Golgi protein-73: A biomarker for assessing cirrhosis and prognosis of liver disease patients
2020-10-09NikolaosGatselisTamasTornaiZakeraShumsKalliopiZachouAsteriosSaitisStellaGabetaRogerAlbesaGaryNormanMariaPappGeorgeDalekos
Nikolaos K Gatselis, Tamas Tornai, Zakera Shums, Kalliopi Zachou, Asterios Saitis, Stella Gabeta, Roger Albesa, Gary L Norman, Maria Papp, George N Dalekos
Abstract
Key Words: Biomarker; Golgi protein-73; Hepatic fibrosis; Cirrhosis; Hepatocellular carcinoma; Hepatitis B; Hepatitis C; Aspartate aminotransferase/Platelets ratio index score
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-associated mortality and the fifth most common cancer globally. However, its early detection is difficult as confounding factors such as the coexistence of inflammation and cirrhosis, may lead to limited predictive values of the existing serological and radiological methods of surveillance[1]. Since the 1970s, alpha-fetoprotein (AFP) has been widely applied as a primary biomarker for HCC screening in cirrhotics. However, its value as a primary screening tool for HCC has been questioned due to its low sensitivity and specificity, especially for early HCC detection[1]. Therefore, novel serum biomarkers for assessing cirrhosis and HCC risk with higher diagnostic accuracy and reasonable costs are still urgently required[2,3].
In this context, Golgi protein-73 (GP73), also called Golgi phosphoprotein-2 (GOLPH2), was originally defined as a resident Golgi type II transmembrane protein expressed in epithelial cells[4]. The N-terminal region of GP73 contains a transmembrane domain that can be cleaved by a proprotein convertase, resulting in the release of GP73 into the extracellular space, which may then be detected in serum and utilized as a novel biomarker[5]. In normal human liver, GP73 expression was found primarily in biliary epithelial cells, with only slight detection in hepatocytes. However, in patients with acute or chronic liver diseases and especially in HCC, the expression of GP73 was significantly up-regulated in hepatocytes[4,5].
So far, there have been several studies examining serum GP73 as a tumor marker for HCC with conflicting results[6-9]. Moreover, few studies have assessed GP73 as a diagnostic marker for liver fibrosis[4,10-12]or as a prognostic factor of disease progression from chronic hepatitis to cirrhosis and subsequent development of liver decompensation and/or HCC development[8,10,13]. Therefore, we designed the present study in order to specifically assess the efficacy of GP73 as a potential diagnostic biomarker for detecting liver cirrhosis and HCC development, and also to evaluate its performance for predicting the progression of chronic liver disease by investigating a large cohort of 632 consecutive patients with chronic viral and non-viral liver diseases collected from two Academic centers in Greece and Hungary.
MATERIALS AND METHODS
Stored serum samples at -20oC from 632 consecutive Greek and Hungarian patients with chronic liver diseases who were prospectively diagnosed and followed at the Department of Medicine and Research Laboratory of Internal Medicine, National Expertise Center of Greece in Autoimmune Liver Diseases, General University Hospital of Larissa, Larissa, Greece (n= 366) and the Department of Internal Medicine, Division of Gastroenterology, University of Debrecen, Debrecen, Hungary (n= 266) were analyzed retrospectively at the time point of initial evaluation. Two hundred and three patients had chronic hepatitis B (CHB), 183 chronic hepatitis C (CHC), 198 alcoholic liver disease, 28 autoimmune cholestatic liver diseases [(26 primary biliary cholangitis (PBC) and 2 primary sclerosing cholangitis (PSC)], 15 autoimmune hepatitis (AIH), and 5 with other liver-related disorders. The baseline demographic, clinical, biochemical, and histological characteristics of the patients are shown in Table 1.
At baseline, 450 out of 632 patients (71.2%) had cirrhosis according to our previous published criteria[2,3,14-19]. Briefly, for patients without available biopsy, the diagnosis of cirrhosis was based on either ultrasonography (nodules in liver, spleen > 12 cm, portal vein > 16 mm), or elastography (liver stiffness > 12 kPa), or endoscopic findings of cirrhosis (varices, portal gastropathy), and/or clinical findings of decompensation (ascites, variceal bleeding, encephalopathy)[17-19]. HCC diagnosis was based on standard histological and/or radiological findings[1]. At initial evaluation, 226 out of 450 cirrhotic patients (50.2%) had already experienced at least one episode of decompensation in the past. Liver biopsy was available for 196 Greek patients and fibrosis was assessed using the METAVIR scoring system[20].
