Endoscopy-based Kyoto classification score of gastritis related to pathological topography of neutrophil activity
2020-10-09OsamuToyoshimaToshihiroNishizawaShuntaroYoshidaYoshikiSakaguchiYousukeNakaiHidenobuWatanabeHidekazuSuzukiChizuTanikawaKoichiMatsudaKazuhikoKoike
Osamu Toyoshima, Toshihiro Nishizawa, Shuntaro Yoshida, Yoshiki Sakaguchi, Yousuke Nakai, Hidenobu Watanabe, Hidekazu Suzuki, Chizu Tanikawa, Koichi Matsuda, Kazuhiko Koike
Abstract
Key Words: Kyoto classification; Helicobacter pylori; Neutrophil activity; Updated Sydney System; Gastritis; Gastric cancer; Endoscopy; Pathology
INTRODUCTION
Stomach cancer is the third leading cause of cancer-related mortality in both sexes worldwide according to the 2018 GLOBOCAN estimates[1]. Thus, the key to obtaining a significant effect on the prognosis of gastric adenocarcinoma and its economic burden is to accurately identify at-risk individuals[2-5].The updated Sydney System is the most widely accepted method for the histological classification and grading of gastritis[6-8]; it can also assess pathologic features related toHelicobacter pylori(H. pylori) infection such as neutrophil activity, chronic inflammation, atrophy, intestinal metaplasia, and gastric cancer[9]. Neutrophil activity is measured by continuing acute inflammation and is linked to tissue damage. The density of intraepithelial neutrophils is correlated with the extent of mucosal damage and intensity ofH. pyloriinfection[6,10]. The topographic distribution of neutrophil activity has been reportedly associated with gastric cancer development[11].
本文从业务逻辑的角度出发,初步建立指标体系。然而在实际应用中,这些指标是否对个人逾期(在数据集中该指标符号为y)有显著的预测作用,或者说,这些指标与因变量y是否相互独立,则需进一步验证;若独立,则意味着这些指标无法起到评价个人信用状况的作用。此外,从另一个角度理解筛选变量的优点,是可以有效地降低数据集的维度,提高鲁棒性,更多的变量在很大程度上意味着收集变量数据所需成本的增加、模型运行速度的下降、模型训练成本的增加。
Endoscopy-led risk stratification is preferable since pathology-based evaluation is more invasive. The endoscopic Kyoto classification of gastritis was advocated by the Japan Gastroenterological Endoscopy Society in 2013. The Kyoto classification was established with the aim to unify the endoscopic diagnosis of gastritis in daily practice and match it with the histological diagnosis. The Kyoto classification score consisted of scores in gastric atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness[12]. Several studies have revealed the association of the Kyoto score withH. pyloriinfection[13-17]and gastric cancer risk[18-20]; however, the consistency of the Kyoto score with pathological findings has not been clarified. Therefore, this study aimed to investigate the relationship between the Kyoto score and pathological findings.
MATERIALS AND METHODS
Study design and subjects
This study was approved by the institutional review board at the Institute of Medical Science, University of Tokyo on September 21, 2013 (approval No. 25-34-0921). All participants provided written informed consent.
This cohort study consisted of participants who underwent esophagogastroduodenoscopy at Toyoshima Endoscopy Clinic from December 2013 to January 2016. Esophagogastroduodenoscopies were performed either for screening, evaluation of present symptoms, or surveillance of previous esophagogastroduodenal diseases. Inclusion criteria were as follows: Patients aged ≥ 20 years without history of gastric neoplasia, surgical gastrectomy, orH. pylorieradication. Exclusion criteria involved a withdrawal of concent.
Demographic characteristics including age, sex, body mass index, smoking history, habitual drinking, and first-degree family history of gastric cancer were obtained[21]. A score of at least 400 on the Brinkman index was defined as positive smoking history. Consumption of at least one alcoholic drink per day was defined as habitual drinking.
Endoscopy-based Kyoto classification score
Endoscopic Kyoto classification score for gastritis, from 0 to 8, is based on the total scores of the following five endoscopic findings: Atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. A high score represents increased risk for gastric cancer andH. pyloriinfection[12].
