Phenolic compounds from Peanut testa
2020-10-14ZhiyongXuYufeiXiXiaoxiaoHuangShaojiangSong
Zhiyong Xu,Yufei Xi,Xiaoxiao Huang*,Shaojiang Song*
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development,Liaoning Province,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract Phytochemical investigation of peanut testa (the seed coat of Arachis hypogaea L.) led to the isolation of eight phenolic compounds,including caffeic acid (1),methyl caffeate (2),ethyl caffeate (3),methyl protocatechuate (4),ethyl protocatechuate(5),butyl protocatechuate (6),(E)-p-hydroxycinnamic acid methyl ester (7),and resveratrol (8).The structures of the compounds were elucidated through spectroscopic data analysis and comparison with the previously reported literature.Among them,compounds 2,3,5,and 6 were obtained from Arachis hypogaeaL.for the first time.
Key words:peanut testa; Arachis hypogaea L.; separation and purification; phenolic compounds
1 Introduction
Peanut is botanically classified asArachis hypogaeaof the family Leguminosae,first found in South America and then distributed all over the world [1].The seed coat ofArachis hypogaeaL.,peanut testa,varies in red,black,or other colors with different cultivars [2].The composition of peanut testa varies too with different cultivars or different growing conditions,but the typical constituents are 20% fat,19% protein,2% ash,18% fiber and 41%others [3].Peanut testa has received increasing attention in recent years,because it is rich in tannins and abundant phenolic compounds beneficial to human health have been found in it,including phenolic acids,stilbenes and flavonoids [4-6].Furthermore,studies have shown that peanut testa displays a variety of biological activities,including antimicrobial,antioxidant,anti-inflammatory and antitumor effects [7-9].