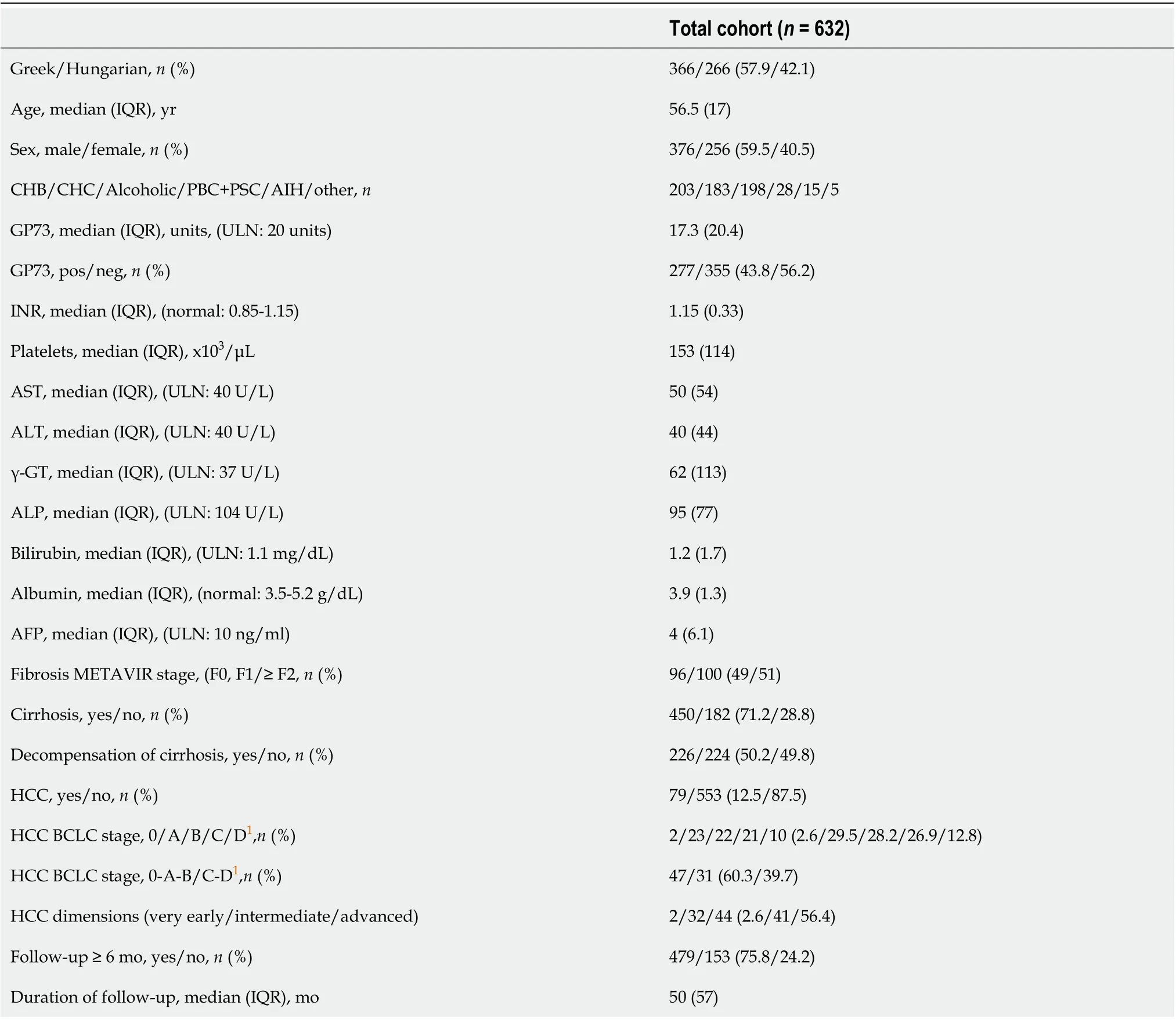
Table 1 Baseline demographic, biochemical, histological and clinical characteristics of patients
Four hundred and seventy-nine patients were followed ≥ 6 mo for a median [interquartile range (IQR)] 50 (57) mo (Figure 1). HCC surveillance was performed regularly with ultrasound imaging and AFP determination every 6 mo in cirrhotic and every 12 mo in non-cirrhotic patients. Development of cirrhosis, liver decompensation and HCC was assessed according to international criteria and in case of death, the cause was recorded[1,21]. Three hundred and twenty-six out of the 479 patients were cirrhotic at baseline, while 14 out of the remaining 153 non-cirrhotic patients developed cirrhosis during follow-up. One hundred and forty-two out of the 326 cirrhotic patients at baseline, had already experienced an event of decompensation prior to initial evaluation, while 66 out of 198 patients (patients with baseline cirrhosisn= 184 and patients who developed cirrhosis during follow-upn= 14) suffered from at least one episode of decompensation during follow-up for the first time (Figure 1).
In total, the diagnosis of HCC was established in 157 subjects; 79 were diagnosed at baseline and 78 during follow-up (Figure 1). Serum samples at the time of HCC diagnosis were available in 130 patients (79 at baseline and 51 at follow-up). Patients with available serum sample at the time of HCC diagnosis were classified according to the Barcelona Clinic Liver Cancer (BCLC) staging system as very early (stage 0,n= 8), early (stage A,n= 41), intermediate (stage B,n= 35), advanced (stage C,n= 34) or terminal stage (stage D,n= 11)[22]. They were also classified according to the tumor dimensions as very early (one single lesion < 2 cm in diameter,n= 8), intermediate (one single lesion 2-5 cm or less than three lesions with diameter < 3 cm each,n= 56), and advanced (n= 65). One patient could not be classified, as HCC diagnosis was established at autopsy. Paired samples for the estimation of GP73 alterations before and after the diagnosis of the tumor were available for 51 patients. Two hundred and twenty-two out of 632 patients (35.1%) died because of liver-related causes (65 patients during the first 6-mo from the initial presentation).
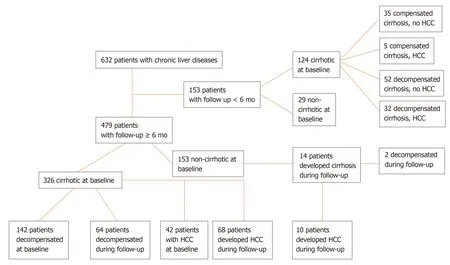
Figure 1 Flow-sheet of the 632 patients participated in the study and liver-related events (development of cirrhosis, liver decompensation, hepatocellular carcinoma development) observed during follow up.
The ethical committees of the two Universities Hospitals approved the protocol which conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected ina prioriapproval by the institution's human research committee. Patients agreed to the use of their data and serum samples for anonymous retrospective analysis by written consent at the time of initial evaluation. In case patients were not able to give consent, a first-degree relative authorized to give consent.
GP73 determination
GP73 antigen was measured using a novel GP73 ELISA (QUANTA Lite®GP73, Inova Diagnostics, Inc., Research Use Only) according to the manufacturer’s instructions. The assay was constructed using proprietary anti-GP73 monoclonal and rabbit polyclonal antibodies developed specifically against a highly purified proprietary fulllength GP73 antigen engineered to maintain glycosylation and conformational integrity. Arbitrary units were calculated using a low positive control as a calibrator. In brief, samples were diluted 1:101 in proprietary diluent and incubated for 1 h at room temperature. Wells were aspirated and washed 3 times with wash buffer followed by addition of 100 uL of horseradish peroxidase conjugated rabbit anti-GP73 antibody. Samples were incubated for 30 min, aspirated, and 100 uL of tetramethylbenzidine was then added. After 30 min of incubation the reaction was terminated by addition of 100 uL of stop solution and plates were then read at 450 nm. Arbitrary units were calculated using a low positive control as a calibrator. According to the manufacturer’s instructions, levels above 20 units were considered positive for GP73. This cut-off was established using a cohort of 518 healthy and disease controls (antiphospholipid syndrome,n= 25; rheumatoid arthritis,n= 55; scleroderma,n= 25; inflammatory bowel disease,n= 10; breast cancer,n= 46; colorectal cancer,n= 50; prostate cancer,n= 45; non-cirrhotic CHC,n= 14; non-cirrhotic CHB,n= 25; human immunodeficiency virus infection,n= 8; syphilis,n= 40, healthy controls,n= 175) along with 719 cirrhotic and 254 HCC patients (Supplementary Figure 1).
