Clinical outcomes of patients with chronic hepatitis C after generic direct-acting antiviral treatments in Vietnam: A retrospective analysis
2020-10-19HuongTTVuAzumiIshizakiQuynhNguyenHuyenNguyenHoiLeKinhNguyenHiroshiIchimura
Huong TT Vu, Azumi Ishizaki, Quynh T Nguyen,, Huyen N Nguyen, Hoi T Le, Kinh V Nguyen, Hiroshi Ichimura
1National Hospital for Tropical Disease, Hanoi, Vietnam
2Department of Viral Infection and International Health, Graduate School of Medical Sciences, Kanazawa University, Japan
ABSTRACT
KEYWORDS: Vietnam; Chronic hepatitis C; Generic direct-acting antiviral; Improvement of liver stiffness
1. Introduction
Chronic hepatitis C (CHC) is a huge global public health issue. Approximately 71 million individuals worldwide have CHC, with 399 000 related deaths being reported in 2015[1]. The elimination of viral hepatitis as a public health threat by 2030: reductions in new infections by 90% and mortality by 65% from 2015 to 2030, is a global target that was endorsed by the World Health Assembly in 2016[2]. One of the key interventions to achieve this goal is to increase the number of individuals who are treated for CHC. New oral direct-acting antivirals (DAA) for hepatitis C virus (HCV) are able to cure more than 90% of patients with CHC. The World Health Organization currently recommends treating all patients with CHC who are 12 years or older irrespective of the disease stage with pan-genotypic DAA[3].
In Vietnam, the number of patients with CHC is estimated to be approximately 1 million, which is 1.1% of the general population[4-7], with 5 300 estimated annual deaths due to the sequela of CHC being reported in 2016[8]. The Ministry of Health Vietnam issued a National Action Plan for viral hepatitis prevention and control between 2015 and 2019 and treatment guidelines for CHC in 2016 to expand access to HCV care and treatment in Vietnam[5]. By 2016, approximately 4 500 individuals, accounting for only 0.45% of patients with CHC, had been treated with DAA[9]. The Ministry of Health Vietnam launched a new policy to reimburse 50% of DAA through health insurance from January 2019 in an attempt to expand the DAA treatment. Vietnam is a country in which mixed HCV genotypes (such as genotypes 1a, 1b, 3, and 6) are circulating[10]. It is important to understand current treatment outcomes in order to build a more effective strategic plan to control CHC in Vietnam; however, limited information is currently available on the outcomes of DAA treatment for Vietnamese CHC in the realworld[11]. Therefore, the aim of the present study was to clarify the current treatment outcomes of CHC with generic DAAs at a tertiary hospital in Hanoi, Vietnam.
2. Materials and methods
2.1. Subjects and methods
The medical records of patients who were treated for CHC at a tertiary hospital in Hanoi, Vietnam, between January 2016 and December 2016 were retrospectively reviewed. The demographic factors of patients, comorbidities, the co-infection status with hepatitis B virus (HBV) and/or human immunodeficiency virus (HIV), risk factors related to HCV acquisition, prior HCV treatment history, HCV genotypes at baseline, DAA regimens and treatment durations, laboratory data, the level of liver stiffness assessed by a transient elastography (TE, FibroScan®, Echosens, Paris, France), and HCV viral loads before and 12 weeks after the end of the treatment were extracted from medical records.
The primary endpoint was a sustained virologic response (SVR) 12 weeks after complete treatment (SVR12) with undetectable HCV RNA <15 IU/mL (Cobas®AmpliPrep/COBAS®TaqMan®HCV Qualitative Test, v2.0, Roche, USA). The secondary outcome was a change of the patients’ liver stiffness level that was measured using TE, a noninvasive method. Based on the TE score, the liver stiffness level was classified into five stages: F0: TE score <5 kilopascals (kPa); F1: 5 ≤TE score <7.1 kPa; F2: 7.1 ≤ TE score <8.7 kPa; F3: 8.7 ≤TE score <14.5 kPa; and F4: TE score ≥ 14.5 kPa. F4 was defined as “cirrhosis” and F0-F3 as “noncirrhosis”. Laboratory data at the baseline and during the treatment period and the magnitudes of changes in laboratory values were calculated and graded from grades 1 to 4 according to the Common Terminology Criteria for Adverse Events version 4.03 by the National Cancer Institute in the United States of America (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Grades 3 and 4 were considered to be serious adverse events. Since patient interviews and examinations were not standardized at the time of consultations, the subjective symptoms of patients documented in medical records were not applied to the assessment of serious adverse events in the present study.