Pathological atrophy is defined as the loss of normal glandular tissue of the gastric mucosa. Endoscopic atrophy was classified according to the extent of mucosal atrophy, as described by Kimuraet al[22,23]. Non-atrophy and C1 were scored as Atrophy score 0, C2 and C3 as Atrophy score 1, and O1 to O3 as Atrophy score 2.
Pathological intestinal metaplasia is defined as a phenotypic change from the normal epithelial cell of the gastric mucosa to an intestinal phenotype. Endoscopically, intestinal metaplasia typically appears as grayish-white and slightly elevated plaques surrounded by mixed patchy pink and pale areas of the mucosa, forming an irregular uneven surface. Villous appearance, whitish mucosa, and rough mucosal surface are useful indicators for endoscopic diagnosis of intestinal metaplasia[24]. Intestinal metaplasia score 0 is defined as the absence of intestinal metaplasia; Intestinal metaplasia score 1 as the presence of intestinal metaplasia within the antrum; and Intestinal metaplasia score 2 as intestinal metaplasia extending into the corpus. The Intestinal metaplasia score is calculated based on the diagnosis using the white light imaging. Intestinal metaplasia diagnosis based on image-enhanced endoscopy and chromo-endoscopy is not included in the Intestinal metaplasia score.
负荷频率控制(Load Frequency Control,LFC)能够有效抑制系统频率偏差[2],使系统频率波动保持在电力工业允许的范围内。柴油发电机是参与负荷频率调节的主要电源,其对稳态功率需求具有明显的优势,而对于瞬时功率波动需求存在劣势。通过柴油机调频可以优化稳态功率波动,而储能装置可以对瞬时功率波动进行快速响应并优化系统频率[3-4]。但是,为了保证柴储混合电力系统的稳定运行,需要设计协调控制策略:对不同的频率管理装置进行协调配合,使得各装置能够充分发挥其频率管理的优势;考虑负荷需求功率的不确定性和储能输出功率的扰动性,设计鲁棒负荷频率控制器以提高系统的稳定性。
An enlarged fold is defined as a width of ≥ 5 mm that is not flattened or is only partially flattened by stomach insufflation. The absence and presence of enlarged folds were scored as Enlarged folds score 0 and 1, respectively.
Nodular gastritis is characterized by a miliary pattern resembling “goose flesh” mainly located in the antrum. The absence and presence of nodularity was scored as Nodularity score 0 and 1, respectively.
Diffuse redness refers to uniform redness with continuous expansion observed in non-atrophic mucosa mainly in the corpus[22]. Regular arrangement of collecting venules (RAC) is a condition where collecting venules are arranged in the corpus. From a distance, it appears like numerous dots; up close, it has the appearance of a regular pattern of starfish-like shapes. The absence of diffuse redness, presence of mild diffuse redness or diffuse redness with RAC, and severe diffuse redness or diffuse redness without RAC were scored as Diffuse redness score 0, 1, and 2, respectively.
Pathology (topographic distribution of neutrophil activity)
We obtained biopsy specimens from two sites, the greater curvature of the corpus and the antrum[25]. One experienced gastrointestinal pathologist diagnosed neutrophil activity score based on the updated Sydney System in hematoxylin and eosin staining. Neutrophil infiltration was graded on a scale of 0-3 (none, 0; mild, 1; moderate, 2; severe, 3).
Based on the topographic distribution of neutrophil infiltration, the patients were divided into four categories: “inactive stomach,” “antrum-predominant gastritis,” “pangastritis,” and “corpus-predominant gastritis.” When neutrophil activity was null for the antrum and the corpus, the diagnosis was “inactive stomach.” When the antrum score was larger than that of the corpus, the diagnosis was “antrumpredominant gastritis.” When neutrophil activity was positive, and the antrum score was equal to that of the corpus, the diagnosis was “pangastritis.” When the corpus score was larger than that of the antrum, the diagnosis was “corpus-predominant gastritis[11,26].”
The serum anti-H. pylori antibody
Patients’ blood samples were obtained on the day of esophagogastroduodenoscopy. The serum antibody titer was measured by an enzyme-linked immunoassay kit using antigens derived from Japanese individuals: E-plate EikenH. pyloriantibody II kit (Eiken Chemical, Tokyo, Japan). We defined a cut-off value of 10 U/mL was identified forH. pyloripositivity as the manufacturer recommended[13-15].