Biochemical parameters
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), bilirubin, albumin, platelets (PLT), international normalized ratio (INR), and AFP levels were determined using standard techniques. AST/PLT ratio index (APRI) was assessed at the relevant time points[23].
Statistical analysis
Normality of the distribution of variables was assessed by Kolmogorov-Smirnov test. Quantitative values are expressed as median (IQR). Data were analyzed by Mann-Whitney U,χ2, Kruskal-Wallis, Wilcoxon signed ranks, Spearman correlation and binary logistic regression analysis, where applicable. Significant interactions and multicollinearity between independent variables were examined using variance inflation factors. Logistic regression predicted value probability was used to calculate the combined effect of two diagnostic markers. Receiver operating characteristic (ROC) curves were constructed, and comparisons were done by the log-rank test. Youden indexes (sensitivity+specificity-1) were calculated to establish optimal cut-offs for GP73. Survival was evaluated with Kaplan-Meier plots for GP73-positive or GP73-negative patients up to the end of follow-up or when patients reached one of the endpoints (development of cirrhosis, liver decompensation, development of HCC, liverrelated death). Two-sidedPvalues less than 0.05 were considered statistically significant. The statistical review of the study was performed by NKG and KZ who have MSc in biostatistics. All data analyses were performed using the statistical software SPSS version 20.0.
RESULTS
Significance of serum GP73 in relation to the baseline characteristics of patients
GP73-positive levels (> 20 units) were found in 277/632 (43.8%) patients. GP73-positivity was significantly related to older age (P< 0.001), increased AST (P< 0.001), γ-GT (P< 0.001), ALP (P< 0.001), INR (P< 0.001), bilirubin (P< 0.001), and lower PLT (P< 0.001) and albumin (P< 0.001) (Table 2). Moreover, GP73 positivity correlated with higher AFP (P< 0.001) and APRI score (P< 0.001), advanced fibrosis (P< 0.001), presence of cirrhosis (P< 0.001), liver decompensation (P< 0.001), HCC presence (P< 0.001) and advanced HCC stage (P= 0.002) (Table 2).
Multivariate analysis revealed that GP73-positivity was independently associated with lower albumin (OR = 0.540, 95%CI: 0.375-0.778,P= 0.001) and presence of cirrhosis at baseline (OR = 7.608, 95%CI: 3.310-17.488;P< 0.001; Table 3). A second multivariate model including only cirrhotic patients, revealed that GP73-positivity was associated with decompensation of liver disease at baseline (OR = 1.812, 95%CI: 1.059-3.101;P= 0.030), but not to the presence of HCC (OR = 1.302, 95%CI: 0.687-2.467;P= 0.419; Table 4). No significant multicollinearity was found between the independent variables.
Of note, GP73 levels progressively increased from non-cirrhotic patients to patients with compensated cirrhosis and finally to patients with decompensated cirrhosis and/or HCC [8.4 (4.6)vs18.7 (15.3)vs29.5 (27.6) units;P< 0.001], as well as between the total number of cirrhotic patients (compensated and decompensated) and HCC patients [22.8 (22.9)vs25 (33.7) units;P= 0.06] (Figure 2). This pattern remained constant among the different groups of liver diseases analyzed (Supplementary Figure 2).
GP73 as a diagnostic marker of cirrhosis
GP73 levels were significantly higher in cirrhotics than in non-cirrhotics [23.3 (23.9)vs8.4 (4.6) units;P< 0.001]. GP73 had a high discriminative ability for detecting cirrhosis [AUC (95%CI): 0.909 (0.885-0.934)], which was superior to the APRI score [0.849 (0.813-0.886);P= 0.003]. The cut-off of 20 units had 59% sensitivity and 95% specificity for diagnosing cirrhosis (PPV: 96%, NPV: 48%). Youden index revealed an optimal cut-off for detecting cirrhosis at 12.7 units with sensitivity and specificity of 85% for both (PPV: 93%, NPV: 70%). The AUC (95%CI) of GP73 for detecting cirrhosis was 0.873 (0.838-0.908) for patients with viral liver diseases and 0.965 (0.929-1.000) for non-viral liver diseases. The AUC (95%) for the diagnosis of cirrhosis in a sub-analysis of patients with available liver biopsy (n= 196; F4 = 66; F3 = 34; F0-F2 = 96) was 0.850 (0.793-0.908) for GP73 and 0.806 (0.745-0.868) for APRI score (P= 0.276).
The combination of GP73 and APRI, further improved the diagnostic accuracy fordetecting cirrhosis [AUC (95%): 0.925 (0.903-0.948)], compared to the respective AUCs of GP73 (P= 0.005) or APRI alone (P< 0.001; Figure 3). In a minority of 118 patients (64 with CHB, 46 CHC, 1 AIH, 7 PBC) with available liver stiffness measurements at the same time point with serum samples, a positive correlation was found between liver stiffness and GP73 values (r= 0.433;P< 0.001).