Peanut testa,a by-product of peanut processing industry,is used as an ingredient of animal feed and functional food [10].In order to utilize peanut testa as a medicinal product and promote further research,this phytochemical study on the drying seed coat ofArachis hypogaeaL.isolated and identified 8 phenolic compounds,including caffeic acid (1),methyl caffeate (2),ethyl caffeate (3),methyl protocatechuate (4),ethyl protocatechuate (5),butyl protocatechuate (6),(E)-p-hydroxycinnamic acid methyl ester (7),and resveratrol (8) (Fig.1).
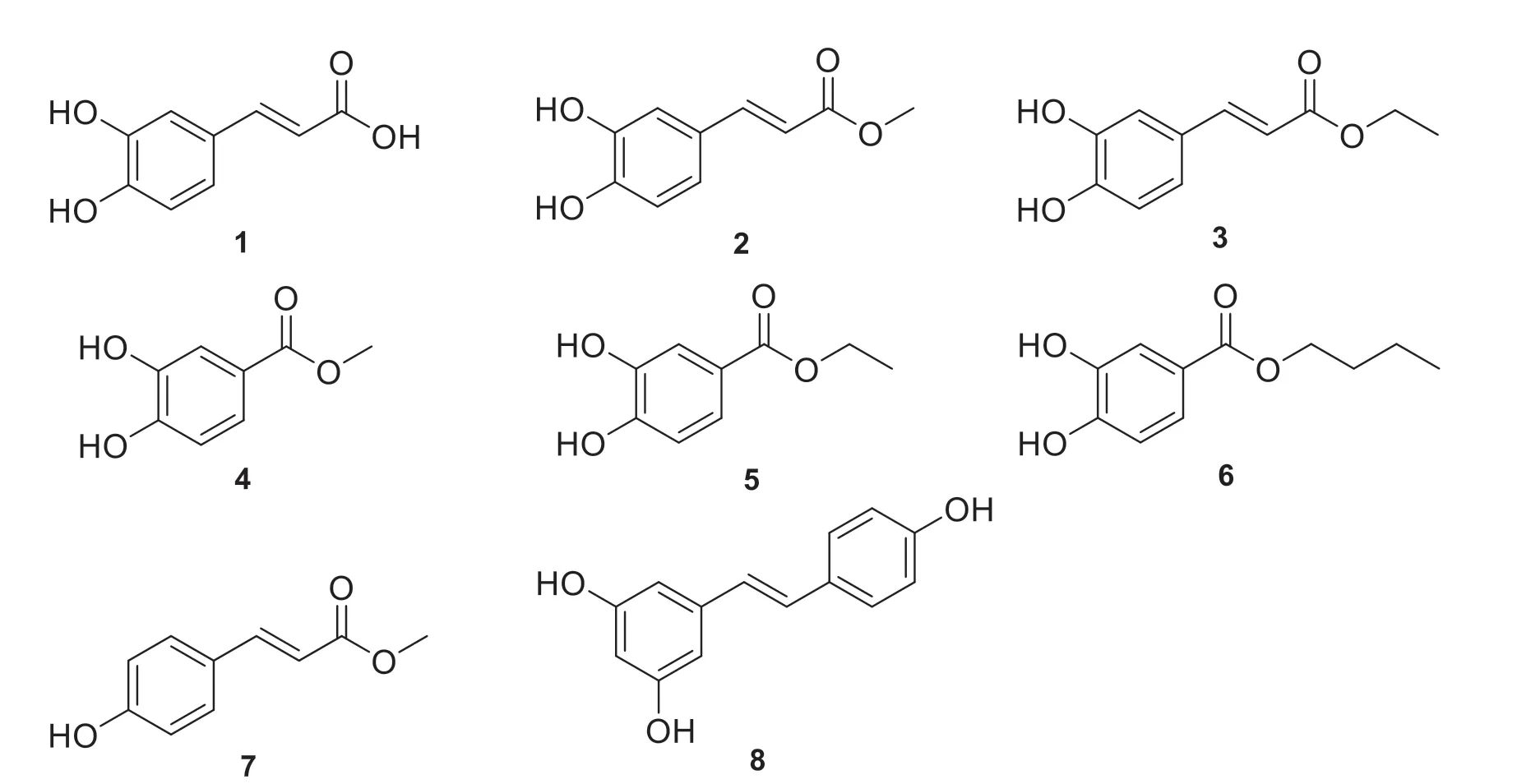
Fig.1 The structures of compounds 1-8
2 Materials and methods
2.1 General experimental procedures
The NMR spectra were recorded by Bruker ARX-400 spectrometers (Bruker Corporation,Bremen,Germany) with TMS as an internal standard and the chemical shifts were expressed in ppm values.Preparative HPLC (detector at 210 nm) was used for isolation and purification with a preparative HPLC YMC ODS-A column (Shimadzu,5µm,250 mm × 10 mm,Japan) and an analytical HPLC YMC ODS-A column (Thermo,5µm,150 mm ×4.6 mm,USA).Silica gel (100-200 and 200-300 mesh,Qingdao Marine Chemical Co.,Qingdao,China),MCI gel (75-150µm,Mitsubishi Chemical Co.,Japan),ODS silica gel (60-80µm,Merck,Germany),and D101 macroporous resin (Hebei Cangzhou Chemical Co.,China) were used to column chromatography and silica gel GF254(Qingdao Marine Chemical Co.,China) was used for TLC.
2.2 Plant material
The seed coats ofArachis hypogaeaL.were collected from Fuyang,Anhui province,People’s Republic of China,in 2016,and identified by Professor Jincai Lu (School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University).A voucher specimen is kept in the herbarium of Shenyang Pharmaceutical University,Liaoning Province,PR China.
