大豆不同生育期叶际光合细菌群落结构特征
2020-11-02孔小婷苏品程菊娥杜晓华罗路云陈丽洁翟忠英张德咏刘勇
孔小婷 苏品 程菊娥 杜晓华 罗路云 陈丽洁 翟忠英 张德咏 刘勇
摘要:【目的】探究大豆不同生育期叶际光合细菌群落结构多样性及其分布特征,揭示大豆生育期与光合细菌群落结构变化间的关系,为促进光合细菌在农业生产中的应用提供参考依据。【方法】对5个生育期(苗期、出枝期、花期、鼓粒期和成熟期)大豆叶际光合细菌的Puf M基因序列进行PCR扩增,并对PCR扩增产物进行Illumina高通量測序,分析大豆叶际光合细菌群落多样性及分布特征。【结果】从大豆5个生育期叶片样品中共检测到光合细菌2门、5纲、36属、84种。不同生育期大豆叶片样品中光合细菌的群落组成和结构存在一定差异,在相对丰度方面表现为成熟期>苗期> 出枝期>花期>鼓粒期;在多样性方面表现为苗期>出枝期>花期>鼓粒期>成熟期。大豆不同生育期的叶际光合细菌在门、纲、属和种分类水平上的优势菌群及所占比例分别为变形菌门(Proteobacteria)(92.11%~99.29%)、α-变形菌纲(Alphaproteobacteria)(75.17%~97.40%)、甲基杆菌属(Methylobacterium)(60.67%~95.09%)和扭脱甲基杆菌(Methylobacterium extorquens)(36.89%~87.88%),随着分类水平逐渐细化,不同生育期对大豆叶际光合细菌群落组成和分布的影响越大。【结论】生育期对大豆叶际光合细菌群落结构有重要影响,实际生产中可通过在大豆不同生育期施用不同的光合细菌以促进优势群落形成,从而促进大豆生长。
关键词: 大豆;生育期;叶际;光合细菌;高通量测序;多样性
中图分类号: S565.1 文献标志码: A 文章编号:2095-1191(2020)08-1977-08
Community structure characteristics of soybean phyllosphere photosynthetic bacteria in different growth stages
KONG Xiao-ting1,2, SU Pin1,2, CHENG Ju-e2, DU Xiao-hua2, LUO Lu-yun2,
CHEN Li-jie1,2, ZHAI Zhong-ying1,2, ZHANG De-yong1,2, LIU Yong1,2*
(1Longping Branch, Graduate School of Hunan University, Changsha 410125, China; 2Institute of Plant Protection,
Hunan Academy of Agriculture Sciences, Changsha 410125, China)
Abstract:【Objective】To explore the phyllosphere photosynthetic bacterial communities structure diversity and distribution of soybean at different growth stages, reveal the relationship between the growth period of soybean and the chan-ges of photosynthetic bacteria community structure, provide a scientific reference for promoting the application of photosynthetic bacteria in agricultural production. 【Method】The Puf M gene region of phyllosphere photosynthetic bacteria in five growth stages of soybean(seedling stages, branching stages, flowering stages, bulging stages and maturity stages)was amplified by PCR, and then the products of PCR amplification were sequenced with Illumina high throughput to analyze the diversity and distribution of phyllosphere photosynthetic bacteria. 【Result】A total of 84 species, 36 genera, 5 classes and 2 phyla of photosynthetic bacteria were detected in the leaves samples from five growth stages . There were some differences in the structure and composition of photosynthetic bacterial community in soybean leaves samples of different growth stages. In terms of relative abundance, maturity stages>seedling stages>branching stages>flowering stages>bulging stages; while in terms of diversity, seedling stages>branching stages>flowering stages>bulging stages> maturity stages. The dominant phyllosphere photosynthetic bacterial community at phyla, class, genus and species levels of five growth stages soybean and their proportions were Proteobacteria(92.11%-99.29%), Alphaproteobacteria(75.17%-97.40%), Methylobacterium(60.67%-95.09%), Methylobacterium extorquens(36.89%-87.88%). However,with the refinement of classification, the influence of growth stages on the composition and distribution of phyllosphere photosynthetic bacteria community became great. 【Conclusion]Soybean growth periods have an important influence on the structure of phyllosphere photosynthetic bacteria community. In agricultural production, different photosynthetic bacteria could be applied in different growth stages of soybean to promote the formation of dominant community and thus promote the growth of soybean.