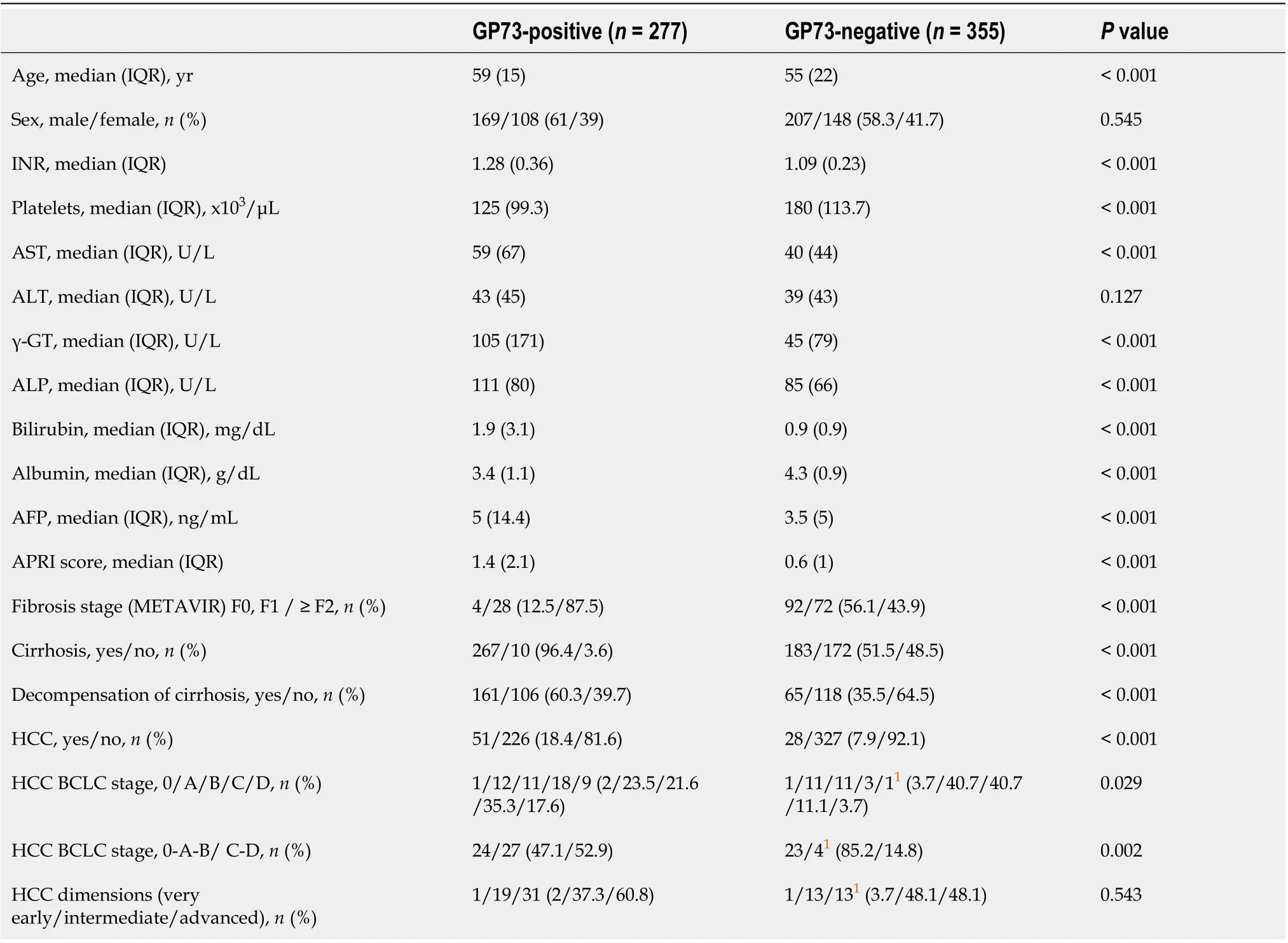
Table 2 Baseline demographic, clinical and laboratory characteristics of patients according to golgi protein 73positivity
GP73 as a diagnostic marker of hepatocellular carcinoma
One hundred and thirty patients had available samples at HCC diagnosis (79 from patients who were diagnosed at presentation and 51 from those who were diagnosed at follow-up visits). GP73 levels were higher in HCC patients compared to non-HCC (n= 502) [22.5 (29.2)vs16 (20.3) units;P< 0.001]. Furthermore, GP73 levels were positively associated with the Barcelona Clinic Liver Cancer (BCLC) stage (P< 0.001) and tumor dimensions (P= 0.004; Figure 4). HCC patients with underlying cirrhosis (n= 124) had also higher GP73 levels compared to HCC without cirrhosis (n= 6) [23.8 (29.9)vs15.5 (16.2) units;P= 0.08].
However, the discriminative ability of GP73 for detecting HCC was relatively low [AUC (95%CI): 0.623 (0.570-0.675)] and significantly lower than that of AFP [AUC (95%CI): 0.767 (0.714-0.821);P= 0.004]. The cut-off of 20 units for GP73 had a sensitivity of 56% and specificity of 59% for diagnosing HCC, with a PPV of 26% and NPV of 83%. According to Youden Index, the optimal cut-off of GP73 for diagnosing HCC was calculated at 12.7 units with a sensitivity of 84% and specificity of 40% (PPV: 27% and NPV: 90%).
The combination of GP73 and AFP did not improve the diagnostic accuracy for detecting HCC in the total population [AUC (95%CI): 0.759 (0.709-0.808)] (Supplementary Figure 3). Superiority of AFP compared to GP73 for detecting HCC remained stable after exclusion of non-cirrhotic patients [AUC (95%CI): 0.738 (0.678-0.798)vs0.500 (0.438-0.562);P< 0.001] and patients with advanced stages of HCC (BCLC stages B, C and D) [AUC (95%CI): 0.644 (0.544-0.744)vs0.376 (0.284-0.468);P< 0.001]. No significant difference was found in GP73 levels before [17.3 (15.6) units] and after HCC development [17.5 (20.7) units] in the 51 patients with available paired samples during follow-up (median: 53; range 8-163 mo; Supplementary Figure 4).