2.3 Extraction and isolation
The compounds of peanut testa were extracted and isolated as shown in Fig.2.The air-dried seed coats ofArachis hypogaeaL.(50 kg) were extracted with 60% EtOH by refluxing (70 °C for 2 h) twice to yield 23.98 kg of crude extract.The concentrated alcohol extract was suspended in H2O and extracted withn-BuOH.Then-BuOH extract (3.98 kg) was then chromatographed with D101 macroporous resin,using a gradient of EtOH/H2O (0:100,30:70,60:40,90:10) to yield three fractions.60% EtOH/H2O extract (723 g) was carried out on a silica gel column under reduced pressure and eluted with a stepwise gradient of CH2Cl2/CH3OH (from 20:1 to 1:1,v/v) to afford four fractions (Fr.A to Fr.D).Fr.A (40 g) was then chromatographed on an ODS column,and eluted with a gradient of EtOH/H2O(from 30:70 to 100:0) to yield sub-fraction (Fr.A1 to Fr.A7).Fr.A1 was then chromatographed on a silica gel column and eluted with a gradient of CH2Cl2/MeOH (from 50:1 to 4:1,v/v) to obtain sub-fractions,and the sub-fractions were chromatographed by preparative HPLC to yield compound 1 (13 mg),while compound 6 (10 mg) was obtained from Fr.A3 and compound 8 (10 mg) was obtained from Fr.A4.Fr.A5 was chromatographed on a silica gel column and eluted with a gradient of CH2Cl2/MeOH(from 50:1 to 2:1,v/v) to obtain sub-fractions,and the sub-fractions were chromatographed by preparative HPLC to yield compound 2 (13 mg) and compound 7 (12 mg),while compound 4 (14 mg)and compound 5 (9 mg) were obtained from Fr.A6.Fr.B (80 g) was then