1. 3. 2 叶际微生物DNA提取、扩增和测序 使用 FastDNA Spin Kit for Soil试剂盒对大豆叶际微生物DNA进行提取,以样品DNA为模板,选用正向引物Puf M F(5'-TACGGSAACCTGTWCTAC-3')和反向引物Puf M WAW(5'-AYNGCRAACCACCANGCCC A-3')(Béjà et al.,2002;Yutin et al.,2005)对样品进行扩增。PCR反应体系50 μL:正、反向引物各2 μL,Mix 25 μL,DNA模板2 μL,无菌水补足至50 μL。扩增程序:94 ℃预变性5 min;94 ℃ 30 s,60 ℃ 30 s,72 ℃ 30 s,进行35个循环;72 ℃延伸10 min,4 ℃保存。PCR扩增产物采用1.5%琼脂糖凝胶进行电泳检测,将目的条带切胶回收后送至北京诺禾致源有限公司进行Illumina高通量测序。
1. 4 数据分析
从下机数据中拆分出各样本数据,截去特异性接头序列和引物序列后使用Flash(V1.2.7)对所有序列进行拼接(Mago? and Salzberg,2011),拼接序列经严格过滤处理(Bokulich et al.,2013)得到高质量的标签序列数据。利用Uparse v7.0.1001(Haas et al.,2011)以97%的相似度将所有标签序列聚类成为分类操作单元(OTU)。以 Puf M功能基因数据库作为注释文库,用Mothur对OTU进行物种注释。最后以样本中数据量最少的OTU数为标准进行均一化处理,得到标准化OTU表,进行后续的α多样性和β多样性分析。
使用QIIME 1.9.1计算物种丰富度指数(Chao1)和多样性指数(Shannon),通过Shannon指数和Chao1指数评估序列文库的α多样性。并采用主坐标分析(PCoA)及多重响应置换程序(MRPP)、相似性分析(Anosim)和非参数检验方法(Adonis)对任意两组间微生物群落的差异进行评估。
1. 5 统计分析
采用SPSS 20.0对所测数据进行单因素方差分析,并应用Duncans新复极差法在P=0.05水平上对大豆不同生育期叶际光合细菌群落组成进行差异显著性分析。
2 结果与分析
2. 1 不同生育期大豆叶际光合细菌群落高通量测序结果
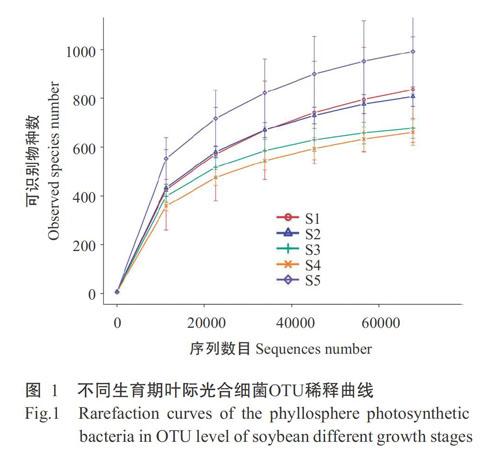
以97%的相似度对OTU进行聚类,共得到3539个OTUs。全部高通量测序结果提交至NCBI SRA (登录号PRJNA593671)。如图1所示,各样品的稀释曲线均趋向平坦,满足测序深度要求。物种注释结果显示本研究共鉴定得到光合细菌2门、5纲、36属、84种。
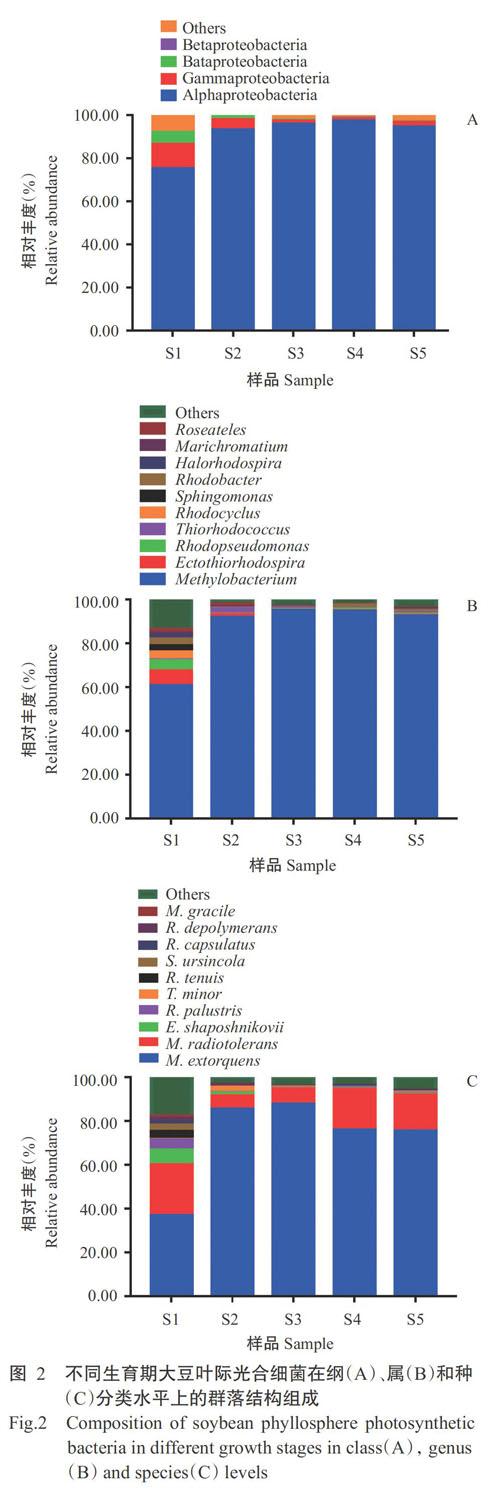