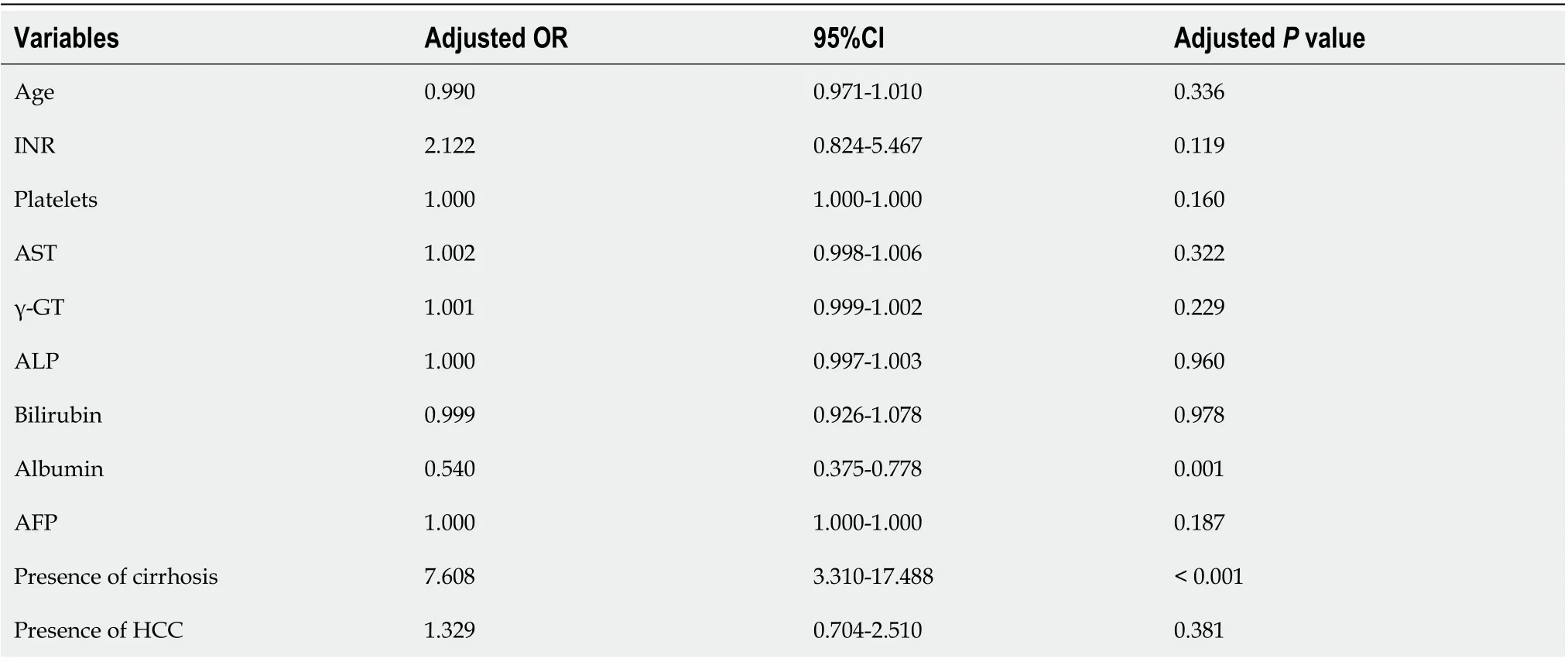
Table 3 Binary logistic regression analysis of baseline factors associated with golgi protein 73 positivity at initial evaluation of patients
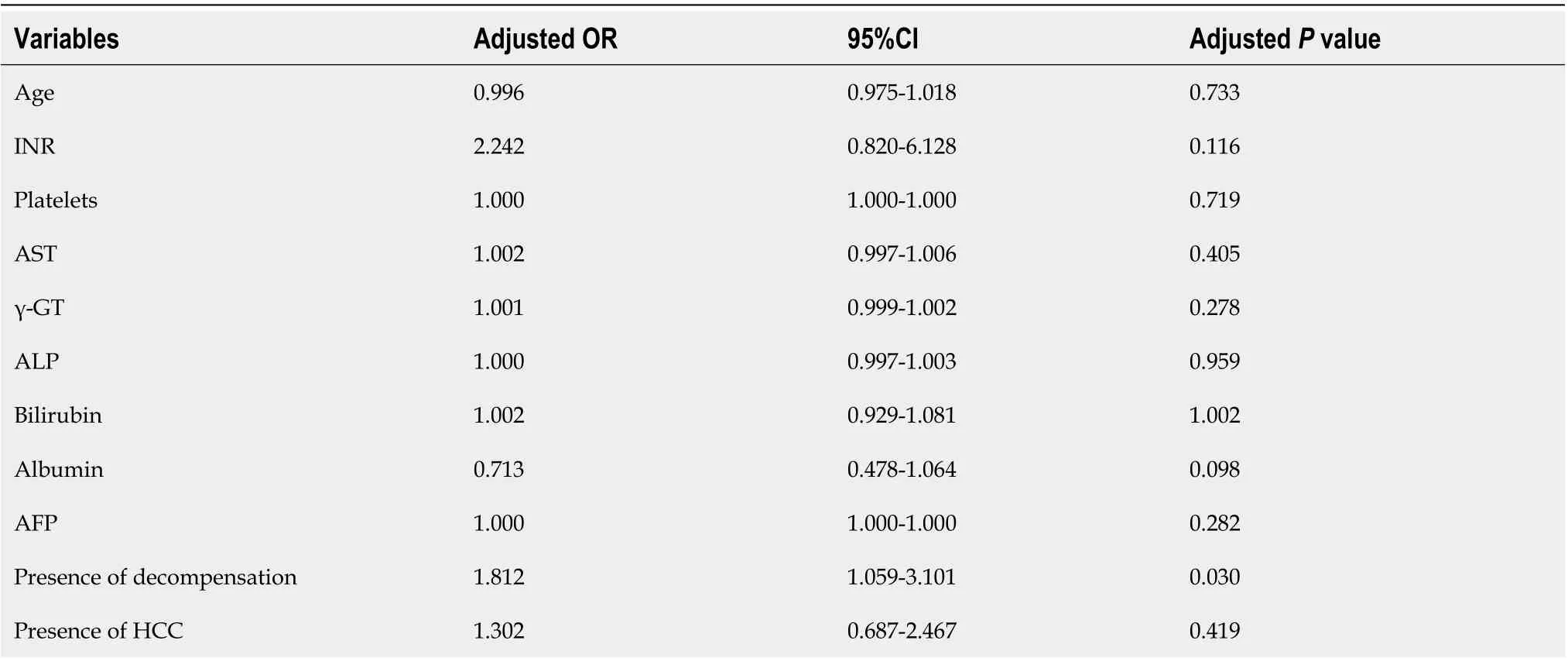
Table 4 Binary logistic regression analysis of baseline factors associated with golgi protein 73 positivity at initial evaluation of the cirrhotic patients
GP73 as a predictive marker of outcome
Kaplan-Meier analysis in patients with available follow-up ≥ 6 mo, showed no differences regarding the development of cirrhosis between GP73-positive and –negative patients (P= 0.432; Figure 5A). In contrast, GP73-positive patients with compensated cirrhosis at baseline and without any previous episode of decompensation had higher rates of liver decompensation (P= 0.036, Figure 5B) and HCC development (total group of patients;P= 0.08; Figure 5C). Of note, liver-related deaths were more common in GP73-positive patients (P< 0.001, Figure 5D) either in the subgroup with available long-term follow-up (≥ 6 mo), or in the group with followup of any duration (P< 0.001; data not shown).
