Finally, these findings reveal that the compounds might have inhibition effectc on the viability of BMMS induced by M-CSF, and directly impact the fusion of osteoclast progenitor cells, thus interfering cellular growth.

Table 3 Effect of five compounds (Tarasaponin IV, Stipuleanoside R2, Araloside C, Araloside A, Chikuselsusaponin IVa) from Aralia echinocaulis on the BMMs

Figure 2 Effect of five compounds (Tarasaponin IV, Stipuleanoside R2, Araloside C, Araloside A, Chikuselsusaponin IVa) from Aralia echinocaulis on the viability of BMMs

Table 4 Effect of nine compounds (Tarasaponin IV, Stipuleanoside R2, Araloside C, Chikuselsusaponin V, Decaisneanaside,Araloside A, Chikuselsusaponin IVa, Narcissiflorine, Ealenduloside E) from Aralia echinocaulis on the BMMs
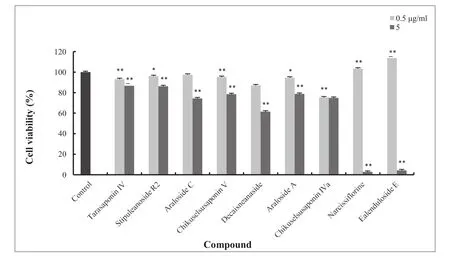
Figure 3 Effect of nine compounds (Tarasaponin IV, Stipuleanoside R2, Araloside C, Chikuselsusaponin V, Decaisneanaside ,Araloside A, Chikuselsusaponin IVa, Narcissiflorine, Ealenduloside E) from Aralia echinocaulis on the viability of BMMs
杂志排行
Global Traditional Chinese Medicine的其它文章
- Rheumatoid arthritis and JAK2/STAT3 signaling pathway: How does the TCM works?
- Correlation between scapulohumeral periarthritis and cervical spondylotic radiculopathy
- Bushen Huoxue methods combined with amiodarone in the treatment of coronary atherosclerotic heart disease with severe frequent premature ventricular contractions: a case report
- Research status, countermeasures and recommendations of emergency management system for TCM prevention and control of major epidemics#
- Mechanism of prevention and treatment of osteoporosis with traditional Chinese medicine