对不同生育期大豆叶际光合细菌样品在门分类水平上进行序列鉴定分析,发现变形菌门(Proteobacteria)在各样品的叶际光合细菌中占主导地位,其在各样品中的相对丰度分别为92.11%(S1)、99.29%(S2)、97.77%(S3)、98.76%(S4)和97.17%(S5)。在纲分类水平上,α-变形菌纲(Alphaproteobacteria)、γ-变形菌纲(Gammaproteobacteria)、Bata变形菌纲(Bataproteobacteria)和β-变形菌纲(Betaproteobacteria)的相对丰度分别为75.17%~97.40%、1.17%~11.46%、0.17%~5.47%和0~0.03%(图2-A)。
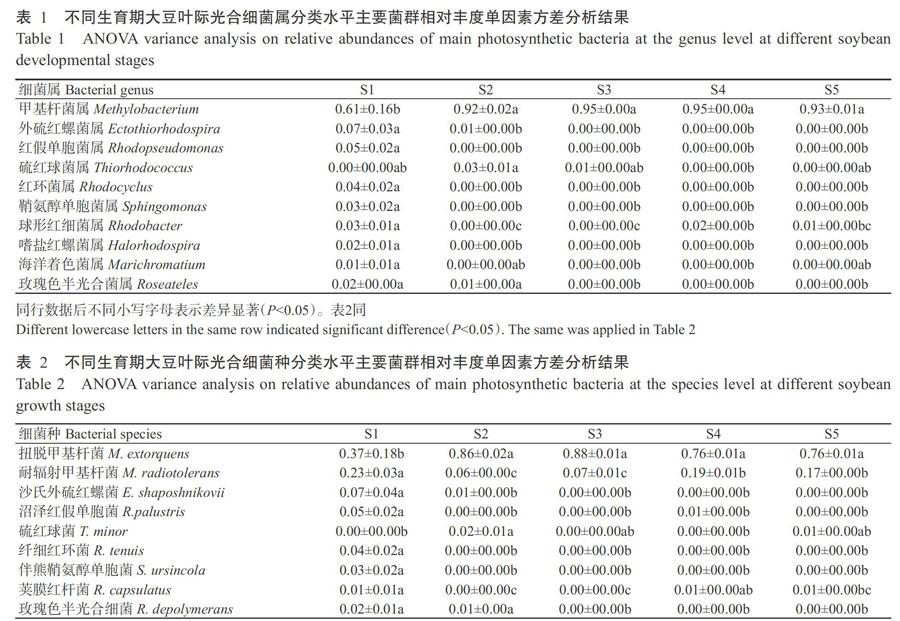
在属分类水平上,S1的优势属为甲基杆菌属(Methylobacterium)(60.67%)、外硫红螺菌属(Ectothiorhodospira)(6.84%)和红假单胞菌属(Rhodopseudomonas)(4.59%);S2的优势属为甲基杆菌属(91.99%)、硫红球菌属(Thiorhodococcus)(2.54%)、外硫红螺菌属(1.22%)和Roseateles(1.29%);S3的优势属为甲基杆菌属(95.09%);S4的优势属为甲基杆菌属(94.82%)和红杆菌属(Rhodobacter)(1.70%);S5的优势属为甲基杆菌属(92.53%)和红杆菌属(1.03%)(图2-B)。综合大豆叶际光合细菌属水平主要菌群相对丰度单因素方差分析结果(表1)可知,甲基杆菌属、外硫红螺菌属和红假单胞菌属在大豆苗期与其他各生长时期均存在显著差异(P<0.05,下同)。甲基杆菌属在大豆生育期呈先升高后降低的变化趋势,外硫红螺菌属和红假单胞菌属则呈逐渐降低并趋于稳定的变化趋势。
在种分类水平上,大豆各生育期的主要优势种为扭脱甲基杆菌(Methylobacterium extorquens)(36.89%~87.88%)和耐辐射甲基杆菌(Methylobacterium radiotolerans)(6.05%~23.12%)(图2-C)。由表2可知,扭脱甲基杆菌在大豆苗期的相对丰度占全部葉际光合细菌的0.37,相对于其他生长发育期均具有显著差异,相对丰度从苗期到成熟期呈先升高后降低的变化趋势;耐辐射甲基杆菌在大豆苗期的相对丰度最高,与其他生育期差异显著,其相对丰度随着大豆的生长发育呈先降低后升高的变化趋势;沙氏外硫红螺菌(E. shaposhnikovii)在大豆苗期相对丰度最高,随后呈现显著降低并趋向平稳的变化趋势。由图3可知,随着大豆的生长发育,S1、S2、S3、S4和S5独有的OTU分别占OTU总量的20.56%、8.84%、5.37%、7.63%和15.42%,所有样品中共检测到433个重合的OTUs,占所有样品OTU总量的12.23%。
