人参根际真菌群落多样性及组成的变化
2017-03-20董林林牛玮浩王瑞徐江张连娟张军
董林林+牛玮浩+王瑞+徐江+张连娟+张军+陈士林


[摘要]连作障碍导致人参减产减收,影响人参产业的可持续发展。土壤真菌群落参与关键生态过程,其多样性及组成的变化与连作障碍相关。该研究采用高通量测序技术,分析人参根际土壤真菌群落多样性及组成的变化,阐述人参栽培模式对根际微生态的影响,为克服连作障碍提供策略。与森林土壤相比,人参根际土壤微生物多样性增加,且随着种植年限的增加,多样性增加趋势下降;真菌群落Sordariomycetes,Alatospora,Eurotiomycetes,Leotiomycetes,Saccharomycetes,Mucorales,Pezizomycetes的丰度增加。皮尔森相关分析表明,土壤理化指标影响人参根际真菌群落的丰度,pH与Dothideomycetes和Alatospora的丰度显著相关,有效钾含量与Dothideomycetes, Alatospora,Mucorales的丰度显著相关,土壤总氮含量与Sordariomycetes,Mucorales的丰度显著相关。结果表明,施肥是影响人参根际微生态的关键因素之一,优化施肥体系是克服人参连作障碍的有效途径之一。
[关键词]人参; 连作障碍; 根际微生态; 真菌; 高通量测序
[Abstract]Continuous cropping obstacles resulted in the yield losses of Panax ginseng, and affected the development of ginseng industry. Soil fungal communities participated in the key ecological process, and their changes of diversity and composition were related to the continuous cropping obstacles. We analyzed the changes of fungal diversity and composition in the rhizosphere of ginseng using the high-throughput sequencing method, stated the effects of ginseng cultivation on the micro-ecology, and provided effective strategies for overcoming continuous cropping obstacles. Compared to those of the forest soils, the fungal diversity of ginseng rhizosphere soils was increased, and the increasing trends were declined with an increasing years of ginseng cultivation; the relative abundance of Sordariomycetes, Alatospora, Eurotiomycetes, Leotiomycetes, Saccharomycetes, Mucorales and Pezizomycetes were increased in the rhizosphere of ginseng. Pearson′s correlation index indicated that soil chemical perporties affected the relative abundance of fungal communities. pH was significantly related to the relative abundance of Dothideomycetes and Alatospora; the content of available potassium was markedly associated with the relative abundance of Dothideomycetes, Alatospora and Mucorales; the content of total nitrogen was significant correlation with the relative abundance of Sordariomycetes and Mucorales. These results indicated that fertilization was one of pivotal factors affecting the rhizosphere micro-ecology of ginseng, and optimization of fertilization system was an effective method to overcome continuous cropping obstacles.