chromatographed on an ODS column,and eluted with a gradient of EtOH/H2O(from 30:70 to 100:0) to yield sub-fractions (Fr.B1,Fr.B2).Fr.B2 was then chromatographed on a gel column to obtain sub-fractions (Fr.B2-1 to Fr.B2-4).Fr.B2-2 was then chromatographed on a silica gel column and eluted with a gradient of CH2Cl2/MeOH(from 50:1 to 4:1,v/v) to obtain compound 3 (12 mg).
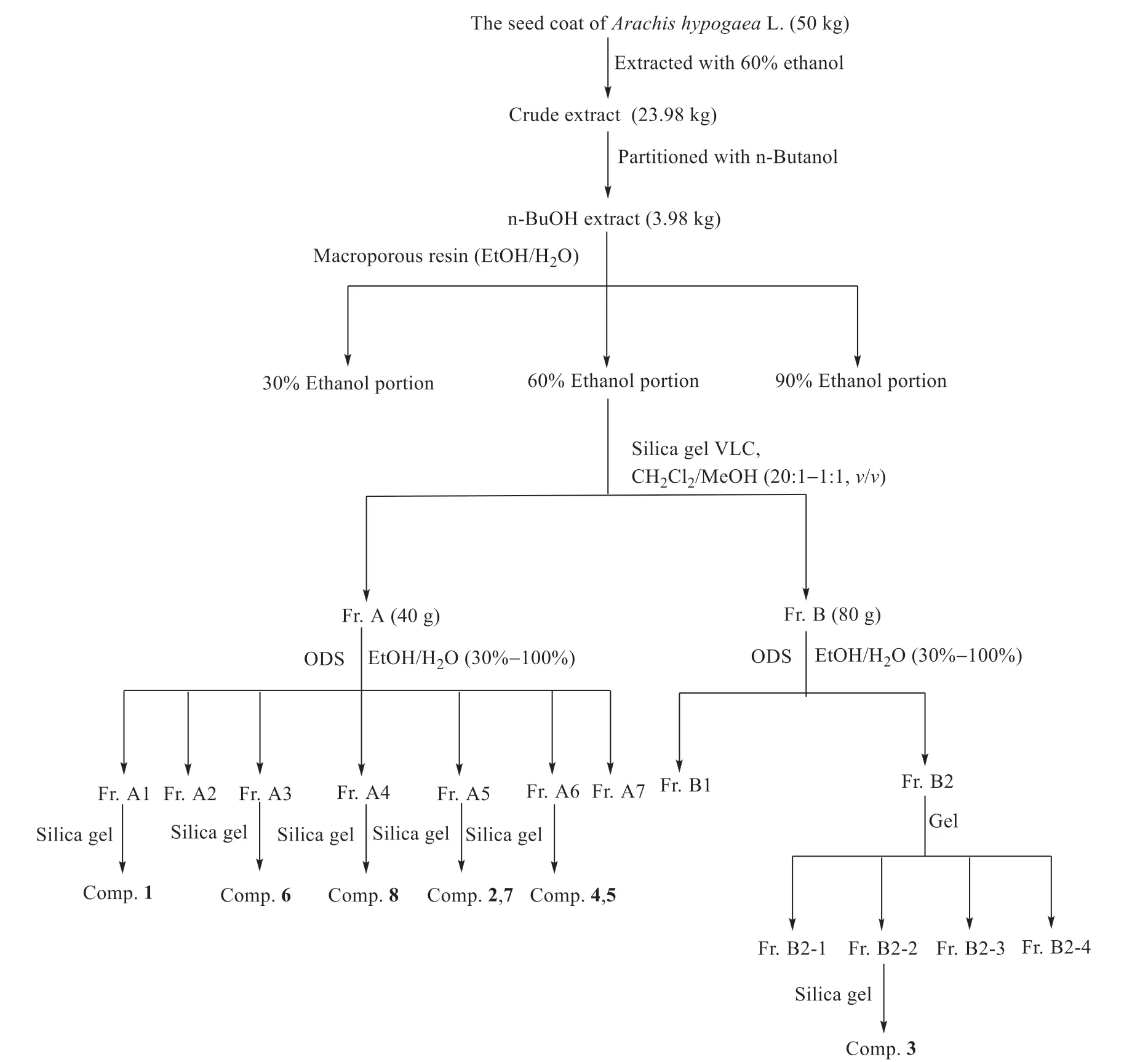
Fig.2 Isolation process of the constituents from peanut testa
2.4 Physical and spectroscopic data of the isolated compounds
Caffeic acid (1):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.29 (1H,d,J=1.9 Hz,H-2),7.10 (1H,dd,J=7.9,1.9 Hz,H-6),6.77 (1H,d,J=8.1 Hz,H-5),7.55 (1H,d,J=15.9 Hz,H-7),6.45 (1H,d,J=15.9 Hz,H-8);13C-NMR (100 MHz,DMSO-d6):δC125.4 (C-1),115.1 (C-2),145.2 (C-3),148.1 (C-4),115.7 (C-5),123.2 (C-6),147.1 (C-7),114.2 (C-8),167.2 (C-9).
Methyl caffeate (2):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.05 (1H,d,J=1.9 Hz,H-2),7.00 (1H,dd,J=8.1,1.9 Hz,H-6),6.76 (1H,d,J=8.1 Hz,H-5),7.48 (1H,d,J=15.9 Hz,H-7),6.26 (1H,d,J=15.9 Hz,H-8),9.39 (2H,br s),3.68 (3H,s);13C-NMR (100 MHz,DMSO-d6):δC125.4 (C-1),115.7 (C-2),145.6 (C-3),148.1 (C-4),114.8 (C-5),121.4 (C-6),145.1 (C-7),113.6 (C-8),166.2 (C-9),51.5 (-OCH3).
Ethyl caffeate (3):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.05 (1H,d,J=1.9 Hz,H-2),6.99 (1H,dd,J=8.1,1.9 Hz,H-6),6.75 (1H,d,J=8.1 Hz,H-5),7.48 (1H,d,J=15.9 Hz,H-7),6.26 (1H,d,J=15.9 Hz,H-8),9.39(2H,br s),3.85 (2H,q,J=7.0 Hz,-OCH2),1.24(3H,t,J=7.0 Hz,-CH3).