根据上述各分类水平的结果,本研究发现随着分类水平逐渐细化,大豆不同生育期对叶际光合细菌群落组成和分布的影响越大。
2. 2 α多样性分析结果
Shannon指数和Chao1指数被用来评估大豆叶际光合细菌群落α多样性。大豆由苗期到成熟期,叶际光合细菌Shannon指数的变化范围为4.27~5.57,在大豆苗期最高、成熟期最低,整体上呈逐渐降低的变化趋势(图4-A)。在大豆生长发育期中,叶际光合细菌群落Chao1指数变化范围为731.61~1115.6,在成熟期最高、花期最低,叶际光合细菌的物种丰富度从苗期到花期呈先逐渐下降再上升的变化趋势(图4-B)。结合图1可知,大豆成熟期叶际光合细菌的物种丰富度高于其他生育期,表明叶际光合细菌群落多样性受大豆生育期的影响。
2. 3 β多样性分析结果
由图5可知,同一生育期的样品均能较紧密地结合在一起,其相似性较高,不同生育期菌群群落可明显地区分开。PCoA分析结果表明,PC1对大豆叶际光合细菌群落结构的差异贡献度为71.73%,PC2对大豆叶际光合细菌群落结构的差异贡献度为13.22%。大豆苗期叶际光合细菌群落所处象限为单独象限,即大豆苗期的叶际微生物群落结构与其他各生长发育阶段存在显著差异;出枝期与花期间距离较近、鼓粒期与成熟期间距离较近,表明彼此间光合细菌群落结构差异相对较小;大豆出枝期与成熟期间的距离相对较远,表明大豆出枝期与成熟期间光合细菌群落结构的差异相对较大。多重响应置换程序、相似性分析和非参数检验方法进一步验证不同生育期具有显著差异(表3),大豆不同生育期能显著影响叶际光合细菌的群落组成和结构。
3 讨论
叶际微生物作为一种生态学指标在生态稳定与环境安全评价中发挥着重要作用(Morris and Monier,2003)。目前,关于叶际微生物的研究主要集中在细菌和真菌多样性等方面(Zhang et al.,2018;Vokou et al.,2019),而对叶际光合细菌多样性的研究较少。光合细菌在农业生产中主要应用于促进农作物生长和品质改善(Koh and Song,2007)。已有研究表明,紫色光合细菌可通过叶面喷施和根际灌溉来促进作物生长(Wu et al.,2013),但光合细菌在植物叶际的存在及作用研究仍较缺乏。本研究利用高通量测序技术对5个不同生育期大豆叶际光合细菌群落组成和结构进行研究,掌握了大豆不同生育期叶际光合细菌的群落结构演替变化规律,对评价生育期对叶际光合细菌多样性的影响具有重要意义。
本研究通过分析5个不同生育期大豆叶际光合细菌群落多样性变化规律,发现在相对丰度方面表现为成熟期>苗期>出枝期>花期>鼓粒期,在多样性方面表现为苗期>出枝期>花期>鼓粒期>成熟期。随着大豆的生长发育,Shannon指数在大豆苗期最高、成熟期最低,整体上呈逐渐降低的变化趋势,由此可知叶际光合细菌群落多样性随着大豆的生长发育呈逐渐降低趋势。