Statistical analysis
We tested the association between the Kyoto classification score, including Atrophy, Intestinal metaplasia, Enlarged folds, Nodularity, and Diffuse redness score, and the four categories of topographic distribution of neutrophil activity by Kruskal–Wallis and Steel–Dwass analysis. A multinomial logistic regression analysis was performed using the four categories of gastritis as objective variables. Sex, age, body mass index, drinking, smoking habit, first-degree family history of gastric cancer, serumH. pyloriantibody, and the Kyoto score were used as explanatory variables. APvalue of < 0.05 was defined as statistical significance. Calculations were carried out using the statistical software Ekuseru-Toukei 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
RESULTS
A total of 327 patients (comprising 50.7% women, with an average age of 50.2 years) were enrolled in this study.H. pyloriinfection rate was 82.9% with a mean Kyoto score of 4.63 (Table 1).
The Kyoto score was associated with the topographic distribution of neutrophil activity (P< 0.001 calculated by Kruskal–Wallis test). Kyoto scores were significantly higher in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (3.05, 4.57, 5.21, and 5.96, respectively; Figure 1). Table 2 shows that each individual score of endoscopic findings (i.e., atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness) was correlated with the topographic distribution of neutrophil activity. On multivariate analysis, the Kyoto score, age, and serumH. pyloriantibody were independently associated with the topographic distribution of neutrophil activity (Table 3).
DISCUSSION
Our study revealed that the Kyoto score was associated with the topographic distribution of neutrophil activity. Uemuraet al[11]reported the significance of gastritis topography, and relative risks of gastric cancer for pangastritis and corpus-predominant gastritis were 15.6 and 34.5, respectively, as compared with antrumpredominant gastritis. Namely, the risk of gastric cancer increased in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis. In this study, Kyoto score also increased in a similar order.