[Key words]Panax ginseng; continuous cropping obstacles; rhizosphere micro-ecology; fungi; high-throughput sequencing
人参 Panax ginseng C. A. Mey为多年生五加科药用植物,享有“百草之王”的美誉,具有大补元气,固脱生津,安神等功效。人参是宿根植物,忌地性极强,栽过一茬人参的土壤要30年后才能再栽参,这已成为参业发展的限制因子[1]。用重茬地继续栽种人参一般在第2年以后存苗率降至30%以下,有大约70%土地上的人参须根脱落、烧须,根周皮烂红色、長满病疤,致使人参地上部分死亡,有的地块几乎全部绝苗[2]。克服连作障碍是保障人参产业可持续发展的基础之一。
自毒作用、土壤理化性状劣变、微生物失衡以及土传病害增加导致作物连作障碍[3-4],其中土壤微生物群落的失衡是导致连作障碍的主要因子[5]。研究表明,连作体系下土壤微生物多样性及组成发生变化,进而影响土壤的生产力[6]。土壤真菌群落参与关键的生态过程,包含大量土传病害的致病因子及拮抗因子[7-9]。连作体系下真菌群落多样性及组成发生变化,致病群落丰度增加,导致花生连作障碍[10]。因此,土壤真菌群落在根际微生态过程、土传病害方面,扮演重要角色,然而关于人参根际真菌群落变化的研究较少,加强根际真菌群落的研究利于阐述人参连作障碍机制。
本研究采用高通量测序的技术,分析人参根际土壤真菌群落多样性及组成的变化,阐述土壤理化性状与土壤真菌群落的相关性,为克服人参连作障碍提供有效的策略。
1 材料与方法
1.1 土壤样品采集 样品采集于中国中医科学院中药研究所靖宇县林下栽参试验基地(126.8°E,42.39°N)。土壤样品采集于移栽后种植年限分别为1,2,3年的人参根际土壤,见图 1。试验小区随机排放且面积为1.5 m×20 m,每个小区随机选取5株人参苗,收集其根际土壤[11],混匀合并为一个样品,并采集参园外的0~20 cm森林土作为对照。12个土壤样品,过2 mm筛,一部分用于理化性状的测定,一部分用于土壤微生物群落的鉴定。
1.2 土壤理化性状的分析
采用水浸提法测定土壤的pH[12];水合热法测定土壤有机质[13];凯氏定氮法测定土壤中氮含量[14];分别采用钼蓝法及火焰光度法测定土壤有效磷及有效钾的含量。
1.3 土壤微生物群落的分析
采用MOBIO PowerSoil Kit(MOBIO,美国)提取土壤总DNA,利用通用引物扩增真菌18S rRNA片段[15],标签序列见表1。序列扩增、纯化、均一化参照Rodrigues 等[16]描述。采用Iron Torrent测序平台获取土壤真菌宏基因组序列,利用QIIME软件进行序列分析[17]。数据预处理,采用Flash的软件融合双末端序列,通过各样品标签序列对数据进行区分并归类,去除非靶区域序列及嵌合体[18]。采用Sliva将序列进行物种分类,对每个样本和每个物种单元分类进行序列丰度计算构建样本和物种分类单元序列丰度矩阵[19]。根据序列相似度(97%)构建操作分类单元(OTU)。通过多样性分析,计算各种物种多样性指数,衡量样本物种多样性[19]。
1.4 数据分析 采用SPSS 11.0软件,在P<0.05水平上分析人参根际土壤与森林土的差异。
2 结果
2.1 土壤理化指标 与森林土相比,人参根际土壤理化性状不同,见表2。与森林土相比,人参根际土壤pH下降3.4%~10.2%;土壤总氮及有效钾含量显著增高112%~135%,63.4%~189%。与对照相比,人参根际土壤有机质及有效钾含量差异均不显著。
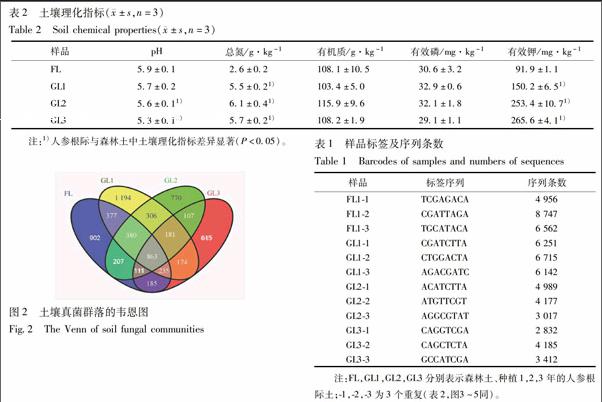
2.2 土壤真菌多样性 12个土壤样品中共获得61 985条可分类的真菌序列,每个样本中平均5 165条,数量范围为2 832~8 747条,见表1。森林土、一年生、二年生及三年生人参根际土壤中个检测到真菌OUT分别为:3 260,3 710,2 925,2 471;森林土与一年生,二年生及三年生人参根际土壤中检测到共有的真菌OUT为863;森林土分别与一年生,二年生及三年生人参根际土壤中检测到共有的真菌OUT为:1 783,1 561,1 394,見图2。
人参根际土壤中真菌群落多样性增加,见图3。对森林土相比,人参根际土壤真菌多样性指数分类多样性,香农指数,Chao 1和群落种类显著增加;随着人参种植年限增加,真菌多样性指数增加趋势而下降。
2.3 土壤真菌组成 人参根际土壤真菌群落发生变化见图4。与对照相比,在门水平人参根际土壤中Ascomycota和Glomeromycota的丰度分别增加了9.5%~22.2%和1.2%~2.4%;Basidiomycota,Blastocladiomycota 和Chytridiomycota丰度分别下降了6.9%~31.6%,27.4%~54.8%,0.7%~33.2%;一年生人参根际Neocallimastigomycota的丰度增加了15.1%,而二年生及三年生人参根际其丰度分别下降了22.1%,45.4%(图4a)。基于分类距离矩阵分析,人参根际土壤真菌结构发生变化,且与种植年限相关(图4b,c)。基于Unifrac距离矩阵分析,第一轴(总变异为20.76%)区分一、二年生人参根际真菌群落与森林土和三年生人参根际真菌群落;而第二轴(总变异为15.76%)区分三年生人参根际与森林土的真菌群落(图4b)。基于Bray-Curtis距离矩阵分析,第一轴(总变异为17.79%)区分了人参根际及森林土真菌群落,第二轴(总变异为15.16%)区分了三年生人参根际真菌群落与一年生、二年生人参根际及森林土真菌群落(图4c)。