2.2. Statistical analysis
Statistical analyses were performed with SPSS version 25. Pearson’s chi-square test and Fischer’s exact test were performed to assess differences in proportions. Mann-Whitney U test was used to compare data among the groups. The Friedman test with Bonferroni correction was conducted to compare paired data from baseline. Pearson’s product-moment correlation coefficient was used to assess relationships. A P-value of less than 0.05 was considered to be significant.
2.3. Ethical consideration
This study protocol was approved by the Ethics Committee of Kanazawa University, Japan (No. 2383-1) and the Ethics Committee of the tertiary hospital in Hanoi, Vietnam (No. 241/NDTW-TCCB).
3. Results
3.1. Demographic and clinical characteristics at baseline
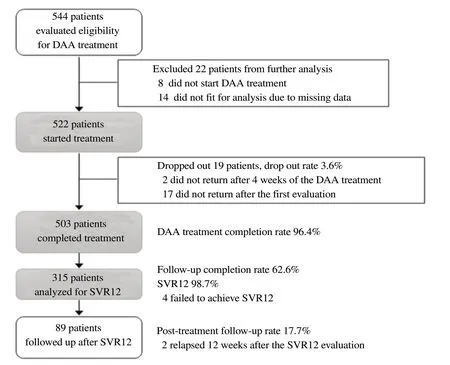
Figure 1. The study flowchart. DAA: direct-acting antivirals; SVR12: sustained virologic response 12 weeks after the end of the treatment.
The study flowchart is shown in Figure 1. We recruited all patients (544 patients) who were evaluated the eligibility to treat with generic DAAs at the site between January 2016 and December 2016 then excluded 8 patients who did not start DAA treatment and 14 patients whose medical records had missing data and not able to analyze further. Finally, the medical records of 522 patients who were treated with generic DAAs at a tertiary hospital in Hanoi, Vietnam were reviewed (Table 1). There were 132 (25.3%) females and 390 (74.7%) males with a median age of 45 years (interquartile range, IQR: 39-55). Female patients with a median age of 52 years (IQR 41-59) were significantly older than male patients with a median age of 43 years (IQR 38-53) (P<0.001).
The median TE score of the patients (n=476) whose TE data were available was 8.2 kPa (IQR 6.1-14.8), and 123 (25.8%) had cirrhosis (F4: TE score ≥ 14.5 kPa). The TE score correlated with age (r=0.31, P<0.001), but the TE score did not differ between female and male (Female: median 8.0 kPa, IQR 5.7-15.1; Male: 8.3 kPa, IQR 6.2-14.8, P=0.38, Table 1).

Table 1. Demographic and clinical characteristics of 522 patients who started DAA.

Table 2. Laboratory data of participants at baseline.
Among the 522 patients with CHC, HCV genotype 6 was most commonly detected (49.4%), followed by genotypes 1a (19.0%), 1b (13.0%), and 3 (5.9%). Of the 519 patients whose data of coinfections were available, 30 (5.8%) were co-infected with HBV and 16 (3.1%) with HIV (Table 1). Thirty patients had a history of interferon treatment.
Laboratory data at baseline (before the treatment) showed mild liver injury (Table 2). Hemoglobin, creatinine, alanine aminotransferase, and gamma-glutamyl transferase levels were significantly lower in females than in males and other laboratory data showed no significant difference between females and males.
3.2. DAA treatments and safety
Among the 522 patients with CHC, 370 (70.9%) started treatment with ledipasvir/sofosbuvir with or without ribavirin, 69 (13.2%) with sofosbuvir/pegylated-interferon + ribavirin, 65 (12.5%) with daclatasvir/sofosbuvir with or without ribavirin, and 18 (3.4%) with sofosbuvir/ribavirin (Table 3).