Methyl protocatechuate (4):colorless,crystal.1H-NMR (400 MHz,DMSO-d6):δH7.40 (1H,d,J=1.9 Hz,H-2),7.35 (1H,dd,J=7.9,1.9 Hz,H-6),6.85 (1H,d,J=8.1 Hz,H-5),9.58 (2H,s),3.79 (3H,s).13C-NMR (100 MHz,DMSO-d6):δC120.7 (C-1),116.4 (C-2),145.2 (C-3),150.5 (C-4),115.4 (C-5),121.9 (C-6),166.3 (C=O),51.6 (-OCH3).
Ethyl protocatechuate (5):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.35(1H,d,J=1.9 Hz,H-2),7.30 (1H,dd,J=8.1,1.9 Hz,H-6),6.80 (1H,d,J=8.1 Hz,H-5),4.22(2H,q,J=7.1 Hz,-OCH2),1.27 (3H,t,J=7.1 Hz,-CH3),9.51 (2H,br s).
Butyl protocatechuate (6):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.36(1H,d,J=1.9 Hz,H-2),7.31 (1H,dd,J=7.9,1.9 Hz,H-6),6.80 (1H,d,J=8.1 Hz,H-5),4.18 (2H,t,J=7.1 Hz,OCH2),0.9-1.8 (7H,m,-CH2CH2CH3);13C-NMR (100 MHz,DMSO-d6):δC120.7 (C-1),116.2 (C-2),145.1 (C-3),150.5(C-4),115.3 (C-5),121.7 (C-6),165.8 (C=O),63.7(-OCH2),30.4 (-CH2CH2CH3),18.8 (-CH2CH3),13.6(-CH3).
(E)-p-hydroxycinnamic acid methyl ester (7):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.59 (2H,d,J=8.4 Hz,H-2,H-6),6.82 (2H,dd,J=8.4 Hz,H-3,H-5),7.50 (1H,d,J=15.9 Hz,H-7),6.40 (1H,d,J=15.9 Hz,H-8),10.1(1H,br s),3.71 (3H,s,-OCH3).
Resveratrol (8):white,amorphous powder.1H-NMR (400 MHz,DMSO-d6):δH7.39 (2H,d,J=8.5 Hz,H-2’,6’),6.38 (2H,d,J=8.7 Hz,H-3’,5’),6.38 (2H,d,J=1.8 Hz,H-2,6),6.11 (1H,s,H-4),6.92 (1H,d,J=16.3 Hz,H-β),6.81 (1H,d,J=16.3 Hz,H-α),9.22 (3H,br s);13C-NMR (100 MHz,DMSO-d6):δC127.8 (C-α),125.8 (C-β),128.1 (C-1’),127.8 (C-2’,6’),115.5 (C-3’,5’),157.2 (C-4’),139.2(C-1),104.3 (C-2,6),158.5 (C-3,5),101.8 (C-4).
3 Results and discussion
The1H-NMR spectrum of compound 1 showed the characteristic signal of a 1,3,4-trisubstituted benzene ring atδH7.29 (1H,d,J=1.9 Hz,H-2),7.10 (1H,dd,J=7.9,1.9 Hz,H-6) andδH6.77 (1H,d,J=8.1 Hz,H-5).δH7.55 (1H,d,J=15.9 Hz,H-7)andδH6.45 (1H,d,J=15.9 Hz,H-8) are hydrogen signals of atrans-double bond.The13C-NMR spectrum revealed 9 carbon signals,including an aromatic ring (δC115.1,115.7,123.2,125.4,145.2,148.1),a double bond (δC114.2,147.1),and a carbonyl carbon (δC167.2).Agreeing well with the1H-and13C-NMR spectrum of caffeic acid,a reported compound [11],compound 1 was identified as caffeic acid (Fig.1).