在β多样性分析中,主坐标分析PCoA和相异性分析结果表明大豆各生育期间的光合细菌群落结构存在显著差异。植物叶际微生物群落组成结构受生长季节的显著影響,植物生长早期叶际微生物受土壤的影响较大,随着植物的生长,植物叶际逐渐形成其独特的微生物群落结构(Copeland et al.,2015)。故推测大豆苗期叶际光合细菌群落结构与其他生育期差异显著的原因是大豆叶际光合细菌群落组成在苗期时受土壤微生物的影响较大,随着大豆的生长发育,叶际光合细菌群落结构逐渐趋向稳定。
物种分析结果表明,变形菌门是大豆不同生育期叶际光合细菌群落中最丰富的细菌类群,与Bulgarelli等(2013)、Dong等(2019)的研究结果一致。本研究中,变形菌门主要包括α-变形菌纲(75.17%~97.4%)和γ-变形菌纲(1.17%~11.46%),且其相对丰度表现为α-变形菌纲>γ-变形菌纲,与Laforest-Lapointe等(2016)研究加拿大温带森林细菌群落丰度分布的结果一致,表明叶际细菌群落组成中α-变形菌纲占据着叶际细菌群落的绝对优势。本研究结果表明,甲基杆菌属是大豆不同生育期叶际光合细菌的主要优势属,甲基杆菌属作为大豆叶际细菌的优势属在其他植物叶际细菌群落的研究中也有报道。Delmotte等(2009)研究表明甲基杆菌属在大豆、三叶草和拟南芥叶际微生物群落中具有较高的丰度;Wellner等(2011)研究发现甲基杆菌是三叶草和耳菜草叶面微生物群落的优势菌。甲基杆菌是最早报道在植物叶片上能进行光合作用的细菌,对植物叶片表面具有重要作用,其能促进植物种子萌发和植物生长(Lidstrom and Chistoserdova,2002;Madhaiyan et al.,2007)。
4 结论
不同生育期大豆叶片中光合细菌的群落组成和结构存在一定差异,变形菌门在大豆不同生育期中均为最优势的光合细菌类群,大豆成熟期叶际光合细菌种类最多,苗期叶际光合细菌分布最均匀,表明大豆生育期对叶际光合细菌群落结构有重要影响。因此,实际生产中可通过在大豆不同生育期施用不同的光合细菌以促进优势群落形成,从而促进大豆生长。
参考文献:
穆金艳,赵兰枝,王振宇. 2017. 不同浓度光合细菌对水培油麦菜产量及品质的影响[J]. 北方园艺,(15):56-60. [Mu J Y,Zhao L Z,Wang Z Y. 2017. Effects of different concentrations of photosynthetic bacteria on yield and quality of Lactuca sativa L.[J]. Northern Horticulture,(15):56-60.]