Of the 522 patients, 503 patients (96.4%) completed the DAA treatment. The reason(s) for the loss of 19 patients to the follow-up during the treatment were not described in their medical records. The treatment duration was 12 weeks (n=465, 92.4%), 16 weeks (n=14, 2.8%), 20 weeks (n=3, 0.6%), and 24 weeks (n=21, 4.2%).
Among the 503 patients who completed DAA treatment, 33 (6.6%) developed serious adverse events with grade 3 or 4: hepatotoxicity (n=20), hyperglycemia (n=5), thrombocytopenia (n=4), anemia (n=3), and renal insufficiency (n=1), during the DAA treatment. These laboratory documented serious adverse events were observed more frequently in the patients who received the daclatasvir containing regimens than those without daclatasvir regimens (14.3%, 9/63 vs. 5.5%, 24/440; P=0.04), while the difference was not significant between the patients who received ribavirincontaining and non-containing regimens (6.3%, 30/478 vs. 12.0%, 3/25; P=0.26). Modifications of the DAA treatment due to these laboratory-documented serious adverse events were not required during the treatment period.
3.3. The SVR12 evaluation, outcomes of the DAA treatment, and further hospital visits
Only 315 out of the 503 patients who completed the DAA treatment (62.6%) returned to the hospital for SVR12 evaluation. Overall SVR12 was 98.7% regardless of HCV genotypes and DAA regimens. The SVR12 stratified by the infected HCV genotypes and DAA regimens is shown in Table 3.
Of the 123 patients with cirrhosis, 119 completed the DAA treatment (lost to the follow-up rate of 3.3%), 75 (63.0%) of the 119 patients who completed the treatment came back for SVR12 evaluation, and 30 patients (25.2%) returned for post-treatment follow-up. Among the 75 patients evaluated for SVR12, 74 (98.7%) reached SVR12. Of the 353 patients with non-cirrhosis, 343 completed the DAA treatment (lost to the follow-up rate of 2.8%),213 (62.1%) of the 343 patients who completed the treatment came back for SVR12 evaluation, and 57 (16.6%) returned for posttreatment follow-up. Among the 213 patients evaluated for SVR12, 210 (98.6%) reached SVR12. There was no significant difference in the lost to the follow-up rate, SVR12 evaluation rate, and SVR12 rate between the patients with and without cirrhosis.

Table 3. The treatment outcome of the 315 patients stratified by the HCV genotypes and the DAA regimens.
Of the 503 who completed the treatment, 89 patients (17.7%) returned for post-treatment follow-up (Figure 1). Among the patients whose TE scores at baseline were available, the post-treatment follow-up rate was higher in the patients with cirrhosis (25.2% vs. 16.6%; P=0.04).
3.4. Changes in laboratory data after the DAA treatment
The liver function tests returned to normal ranges after the DAA treatment among patients of whom the data before and after treatment were available (Table 4). The median TE scores of the 101 patients, whose TE data were available at three points, significantly decreased from 10.2 kPa (IQR 6.8-16.9) before the treatment to 8.6 kPa (IQR 6.1-14.8) at the end of the treatment, and then to 6.3 kPa (IQR 4.9-11.7) at 12 weeks after the end of the treatment (P<0.001, Table 4). Comparing with the baseline data, the fibrosis stage was improved in 53 of the 101 patients, did not change in 38 patients, and got worse in 10 patients at 12 weeks after the end of the treatment.
4. Discussion
In the present study, the treatment outcome of generic DAA for Vietnamese CHC patients treated in 2016 were evaluated. The rate of SVR12 was as high as 98.7% regardless of HCV genotypes and DAA regimens with the attenuation of liver stiffness from 10.2 kPa before the treatment to 6.3 kPa at the 12 weeks after the end of DAA treatment. This is a study to describe the improvement of liver stiffness and the normalization of liver function tests in CHC patients after DAA treatment in the community in Vietnam.
Liver stiffness measured by a transient electrography is a surrogate marker of histological liver fibrosis[12,13] that is associated with the development of decompensated cirrhosis and hepatocellular carcinoma (HCC) as well as with mortality[14]. Several prospective studies demonstrated that the eradication of HCV reduced the progression rates of liver fibrosis and the risk of developing HCC[15- 17]. In the present study, a significant reduction in liver stiffness and the normalization of liver function tests were observed in patients who achieved SVR12[18,19]. The result provides a strong rationale for the initiation of DAAs for all patients with CHC including those who have cirrhosis in Vietnam.