Although there is a resemblance between 1D NMR signals of compound 2 and 1,they differ in structures since the methoxy signal atδC51.5/δH3.68 observed in compound 2 was absent in compound 1.Analysis of1H-NMR signals of compound 2 showed the characteristic signals of 1,3,4-trisubstituted benzene ring atδH7.05 (1H,d,J=1.9 Hz,H-2),7.00 (1H,dd,J=8.1,1.9 Hz,H-6) andδH6.76 (1H,d,J=8.1 Hz,H-5),onetrans-double bond signal atδH7.48 (1H,d,J=15.9 Hz,H-7) andδH6.26 (1H,d,J=15.9 Hz,H-8),one methoxy signal atδH3.68(3H,s),and two hydroxyl signals atδH9.39 (2H,br s).The13C-NMR spectrum revealed 10 carbons involved in an aromatic ring (δC114.8,115.7,121.4,125.4,145.6,148.4),a double bond (δC113.6,145.1),a carbonyl carbon (δC166.2),and a methoxy (δC51.5).Correlated with the experimental spectroscopic data in the reported literature [12],compound 2 was identified as methyl caffeate (Fig.1).
A comparison of the1H NMR data of compound 3 with those of compound 2 revealed that the two compounds have similar proton signals.The1H-NMR data of compound 3 showed the characteristic signals of 1,3,4-trisubstituted benzene ring atδH7.05 (1H,d,J=1.9 Hz,H-2),6.99 (1H,dd,J=8.1,1.9 Hz,H-6) andδH6.75 (1H,d,J=8.1 Hz,H-5).δH7.48 (1H,d,J=15.9 Hz,H-7) andδH6.26 (1H,d,J=15.9 Hz,H-8) were hydrogen signals of atrans-double bond.Furthermore,one oxygenated methylene signal atδH3.85 (2H,q,J=7.0 Hz,-OCH2),one methyl signal at 1.24 (3H,t,J=7.0 Hz,-CH3) and two hydroxys signals atδH9.39(2H,br s) were showed in the1H-NMR spectrum.Agreeing well with the1H-NMR data of ethyl caffeate in the reported literature [13],compound 3 was identified as ethyl caffeate (Fig.1).
The1H-NMR spectrum of compound 4 showed the characteristic signals of 1,3,4-trisubstituted benzene ring atδH7.40 (1H,d,J=1.9 Hz,H-2),7.35 (1H,dd,J=7.9,1.9 Hz,H-6) andδH6.85(1H,d,J=8.1 Hz,H-5),one methoxy signal atδH3.79 (3H,s),two hydroxyl signals atδH9.58(2H,s).The13C-NMR spectrum revealed 8 carbon signals including an aromatic ring (δC115.4,116.4,120.7,121.9,145.2,150.5),one carbonyl carbon(δC166.3),one methoxy (δC51.6).Compared with the1H-and13C-NMR data reported in the literature [14],compound 4 was identified as methyl protocatechuate (Fig.1).
The1H-NMR spectrum of compound 5 showed characteristic signals of 1,3,4-trisubstituted benzene ring atδH7.35 (1H,d,J=1.9 Hz,H-2),7.30 (1H,dd,J=8.1,1.9 Hz,H-6) andδH6.80 (1H,d,J=8.1 Hz,H-5),two hydroxyl signals atδH9.51 (2H,br s),one oxygenated methylene signal atδH4.22(2H,q,J=7.1 Hz,-OCH2) and one methyl signal at 1.27 (3H,t,J=7.1 Hz,-CH3).Agreeing well with the1H-NMR data of ethyl protocatechuate in the reported literature [15],compound 5 was verified as ethyl protocatechuate (Fig.1).