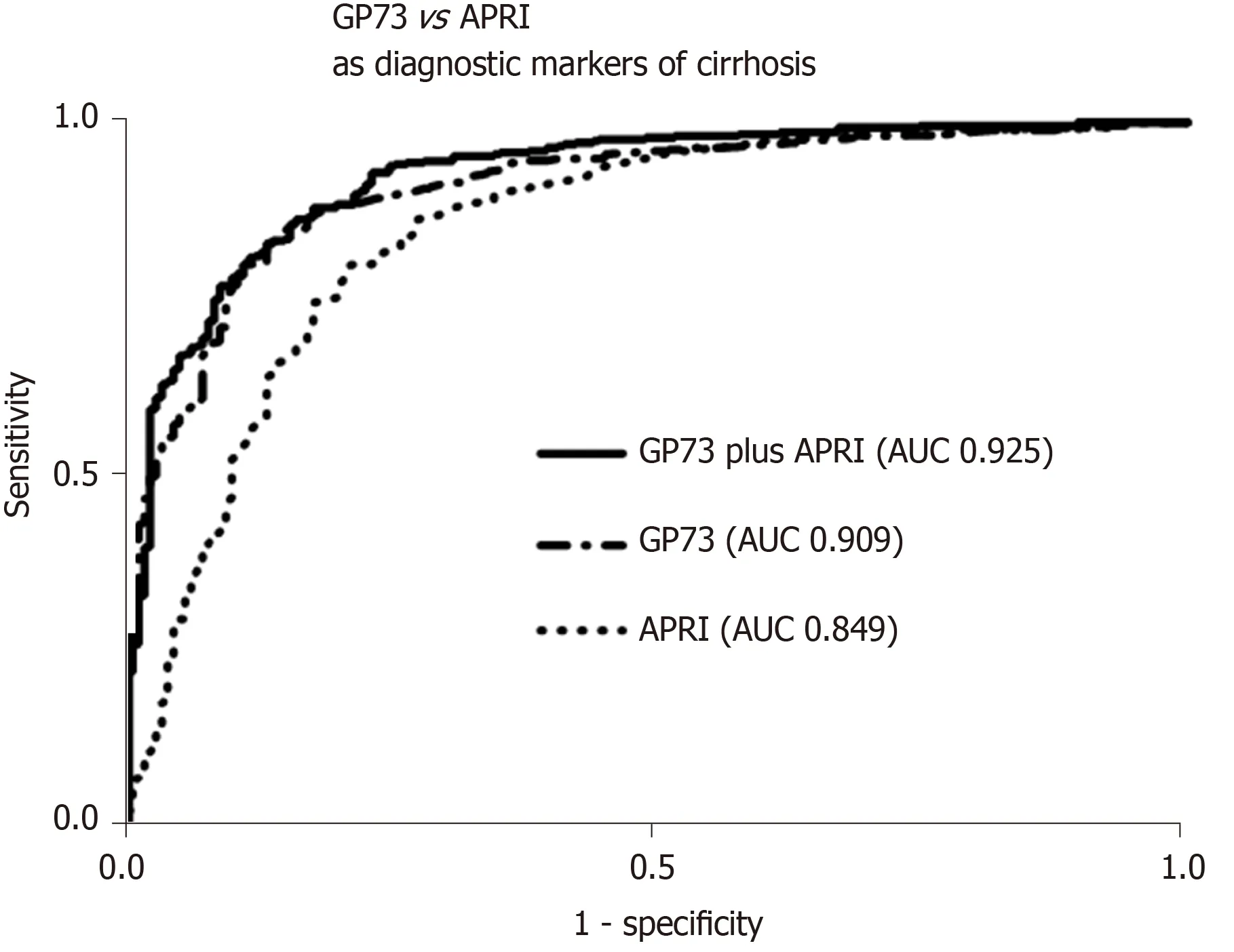
Figure 3 Golgi protein 73 vs aspartate aminotransferase to platelets index as diagnostic markers of cirrhosis. Receiver operating characteristic curves for the prediction of cirrhosis according to Golgi protein 73 (GP73) levels and aspartate aminotransferase to platelets index (APRI) score. Combination of GP73 and APRI had a higher diagnostic accuracy for cirrhosis), compared to GP73 (P = 0.005) or APRI score alone (P < 0.001). GP73: Golgi protein 73; APRI: Aspartate aminotransferase to platelets index; AUC: Area under the curve.
DISCUSSION
In the current study, we showed that GP73-positivity is strongly associated with the presence of cirrhosis, while increased GP73 appear to identify patients at high risk of disease progression to liver decompensation, HCC development, and liver-related mortality. Importantly, multivariate analysis revealed that GP73-positivity was independently associated only with the presence of cirrhosis irrespective of the levels of aminotransferases. Furthermore, the ROC curves confirmed the high diagnostic accuracy of GP73 for detecting cirrhosis and its superiority to the APRI score. In addition, GP73 was associated with the presence of HCC, BCLC stage, and tumor dimensions, although its diagnostic accuracy for HCC was lower than that of AFP.