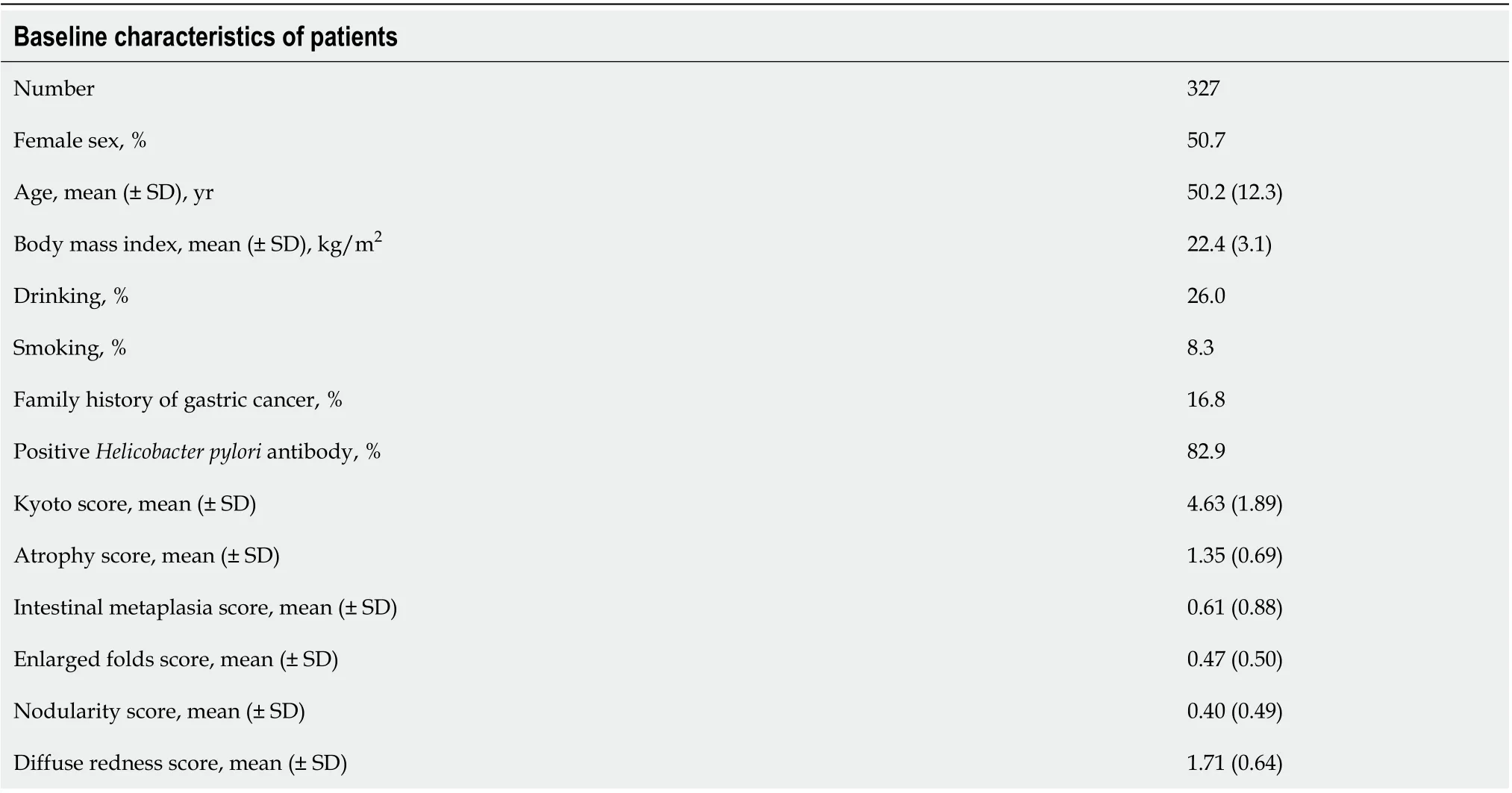
Table 1 Baseline characteristics of patients
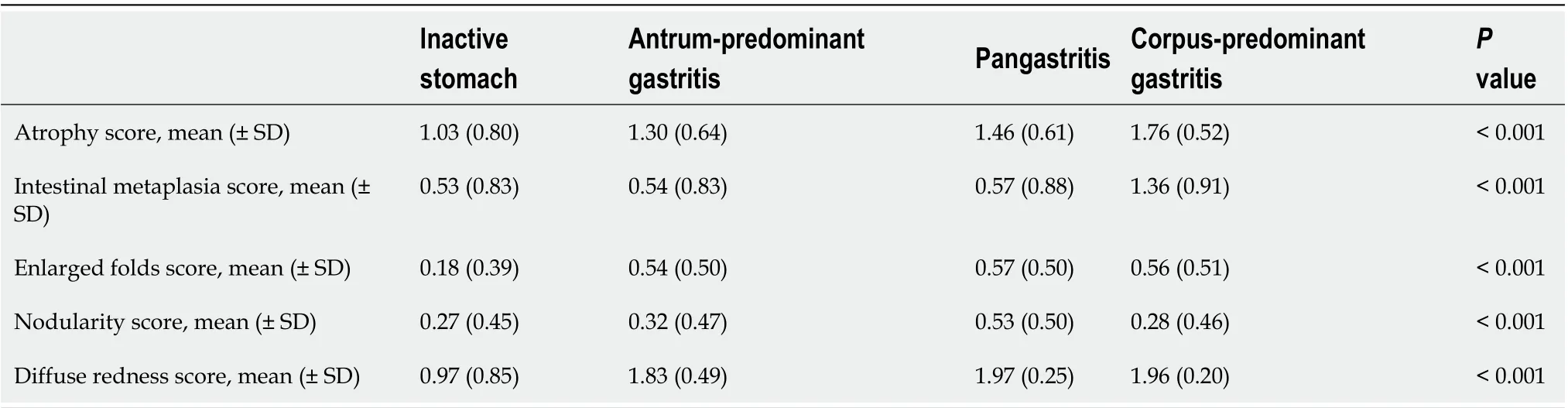
Table 2 Association between Kyoto classification score and the topographic distribution of neutrophil activity
Our data also showed that age, endoscopic atrophy score, and endoscopic intestinal metaplasia score increased in the order of pathologically antrum-predominant active gastritis, pangastritis, and corpus-predominant gastritis. Correa’s cascade inH. pyloriassociated gastritis follows the following consecutive steps: (1) Normal gastric mucosa; (2) Nonatrophic antrum-predominant active gastritis; (3) Atrophy; (4) Intestinal metaplasia; (5) Dysplasia; and (6) Cancer[27]. Atrophic gastritis progresses from the antrum to the corpus[22,23]. In the early phase of atrophic gastritis, neutrophils are mainly infiltrated in the antrum. This condition could correspond to antrumpredominant active gastritis. When atrophic gastritis progresses from the antrum to the corpus, neutrophil infiltration in antrum and corpus would be similar. This condition could correspond to pangastritis. Along with the atrophic gastritis progression, intestinal metaplasia occurs, especially in the antrum. Since intestinal metaplasia could be a harsh environment forH. pylori, topographic distribution ofH. pyloricould alter from the antrum to the corpus[28]. The density ofH. pyloriwas also correlated with neutrophil activity[10,29-31]. After the emergence of antral intestinal metaplasia, neutrophil activity decreases in the antrum, and the status could be categorized as corpus-predominant gastritis. Namely, pathologically active gastritis could progress in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (Figure 2). Imagawaet al[32]also suggested that gastritis topography changes in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis as age advances. In our study, endoscopic atrophy and intestinal metaplasia were associated with the development of pathologically active gastritis.