The1H-NMR spectrum of compound 6 showed characteristic signals of 1,3,4-trisubstituted benzene ring atδH7.36 (1H,d,J=1.9 Hz,H-2),7.31 (1H,dd,J=7.9,1.9 Hz,H-6) andδH6.80 (1H,d,J=8.1 Hz,H-5),and one hydrogen signal of one oxygenated methylene atδH4.18 (2H,t,J=7.1 Hz,OCH2).The13C-NMR spectrum revealed 11 carbons,including an aromatic ring (δC115.3,116.2,120.7,121.7,145.1,150.5),one carbonyl carbon (δC165.7),one oxygenated carbon (δC63.7),two methylene carbons (δC18.8,30.4),one methyl carbon (δC13.6).A group of carbons (δC63.7,30.4,18.8,13.6),combined with the1H-NMR spectrum (δH4.18,δH0.9-1.7),showed the existence of butoxy.Consistent with the1H-and13C-NMR data reported for butyl protocatechuate [16],compound 6 was verified as butyl protocatechuate (Fig.1).
The1H-NMR spectrum of compound 7 showed characteristic signals of 1,4-disubstituted benzene ring atδH7.59 (2H,d,J=8.4 Hz,H-2,H-6) andδH6.82 (2H,dd,J=8.4 Hz,H-3,H-5),as well as onetrans-double bond atδH7.55 (1H,d,J=15.9 Hz,H-7) andδH6.45 (1H,d,J=15.9 Hz,H-8).Furthermore,one methoxy signal atδH3.71 (3H,s)and one hydroxyl signal atδH10.1 (1H,br s) were showed in the1H-NMR spectrum.Consistent with reported1H-NMR data in the literature [17],compound 7 was verified as (E)-p-hydroxycinnamic acid methyl ester (Fig.1).
The1H-NMR spectrum of compound 8 showed characteristic signals of 1,4-disubstituted benzene ring atδH7.39 (2H,d,J=8.5 Hz,H-2’,6’) andδH6.38 (2H,d,J=8.7 Hz,H-3’,5’),as well as 1,3,5-trisubstituted benzene ring atδH6.38 (2H,d,J=1.8 Hz,H-2,6) andδH6.11 (1H,s,H-4).Furthermore,δH7.55 (1H,d,J=15.9 Hz,H-7) and 6.45 (1H,d,J=15.9 Hz,H-8) were hydrogen signals of onetrans-double bond andδH9.22 (3H,br s)were three hydroxyl signals.The13C-NMR spectrum revealed 14 carbons including onep-disubstituted aromatic ring (δC115.5 × 2,127.8 × 2,128.1,157.2),onem-trisubstituted aromatic ring (δC101.8,104.3 ×2,157.2,158.5 × 2),and one double bond (δC125.8,127.8).Furthermore,two aromatic rings were connected by the double bond.The1Hand13C-NMR data agreed well with the reported compound [18].Thus,compound 8 was identified as resveratrol (Fig.1).
4 Conclusion
In summary,eight phenolic compounds,including four cinnamic acid derivatives (1-3,7),three protocatechuic acid derivatives (4-6) and resveratrol (8),were isolated from 60% EtOH exact of dried seed coats ofArachis hypogaeaL.These compounds were identified,through comparison with the spectroscopic data in the reported literature,as caffeic acid (1),methyl caffeate (2),ethyl caffeate (3),methyl protocatechuate (4),ethyl protocatechuate (5),butyl protocatechuate (6),(E)-p-hydroxycinnamic acid methyl ester (7),and resveratrol (8).Among them,compounds 2,3,5,and 6 were isolated fromArachis hypogaeaL.for the first time.
杂志排行
Asian Journal of Traditional Medicines的其它文章
- Antimicrobial activities and analysis of the crude extract,fractions and compounds from the Lomatogonium rotatum
- Flavonoids with cytotoxicities from the seeds of Pongamia pinnata (L.) Pierre
- Impact of polyherbal formulations MEF-4 and MEF-8 on high fat diet induced obesity in SD rats
- Study on chemical composition of Viscum coloratum and its HPLC fingerprint in different harvest periods
- A review of research on coumarins from Radix Glehniae
- A review of phytochemistry and pharmacology perspectives of Gentiana rhodantha Franch.ex Hemsl.