吴太虎,毛佳文,陈锋,顾彪,李抄. 2015. 痕量微生物快速检测系统[J]. 光学精密工程,23(11): 3061-3068. [Wu T H,Mao J W,Chen F,Gu B,Li C. 2015. Rapid trace microbia detection system[J]. Optics and Precision Engineering,23(11): 3061-3068.]
徐華东,狄亚楠,曹延珺,王立海,王海滨. 2019. 可培养土壤微生物数量与红松活立木干基腐朽程度的关系[J]. 东北林业大学学报,47(1): 52-55. [Xu H D,Di Y N,Cao Y J,Wang L H,Wang H B. 2019. Relationship between cultural soil microbial quantity and butt decay degree of Pinus koraiensis standing trees[J]. Journal of Northeast Forestry University,47(1): 52-55.]
杨芳,田俊岭,杨盼盼,冯宏,贺广生,陈旭东,卢钰升,谭志远,彭桂香. 2014. 高效光合细菌菌剂对番茄品质、土壤肥力及微生物特性的影响[J]. 华南农业大学学报,35(1): 49-54. [Yang F,Tian J L,Yang P P,Feng H,He G S,Chen X D,Lu Y S,Tan Z Y,Peng G X. 2014. Effects of inoculant of photosynthetic bacteria on tomato quality,soil fertility and soil microbial characteristics[J]. Journal of South China Agricultural University,35(1): 49-54.]
曾益波,刘骏,赵国盛,吴希阳,陈岳,苏品,张德咏,刘勇. 2018. 光合细菌PSB06浸种对水稻促生作用研究[J]. 杂交水稻,33(3): 50-53. [Zeng Y B,Liu J,Zhao G S,Wu X Y,Chen Y,Su P,Zhang D Y,Liu Y. 2018. Promoting effects of soaking seed with photosynthetic bacterium PSB06 on rice growth[J]. Hybrid Rice,33(3): 50-53.]
Atamna-Ismaeel N,Finkel O,Glaser F,von Mering C,Vorholt J A,Koblí?ek M,Belkin S,Béjà O. 2012. Bacterial anoxygenic photosynthesis on plant leaf surfaces[J]. Environmental Microbiology Reports,4(2): 209-216.