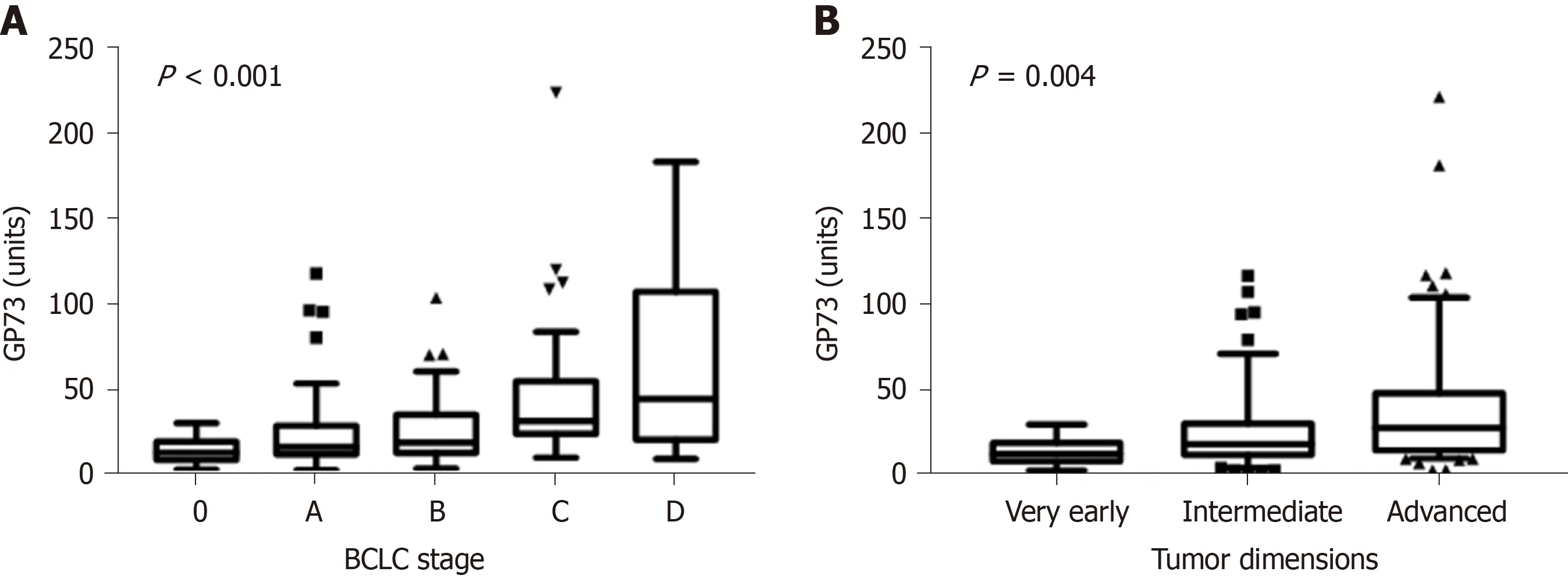
Figure 4 Golgi protein values distribution among hepatocellular carcinoma stages according to. A: BCLC staging system [stage 0, 13.9 (10.8) units; stage A, 17.1 (16.8) units; stage B, 19.6 (22.3) units; stage C, 32.2 (30.8) units; stage D, 45.3 (86.6) units; P < 0.001]. B: Tumor dimensions [very early, 13.9 (10.8) units; intermediate, 19.6 (18.4) units; advanced, 29.1 (33.6) units; P = 0.004] (Tukey box-and-whisker plots). GP73: Golgi protein 73; BCLC: Barcelona clinic liver cancer.
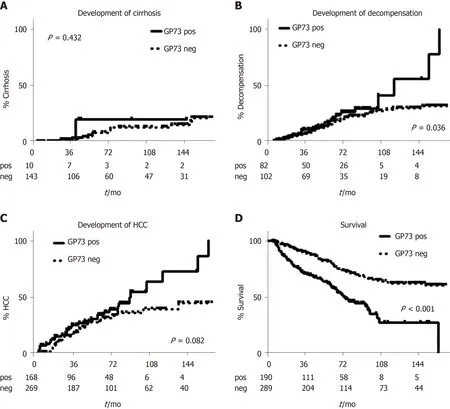
Figure 5 Kaplan-Meier analysis of patients according to Golgi protein 73 detection. Only 14 patients (one GP73 positive and 13 GP73 negative) developed cirrhosis during follow-up A. GP73 positive patients were characterized by higher decompensation B, HCC development C and liver-related mortality rates D. GP73: Golgi protein 73 detection; HCC: hepatocellular carcinoma.
GP73 is a 73-kDa transmembrane glycoprotein that resides in epithelial cells of many human tissues, within the cis-Golgi complex[4,5]. Wrightet al[24]described significant hepatocellular injury, including microvesicular hepatic steatosis, hepatocyte nuclear membrane irregularities and intranuclear inclusions in a transgenic mice model of GP73 deficiency, indicating that GP73 is essential for normal survival. Recent knock-down genetic studies in HepG2.2.15 cell lines provide further support as they proved that GP73 inhibition is related to a reduction of the inside surface area of the Golgi complex[25,26]. Additionally, previous reports showed that GP73 promotes HCC cell migration[27]and invasion[28], while GP73 N-glycosylation reduces HCC cell functions like motility and invasiveness[29,30], suggesting GP73 as a potential target of novel therapies for the inhibition of tumor proliferation and metastasis.
Our finding of a strong association of GP73-positivity with cirrhosis is in line with the initial manufacturer’s performance data which showed a sensitivity of 50.2% with a high specificity (96.9%) and also with previous studies which showed that GP73 is not overexpressed only in hepatocytes and liver cancer cells, but also in activated hepatic stellate cells which are the primary drivers of hepatic fibrosis[4,31,32]. In addition, previous studies have shown greater GP73 concentrations in cirrhosis rather than in HCC[33]. Our results provide strong support indicating that GP73 could be used as a tool to evaluate the presence of cirrhosis irrespective of the etiology of liver disease and very importantly, in contrast to other complex and more expensive models for staging fibrosis such as FibroMeter®, FibroTest®and HepaScore®[34], it is a single marker which can be rapidly determined by a simple ELISA, using equipment found in every clinical laboratory worldwide. Moreover, GP73 could also be combined with other novel"easy-to-use"markers of fibrosis like cartilage oligomeric matrix protein (COMP) already published by our group[2,3,35]. Indeed, in a subgroup of 288 patients with available measurements of both GP73 and COMP, the combination of GP73 with COMP had the higher discriminative ability to detect cirrhosis [AUC (95%CI): 0.881 (0.840-0.922)] compared to GP73 [AUC (95%CI): 0.846 (0.799-0.893)], APRI [AUC (95%CI): 0.834 (0.788-0.880) or COMP [AUC (95%CI): 0.767 (0.710-0.823)] alone, while the addition of APRI did not change the diagnostic performance of detecting cirrhosis [GP73 + COMP + APRI AUC (95%CI): 0.886 (0.846-0.926); Dalekos, Gatselis data in preparation].
Recently, Caoet al[11,12]found a gradual increase of GP73 through liver fibrosis stages and proposed that serum GP73 might be a complementary tool to transient elastography in an attempt to estimate significant fibrosis in CHB patients. In this context, a proposed algorithm including GP73 values and liver stiffness has been recently suggested to have better diagnostic accuracy than currently available approaches[12]. Our study verified a positive correlation between liver stiffness and GP73 values in a subset of patients with available measurements, although the retrospective nature of the study did not allow us for further statistical analysis due to the low number of patients with simultaneous elastography determinations and liver biopsy. Our findings of a gradual increase of GP73 levels from chronic hepatitis to cirrhosis and finally to decompensation of liver disease along with the predictive ability of GP73-positivity for a worse outcome, suggest that GP73 might be implicated in a central cellular pathway of liver diseases and not only in HCC development.