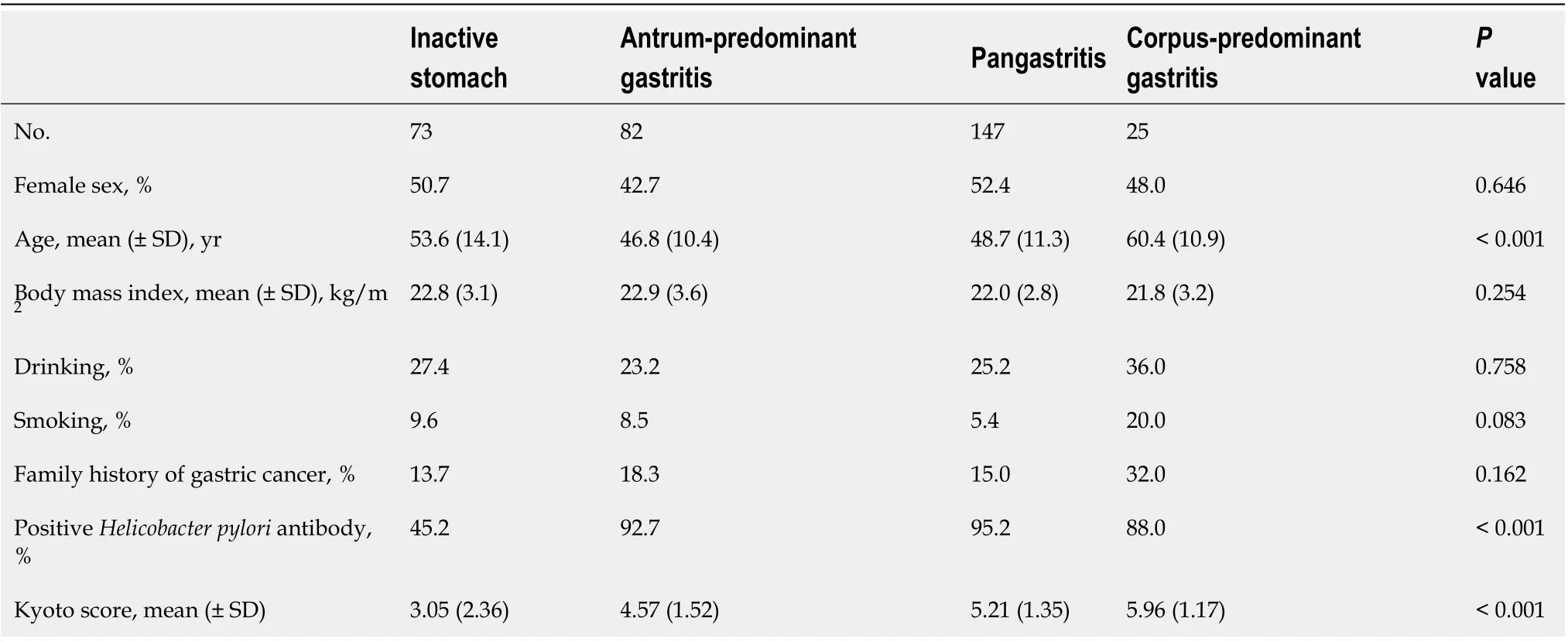
Table 3 Multivariate analysis for the topographic distribution of neutrophil activity
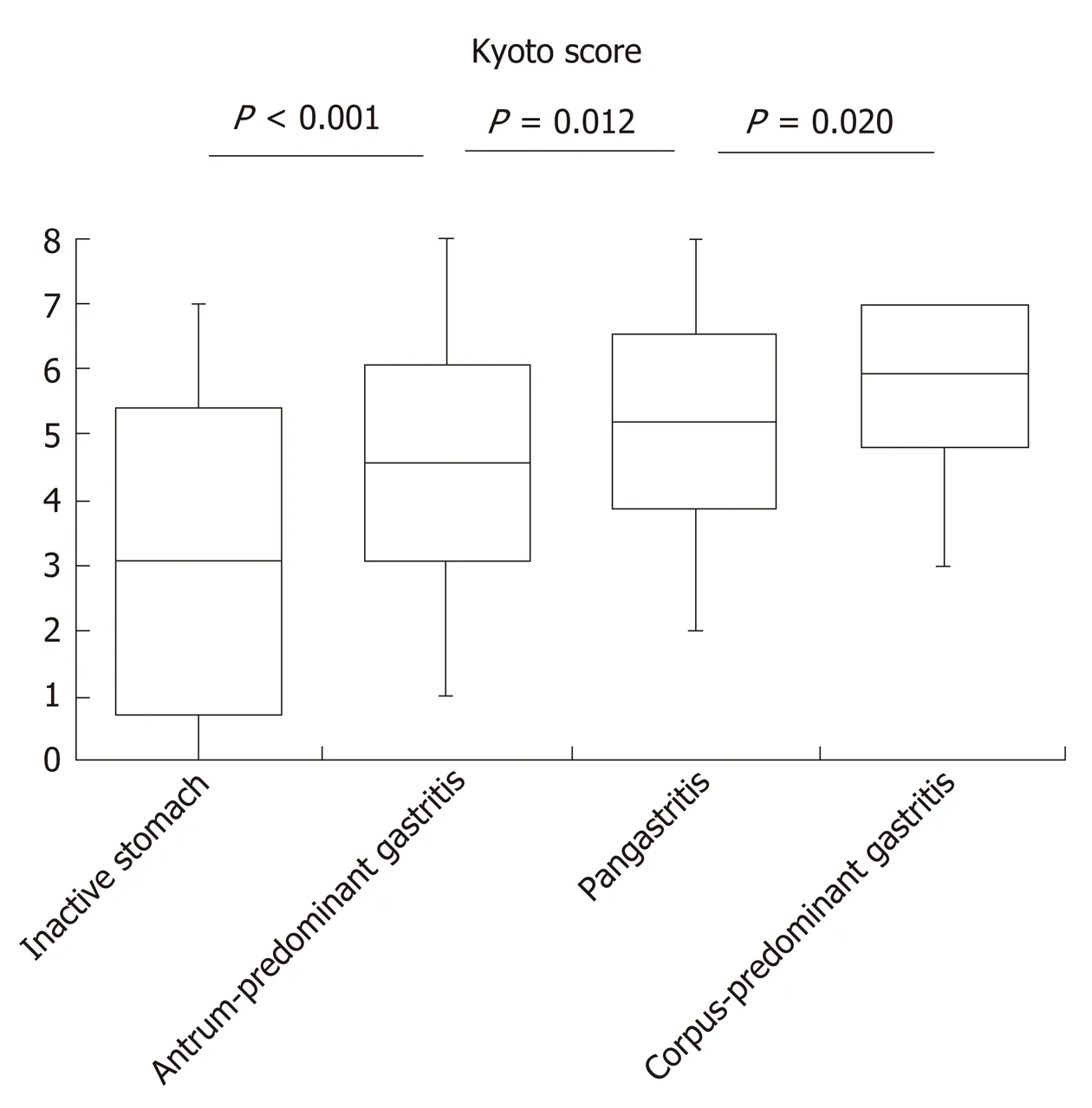
Figure 1 Kyoto score according to the topographic distribution of neutrophil activity. Box-plots depicting the average Kyoto score. P value was calculated using the Steel–Dwass test.