Béjà O,Suzuki M T,Heidelberg J F,Nelson W C,Preston C M,Hamada T,Eisen J A,Fraser C M,DeLong E F. 2002. Unsuspected diversity among marine aerobic anoxy-genic phototrophs[J]. Nature,415(6872): 630-633.
Bokulich N A,Subramanian S,Faith J J,Gevers D,Gordon J I,Knight R,Mills D A,Caporaso J G. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing[J]. Nature Methods,10(1): 57-59.
Bulgarelli D,Schlaeppi K,Spaepen S,Ver Loren van Themaat E,Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants[J]. Annual Review of Plant Biology,64(1): 807-838.
Compant S,Duffy B,Nowak J,Clément C,Barka E A. 2005. Use of plant growth-promoting bacteria for biocontrol of plant diseases:Principles,mechanisms of action,and future prospects[J]. Applied and Environmental Microbio-logy,71(9): 4951-4959.
Copeland J K,Yuan L,Layeghifard M,Wang P W,Guttman D S. 2015. Seasonal community succession of the phyllosphere microbiome[J]. Molecular Plant-Microbe Interactions,28(3):274-285.
Csotonyi J T,Swiderski J,Stackebrandt E,Yurkov V. 2010. A new environment for aerobic anoxygenic phototrophic bacteria: Biological soil crusts[J]. Environmental Microbiology Report,2(5): 651-656.
Danhorn T,Fuqua C. 2007. Biofilm formation by plant-associa-ted bacteria[J]. Annual Review of Microbiology,61(1): 401-422.
Delmotte N,Knief C,Chaffron S,Innerebner G,Roschitzki B,Schlapbach R,von Mering C,Vorholt J A. 2009. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America,106(38): 16428-16433.
Dong C J,Wang L L,Li Q,Shang Q M. 2019. Bacterial communities in the rhizosphere,phyllosphere and endosphere of tomato plants[J]. PLoS One,14(11): e0223847.
Du H L,Jiao N Z,Hu Y H,Zeng Y H. 2006. Real-time PCR for quantification of aerobic anoxygenic phototrophic bacteria based on pufM gene in marine environment[J]. Journal of Experimental Marine Biology,329(1): 113-121.
Haas B J,Gevers D,Earl A M,Feldgarden M,Ward D V,Gia-nnoukos G,Ciulla D,Tabbaa D,Highlander S K,Sodergren E,Methé B,DeSantis T Z,The Human Microbiome Consortium,Petrosino J F,Knight R,Birren B W. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons[J]. Genome Research,21(3): 494-504.
Haas J C,Streeta N R,Sj?din A,Lee N M,H?gberge M N,N?sholm T,Hurry V. 2018. Microbial community response to growing season and plant nutrient optimisation in a boreal Norway spruce forest[J]. Soil Biology and Biochemistry,125:197-209.
Hirano S S,Upper C D. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae-a pathogen,ice nucleus,and epiphyte[J]. Microbiology and Molecular Bio-logy Reviews,64(3): 624-653.
Hirose S,Nagashima K V P,Matsuura K,Haruta S. 2012. Diversity of purple phototrophic bacteria,inferred from puf M gene,within epilithic biofilm in Tama River,Japan[J]. Microbes and Environments,27(3): 327-329.
Jackson C R,Denney W C. 2011. Annual and seasonal variation in the phyllosphere bacterial community associated with leaves of the southern magnolia(Magnolia grandiflora)[J]. Microbial Ecology,61(1):113-122.
Koh R H,Song H G. 2007. Effects of application of Rhodopseudomonas sp. on seed germination and growth of tomato under axenic conditions[J]. Journal of Microbiology and Biotechnology,17(11): 1805-1810.