Nineteen out of the 522 patients (3.6%) were lost to the follow-up during the DAA treatment. Among the 503 patients who completed the DAA treatment, 62.6% returned to the hospital for the SVR12 evaluation, and only 17.7% were followed up after treatment period. Missing SVR12 evaluation and no further follow-up may increase the risk to miss relapse and re-infection of HCV infection. Regular screening for HCC is recommended as there is a risk to develop HCC even after the cure of HCV infection[3]. Previous studies reported that younger age, prison-based treatment, a history of mental health disease, active drug use, and type of insurance are factors related to being lost to the follow-up[20-22]. However, in the present study, the related factors were not sufficiently investigated, since it was conducted retrospectively. Further assessments are urgently needed to elucidate the reason(s) for being lost to the follow-up and missing the SVR12 evaluation and assessment of HCC development particularly in persons with cirrhosis in order to improve patient care for CHC in Vietnam.
As the rate of laboratory documented serious adverse events indaclatasvir containing regimen was higher than that in the other regimen groups in the current study, treating physician may need to take an appropriate awareness-raising measure for the patient treating with daclatasvir for proper patient care.
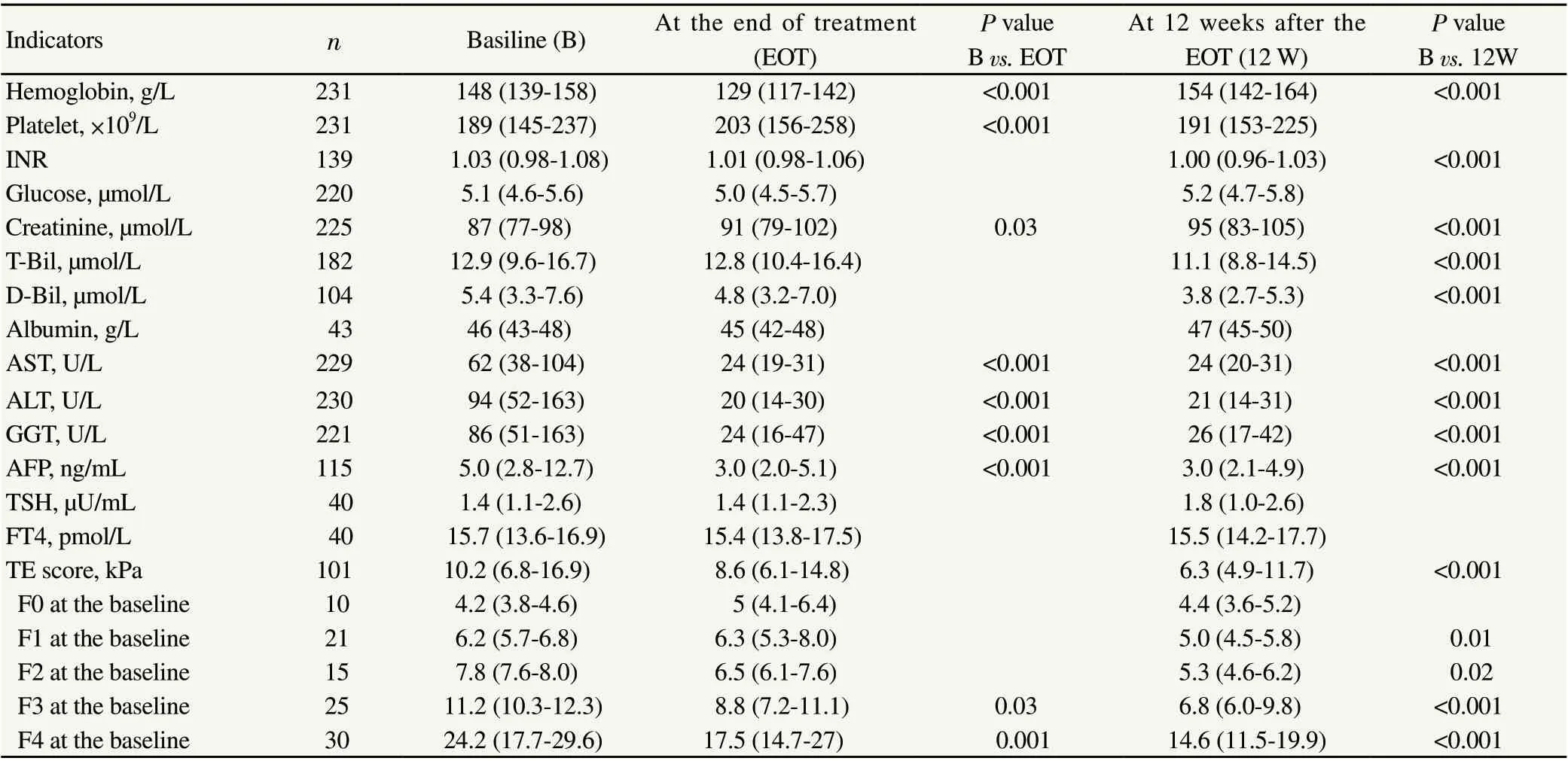
Table 4. The change of laboratory data of participants of whom all data at baseline and the other follow-up periods were available.