Indeed, recently Yaoet al[36]found that GP73 can be induced by interleukin-6 through the STAT3 signal pathway, implicating a positive role of GP73 in liver regeneration and that enhanced GP73 expression may reflect the proliferative potential for hepatic parenchymal cells. In line with our findings, Xuet al[10]demonstrated that changes in serum GP73 levels were closely associated with changes in liver injury severity, and therefore, GP73 may be an effective biomarker for the evaluation of disease progression. Further support of the role of GP73 as an important promoter of HCC progression was just published by Weiet al[37], where they suggest that increased serum GP73 contributes to HCC development in a mouse model. The induction of GP73 expression in cell lines transfected by adenovirus, HBV and HCV, and a highlevel of GP73 expression in a variety of acute and chronic liver diseases has also been described[4,29,32,38]. Saiet al[39]showed a shorter survival in HCC patients with higher GP73 levels than that of patients with low GP73 expression and proposed that an increased GP73 level in liver cancer cells might be associated with cell survival by protecting them from apoptosis. Furthermore, other studies have evaluated the utility of GP73 for monitoring the efficacy of several therapeutic procedures in HCC or disease recurrence such as, the radiofrequency ablation, surgical resection or transcatheter arterial chemoembolization[7,40,41]. Indeed, further research is needed to define the diagnostic value of GP73 in cirrhosis, including also patients with nonalcoholic fatty liver disease.
In our patients, we failed to detect any advantage of GP73 in HCC diagnosis over AFP, even when both markers were combined. Moreover, we did not manage to detect any benefit regarding the efficacy of GP73 at early detection of HCC, although GP73 levels were positively associated with BCLC stage and the tumor dimensions. Our findings are in accordance with a recent study from China which showed that AFP remained the best biomarker for the very early diagnosis of HBV-associated HCC, and the combination of one or more markers including GP73 did not significantly improve the diagnostic accuracy for HCC[42]. In this context, multiple studies have investigated the utility of GP73 for detecting HCC with conflicting results[13,33,43-47]. A recent metaanalysis reviewed the high heterogeneity of the studies with opposing or ambiguous results[9]. Of interest, Liuet al[48]demonstrated that serum GP73 increased in cirrhotic HCC patients, but not in those without cirrhosis, suggesting that the underlying cirrhosis, but not HCCperse, was related to the GP73 upregulation. Further support to this assumption raised from another study which showed that serum GP73 did not change after tumor resection in contrast to AFP levels[49]. In support of these findings, we failed to detect any difference before and after HCC development, while GP73 levels in our HCC patients were higher in cirrhotic HCC patients compared to noncirrhotic. Furthermore, other studies found elevated GP73 levels or protein expression in liver tumor samples from patients with benign liver lesions such as, hepatocellular adenoma, focal nodal hyperplasia and hepatic cysts[7,8]. Taken together, these findings indicate that while GP73 is unlikely to be useful as a specific marker for HCC diagnosis, it may be of significant value to identify patients with cirrhosis and those potentially progressing towards HCC.
It is likely that technical issues contribute significantly to the conflicting results reported by various studies, as different detection methods and importantly, different antigens and monoclonal reagents have been used in various studies. Methodological approaches followed in each study may also differ, especially the patient inclusion criteria. Since our results suggest the importance of GP73 in disease progression, differences in the time of specimen collection in relation to the patient’s disease course may also impact the strength of the observed results. Importantly, many studies used healthy subjects as the control group, rather than patients with chronic hepatitis or liver cirrhosis. Discordances between studies may also be due to variant differentiation of HCC cells with different capacity to produce and secrete GP73 protein. In addition, different regulatory mechanisms of GP73 between benign and malignant liver diseases may be implicated[13]. Sunet al[6]showed that only tissue GP73 protein levels and not serum levels were positively associated with aggressive behaviour of HCC, while Wanget al[40]and Maoet al[7]found that serum levels of GP73 in HCC patients were not consistently influenced by tumor size and differentiation. On the other hand, Shanet al[13]found that both tissue and serum GP73 levels in HCC patients were lower than that in patients with cirrhosis and negatively associated with tumor size and tumornode-metastasis stage, while tissue GP73 levels were positively correlated with tumor differentiation. In addition, they observed that serum GP73 levels contribute little to the prognosis of HCC, because the majority of HCC patients are already cirrhotic, resulting in simultaneous secretion of GP73 from both tumorous and cirrhotic tissue.
CONCLUSION
In conclusion, although GP73 does not appear to be a specific marker of HCC, its determination by a low cost, easy to perform, non-invasive blood test method that can be done in any clinical laboratory, appears to offer better diagnostic accuracy than the APRI score for detecting liver cirrhosis in patients with both viral and non-viral chronic liver diseases. Unlike APRI, GP73 is not affected by transaminase levels and very importantly, unlike transient elastography, the assay needs no specialized equipment. This is a critically important point as special equipment may not be available in regions where testing is most needed. Of note, GP73 in combination with APRI score further improved the diagnostic accuracy for diagnosing cirrhosis compared to GP73 or APRI alone. Most importantly however, GP73 proved efficient to predict a worse outcome of patients with chronic liver diseases as attested by the increased risk of progression to decompensated liver disease, HCC development and liver-related mortality. While our results, indicate a potential role of GP73 testing in patient management, future large-scale studies with prospectively collected serum samples will be needed to validate these findings.
ARTICLE HIGHLIGHTS
杂志排行
World Journal of Gastroenterology的其它文章
- Role of succinate dehydrogenase deficiency and oncometabolites in gastrointestinal stromal tumors
- Acupuncture improved lipid metabolism by regulating intestinal absorption in mice
- Experimental model standardizing polyvinyl alcohol hydrogel to simulate endoscopic ultrasound and endoscopic ultrasound-elastography
- Efficacy of pancreatoscopy for pancreatic duct stones: A systematic review and meta-analysis
- Construction of a convolutional neural network classifier developed by computed tomography images for pancreatic cancer diagnosis
- Mixed epithelial endocrine neoplasms of the colon and rectum – An evolution over time: A systematic review