Laforest-Lapointe I,Messier C,Kembel S W. 2016. Host species identity,site and time drive temperate tree phyllosphere bacterial community structure[J]. Microbiome,4(1): 27.
Lidstrom M E,Chistoserdova L. 2002. Plants in the pink: Cytokinin production by methylobacterium[J]. Journal of Bacteriology,184(7): 1818.
Lindow S E,Brandl M T. 2003. Microbiology of the phyllosphere[J]. Applied and Environmental Microbiology,69(4): 1875-1883.
Madhaiyan M,Poonguzhali S,Sa T. 2007. Characterization of 1-aminocyclopropane-1-carboxylate(ACC) deaminase con-taining Methylobacterium oryzaeand interactions with auxins and ACC regulation of ethylene in canola(Brassica campestris)[J]. Planta,226(4): 867-876.
Mago? T,Salzberg S L. 2011. FLASH: Fast length adjustment of short reads to improve genome assemblies[J]. Bioinformatics,27(21): 2957-2963.
Manching H C,Carlson K,Kosowsky S,Smitherman C T,Stapleton A E. 2018. Maize phyllosphere microbial community niche development across stages of host leaf growth[J]. F1000 Research,6:1698.
Morris C E,Monier J M. 2003. The ecological significance of biofilm formation by plant-associated bacteria[J]. Annual Review of Phytopathology,41: 429-453.
Sapkota R,Knorr K,Jorgensen L N,O'Hanlon K A,Nicolaisen M. 2015. Host genotype is an important determinant of the cereal phyllosphere mycobiome[J]. The New Phytologist,207(4): 1134-1144.
Su P,Tan X Q,Li C G,Zhang D Y,Cheng J,Zhang S B,Zhou X G,Yan Q P,Peng J,Zhang Z,Liu Y,Lu X Y. 2017. Photosynthetic bacterium Rhodopseudomonas palus-tris GJ-22 induces systemic resistance against viruses[J]. Microbial Biotechnology,10(3):612-624.
Vokou D,Genitsaris S,Karamanoli K,Vareli K,Zachari M,Voggoli D,Monokrousos N,Halley J M,Sainis I. 2019. Metagenomic characterization reveals pronounced seasona-lity in the diversity and structure of the phyllosphere bacterial community in a mediterranean ecosystem[J]. Microorganisms,7(11): 518.
Wellner S,Lodders N,K?mpfer P. 2011. Diversity and biogeo-graphy of selected phyllosphere bacteria with special emphasis on Methylobacterium spp.[J]. Systematic and Applied Microbiology,34(8): 621-630.
Whipps J M,Hand P,Pink D,Bending G D. 2008. Phyllosphere microbiology with special reference to diversity and plant genotype[J]. Journal of Applied Microbiology,105(6): 1744-1755.
Wu J,Wang Y M,Lin X G. 2013. Purple phototrophic bacterium enhances stevioside yield by stevia rebaudiana bertoni via foliar spray and rhizosphere irrigation[J]. PLoS One,8(6): e67644.
Yutin N,Suzuki M T,Beja O. 2005. Novel primers reveal wider diversity among marine aerobic anoxygenic phototrophs[J]. Appl Environ Microbiol,71(12): 8958-8962.
Zhang Z,Luo L Y,Tan X Q,Kong X,Yang J G,Wang D H,Zhang D Y,Jin D C,Liu Y. 2018. Pumpkin. powdery mildew disease severity influences the fungal diversity of the phyllosphere[J]. PeerJ,6: e4559.
(責任编辑 麻小燕)
收稿日期:2019-12-11
基金项目:国家自然科学基金青年科学基金项目(31701764)
作者简介:*为通讯作者,刘勇(1966-),研究员,主要从事田间蔬菜病虫害防治研究工作,E-mail:haoasliu@163.com。孔小婷(1993-),主要从事植物保护研究工作,E-mail:569079899kxt@hnu.edu.cn