A number of studies have shown that pathological topography of neutrophil infiltration was correlated with gastric cancer risk[32]. Sakitaniet al[33]demonstrated that neutrophil infiltration in the corpus was a risk factor for gastric cancer, especially for the diffuse-type cancer. Matsuhisaet al[34]showed that amongH. pylori-positive patients, corpus-predominant gastritis was common in elderly Japanese and Chinese whose prevalence of gastric cancer were high, whereas antrum-predominant gastritis was prevalent in Thailand and Vietnam, which had low incidence of gastric cancer. We previously reported that corpus-predominant gastritis and pangastritis were associated with the risk allele ofProstate Stem Cell Antigengene, a gastric cancer-related single nucleotide polymorphism. The patients with corpus-predominant gastritis and the pangastritis had low expression ofProstate Stem Cell Antigenin the gastric mucosa, as did the stomach cancer patients did[26]. It has also been reported that the presence of neutrophil infiltration in the corpus is related to metachronous gastric cancer[35]and that activated neutrophils generate reactive oxygen and nitrogen species, which are mutagenic and carcinogenic[36,37]. Although gastritis topography for gastric cancer risk has ample evidence, Kyoto score for gastric cancer risk has not been fully assessed yet[18,38]. Thus, this study provides evidentiary support that Kyoto score is useful for assessing gastric cancer risk.
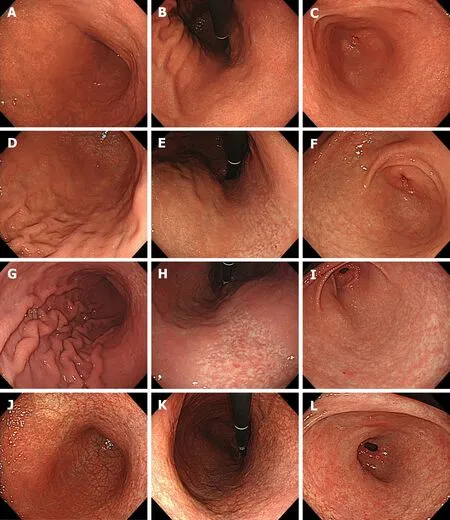
Figure 2 Representative images of four categories of gastritis. A-C: Inactive stomach. A 49-year-old woman. Kyoto score: 3; Atrophy score: 1; Intestinal metaplasia score: 0; Enlarged folds score: 0; Nodularity score: 0; Diffuse redness score: 2; D-F: Antrum-predominant gastritis. A 37-year-old man. Kyoto score: 4; Atrophy score: 1; Intestinal metaplasia score: 0; Enlarged folds score: 1; Nodularity score: 0; Diffuse redness score: 2; G-I: Pangastritis. A 45-year-old man. Kyoto score: 5; Atrophy score: 1; Intestinal metaplasia score: 0; Enlarged folds score: 1; Nodularity score: 1; Diffuse redness score: 2; J-L: Corpus-predominant gastritis. A 51-year-old woman. Kyoto score: 6; Atrophy score: 2; Intestinal metaplasia score: 2; Enlarged folds score: 0; Nodularity score: 0; Diffuse redness score: 2. Greater curvature of the corpus (A, D, G and J); Lesser curvature of the corpus (B, E, H and K); Antrum (C, F, I and L).
This study has several limitations. First, this was a retrospective, single-center study. Second, interobserver agreement and reproducibility of the Kyoto classification have not been demonstrated. Third, the category of inactive stomach included subjects who had not been infected withH. pyloriand those with spontaneous disappearance ofH. pylori. Since cancer risks of the two types of subjects are markedly different, it is advisable that these patients will be investigated separately in the future[39,40].
CONCLUSION
In conclusion, the Kyoto classification score was associated with topographic distribution of neutrophil activity.
ARTICLE HIGHLIGHTS
猜你喜欢
杂志排行
World Journal of Gastroenterology的其它文章
- Role of succinate dehydrogenase deficiency and oncometabolites in gastrointestinal stromal tumors
- Acupuncture improved lipid metabolism by regulating intestinal absorption in mice
- Experimental model standardizing polyvinyl alcohol hydrogel to simulate endoscopic ultrasound and endoscopic ultrasound-elastography
- Efficacy of pancreatoscopy for pancreatic duct stones: A systematic review and meta-analysis
- Construction of a convolutional neural network classifier developed by computed tomography images for pancreatic cancer diagnosis
- Mixed epithelial endocrine neoplasms of the colon and rectum – An evolution over time: A systematic review