It is also important to note that the number of female patients who sought DAA treatment was less than that of males (female, n=132; male, n=390) and females were older (P<0.001) than males. These differences may be attributed to the difference in HCV prevalence and screening bias by gender or late attendance to a hospital because of the slow rate of disease progression in females[23-25]. Kanwal et al. revealed that young female patients with CHC were treated with DAA less frequently than male patients and suggested the necessity of targeted interventions for this population[26]. To address the factor(s) related to gender and age differences in CHC patients in the present study, a population survey on the prevalence of HCV and a risk factor analysis at the national level are needed to identify specific populations that need intensive interventions.
The present study had several limitations. It was conducted at one tertiary treatment hospital in northern Vietnam, which may be associated with a referral bias. Thus, the present results cannot be generalized to the whole area of Vietnam. Furthermore, this was a retrospective analysis and important information, such as treatment information for HIV and HBV infection, use of opioid therapy, ongoing drug use, level of alcohol consumption, and reasons for being lost to follow-up during treatment and missing the SVR12 evaluation, was not always documented properly in medical records, which may have resulted in underestimations. Alcohol use disorder is one of the risk factors for decompensated cirrhosis in CHC[27]. Alcohol dependence (5.9% in the male population) and injecting drug use are serious issues in Vietnam[28,29]. In addition, nonalcoholic fatty liver disease is a major factor associated with the persistent alternation of liver transaminases and liver stiffness after the achievement of a cure from HCV infection[30,31]. These risks, which may compromise the prognosis of patients with CHC were not assessed well in the present study. Further studies to address the incidence and risk of HCC development after the achievement of SVR are also needed in Vietnam.
In conclusion, the overall SVR12 with generic DAAs was sufficiently high regardless of HCV genotypes or DAA regimens with a high treatment completion rate in Vietnam. The liver stiffness significantly decreased after the DAA treatment. This study highlights the feasibility of implementing DAA treatment to cure all CHC patients at any level of liver fibrosis including cirrhosis in Vietnam as well as other low- and middle-income countries.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
We would like to acknowledge the participants of this study and Trung Vu Nguyen who supported the clinical evaluation of patients.
Funding
This study was supported by the Japan Society for the Promotion of Science KAKENHI Grant Number JP 15H05289.
Authors’ contributions
HTTV and AI designed the study with input from HNN, HTL, and HI. QTN, HTTV, and AI were responsible for overall data collection with input from HNN. HTTV and AI conducted data analysis and interpretation. HTTV drafted the first manuscript. AI and HI revised the manuscript critically. All authors read and approved the final manuscript. KVN and HI supervised the project.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- ARIMA models forecasting the SARS-COV-2 in the Islamic Republic of Iran
- Genetic variation and phylogenetic relationship of Hypoderaeum conoideum (Bloch, 1782) Dietz, 1909 (Trematoda: Echinostomatidae) inferred from nuclear and mitochondrial DNA sequences
- Effect of climate change on spatial distribution of scorpions of significant public health importance in Iran
- Phytochemical profiling and biological activity of Plectranthus amboinicus (Lour.) mediated by various solvent extracts against Aedes aegypti larvae and toxicity evaluation
- Convalescent plasma: A potential therapeutic option for COVID-19 patients