人参连续种植导致科水平真菌群落丰度的变化,见图5。与对照相比,在科水平上,人参根际土壤真菌群落Sordariomycetes,Alatospora,Eurotiomycetes,Leotiomycetes,Saccharomycetes,Mucorales,Pezizomycetes的丰度增加,分别增加了10.4%~33.7%,2.0%~32.1%,15.8%~44.0%,39.1%~41.2%,40.4%~46.7%,18.3%~76.9%,9.0%~38.2%;真菌群落Agaricomycetes和Dothideomycetes丰度分别下降了4.8%~33.8%;一年生及二年生人参根际土壤真菌群落Tremellomycetes丰度增加了8.9%~16.8%;一年生及三年生人参根际真菌群落Microbotryomycetes的丰度增加了56.2%~93.6%,而二年生人参根际其丰度下降了52.5%。
2.4 土壤理化性狀影响真菌群落的丰度 皮尔森相关分析表明,真菌群落的丰度与土壤理化性状相关,见表3。Dothideomycetes的丰度与土壤pH(r=0.712,P<0.05)及有效钾含量(r=-0.746,P<0.05)显著相关;Sordariomycetes的丰度与土壤总氮含量显著正相关(r=0.719,P<0.05);Alatospora的丰度与土壤pH(r=-0.669,P<0.05)及有效钾含量(r=0.737,P<0.05)显著相关;Mucorales的丰度与土壤总氮(r=0.624,P<0.05)及有效钾含量(r=0.781,P<0.05)显著正相关。结果表明,土壤理化指标影响人参根际土壤真菌群落的丰度。
3 讨论
人参根际土壤真菌群落多样性下降,随着种植年限的增加,真菌多样性指数下降。人参连续种植导致根际土壤真菌群落组成发生变化,在门的水平,丰度最大群落Ascomycota的丰度增加,而真菌群落Basidiomycota,Blastocladiomycota和Chytridiomycota(丰度<1%)的丰度下降;在科的水平,真菌群落(丰度>0.5%)Sordariomycetes,Alatospora,Eurotiomycetes,Leotiomycetes,Saccharomycetes,Mucorales和Pezizomycetes的丰度增加。真菌群落丰度的变化与土壤理化指标相关,pH与Dothideomycetes和Alatospora的丰度显著相关,有效钾含量与Dothideomycetes,Alatospora,Mucorales的丰度显著相关,土壤总氮含量与Sordariomycetes,Mucorales的丰度显著相关,结果表明,土壤理化指标影响人参根际土壤真菌群落的丰度。
土壤微生物多样性及组成的变化打破了根际微生态系统功能,破坏土壤健康度,土传病害增加,进而影响作物的产量及品质[20-22]。连续种植模式改变了土壤微生物多样性及组成,影响了土壤的生产力[5,10]。研究表明,连作体系下,真菌多样性及组成发生变化,且病原菌增加,益生菌减少[10,21]。三七连续种植体系下,死苗率与真菌多样性显著相关,致病群落Fusarium oxysporum丰度增加[23]。因此,微生物多样性及组成的变化是土壤微生态失衡的因素之一。
土壤微生物群落多样性及组成的变化与作物类型、施肥等因素相关[24]。研究表明,氮肥影响了土壤微生物群落的组成,随着氮肥浓度的提高,富养性的分类群落Proteobacteria和Bacteroidetes丰度增加[25],长期平衡施肥降低AMF真菌多样性,改变AMF群落结构[26]。与施用有机肥相比,施用生物有机肥增加有益微生物(Trihoderma,Hypoxylon,Tritirachium
,Paenibacillus,Bacillus,Haliangium,Streptomyces)的丰度[27]。研究表明,土壤微生物群落的结构及丰度与土壤理化性状相关,Sordariomycete的丰度与有效磷含量相关[28]。土壤pH与真菌Helotiales(R2=0.53,P<0.01)、Hypocreales(R2=0.63,P<0.01)的丰度显著相关[29]。与森林土相比,人参根际土壤pH下降,总氮、有效钾含量显著增加,且土壤理化指标与真菌群落的丰度相关。结果表明,施肥是驱动根际微生物群落变化的因素之一,优化施肥体系是改善人参根际微生态的有效措施。此外,新参地资源的开发,如利用传统田地进行休耕种植人参,可实现传统作物与人参轮作,是人参种植的发展方向[30]。因此,强化连作障碍机制的研究结合新参地资源的开发是克服人参连作障碍的有效途径。
[参考文献]
[1]张连学,陈长宝,王英平,等. 人参忌连作研究及其解决途径[J]. 吉林农业大学学报,2008(4):481.
[2]吴连举,赵亚会,关一鸣,等. 人参连作障碍原因及其防治途径研究进展[J]. 特产研究,2008(2):68.
[3]Yu J Q, Shou S Y, Qian Y R, et al. Autotoxic potential of cucurbit crops[J]. Plant Soil, 2000, 223(12):149.
[4]Singh B K, Munro S, Reid E, et al. Investigating microbial community structure in soils by physiological, biochemical and molecular fingerprinting methods[J]. Eur J Soil Sci, 2006, 57(1):72.
[5]Nayyar A, Hamel C, Lafond G, et al. Soil microbial quality associated with yield reduction in continuous-pea[J]. Appl Soil Ecol, 2009, 43(1):115.
[6]Li C, Li X, Kong W, et al. Effect of monoculture soybean on soil microbial community in the Northeast China[J]. Plant Soil, 2009, 330(1):423.
[7]Stajich J E, Berbee M L, Blackwell M, et al. The fungi[J]. Curr Opin Chem Biol, 2009, 19(18):840.
[8]Singh P K,Singh M,Vyas D. Biocontrol of Fusarium wilt of chickpea using arbuscular mycorrhizal fungi and Rhizobium leguminosorum Biovar[J]. Caryologia, 2014, 63(4):349.
[9]Vor1skova′J, Baldrian P. Fungal community on decomposing leaf litter undergoes rapid successional changes[J]. ISME J, 2013, 7(3):477.
[10]Chen M, Li X, Yang Q, et al. Soil Eukaryotic microorganism succession as affected by continuous cropping of peanut-pathogenic and beneficial fungi were selected[J]. PLoS ONE, 2012, 7(7):e40659.
[11]Wu L K, Wang H B, Zhang Z X, et al. Comparative metaproteomic analysis on consecutively Rehmannia glutinosa-monocultured rhizosphere soil[J]. PLoS ONE, 2011, 6(5):9055.
[12]張敏, 谢运球, 冯英梅, 等. 浸提用水对测定土壤pH值的影响[J]. 河南农业科学,2008(6):58.
[13]Rowell D L. Soil science: methods and applications[M]. Utrecht the Netherlands:Longman,1994: 573.
[14]Keeney D R, Nelson D W. Nitrogen-inorganic forms. Methods of soil analysis-part 2-chemical and microbiological properties[M]. Madison: Amer Soc Agronomy, 1982:595.
[15]Johannes R, Erland B, Philip C B, et al. Soil bacterial and fungal communities across a pH gradient in an arable soil[J]. ISME J, 2010, 4(10):1340.
[16]Jorge L M R, Vivian H, Pellizarib, et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities[J]. Proc Natl Acad Sci USA, 2013, 110(3):988.
[17]Caporaso J G, Bittinger K, Bushman F D, et al. PyNAST: a flexible tool for aligning sequences to a template alignment[J]. BMC Bioinformatics, 2010, 26(2):266.
[18]Caporaso J G, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data[J]. Nat Met, 2010, 7(5):335.
[19]Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools[J]. Nucl Acid Res, 2013, 41:D590.
[20]Mazzola M, Manici L M. Apple replant disease: role of microbial ecology in cause and control[J]. Annu Rev Phytopathol, 2012, 50(1):45.
[21]Zhou X G, Wu F Z. Dynamics of the diversity of fungal and Fusarium, communities during continuous cropping of cucumber in the greenhouse[J]. FEMS Microbiol Ecol, 2012, 80(2):469.
[22]Dong L, Yao H, Li Q, et al. Investigation and integrated molecular diagnosis of root-knot nematodes in Panax notoginseng root in the field[J]. Eur J Plant Pathol, 2014, 137(4):667.
[23]Dong L, Xu J, Feng G, et al. Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system[J]. Sci Rep, 2016, 6: 31802.
[24]Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere[J]. FEMS Microbiol Ecol, 2009, 68(1):1.
[25]Fierer N, Lauber C L, Ramirez K S, et al. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients[J]. ISME J, 2012, 6(5): 1007.
[26]Lin X, Feng Y, Zhang H, et al. Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing[J]. Environ Sci Technol, 2012, 46(11):5764.
[27]Qiu M, Zhang R, Xue C, et al. Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil[J]. Biol Fertil Soils, 2012, 48: 816.
[28]Lauber C L, Strickland M S, Bradford M A, et al. The influence of soil properties on the structure of bacterial and fungal communities across land-use types[J]. Soil Biol Biochem, 2008, 40: 2415.
[29]Rousk J, Bth E, Brookes P C, et al. Soil bacterial and fungal communities across a pH gradient in an arable soil[J]. ISME J 2010, 1:12.
[30]沈亮, 徐江, 董林林, 等. 人參栽培种植体系及研究策略[J]. 中国中药杂志,2015,40(17):3367.
[责任编辑 吕冬梅]
