Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome: A comprehensive review
2020-10-09RiccardoInchingoloAlessandroPosaMartinMariappanTiagoKojunTibanaThiagoFranchiNunesStavrosSpiliopoulosEliasBrountzos
Riccardo Inchingolo, Alessandro Posa, Martin Mariappan, Tiago Kojun Tibana, Thiago Franchi Nunes, Stavros Spiliopoulos, Elias Brountzos
Abstract Budd-Chiari syndrome (BCS) is a relatively rare clinical condition with a wide range of symptomatology, caused by the obstruction of the hepatic venous outflow. If left untreated, it has got an high mortality rate. Its management is based on a step-wise approach, depending on the clinical presentation, and includes different treatment from anticoagulation therapy up to Interventional Radiology techniques, such as transjugular intrahepatic portosystemic shunt (TIPS). TIPS is today considered a safe and highly effective treatment and should be recommended for BCS patients, including those awaiting orthotopic liver transplantation. In this review the pathophysiology, diagnosis and treatment options of BCS are presented, with a special focus on published data regarding the techniques and outcomes of TIPS for the treatment of BCS. Moreover, unresolved issues and future research will be discussed.
Key Words: Budd-Chiari syndrome; Liver; Transjugular intrahepatic portosystemic shunt; Orthotopic liver transplantation; Interventional radiology; Portal hypertension
INTRODUCTION
Budd-Chiari syndrome (BCS), first described by the British physician George Budd in 1845 and the Austrian pathologist Hans Chiari in 1898, is a relatively rare clinical condition with a wide range of symptomatology, caused by the obstruction of the hepatic venous outflow, involving small hepatic venules, larger hepatic veins, the entire inferior vein cava (IVC) or all of above[1-3]. Based on the nature of obstruction, BCS is classified as primary if the obstruction is attributed to venous pathology such as thrombosis, webs, or endophlebitis and secondary in cases of extraluminal compression such as tumours, abscess, cysts, and pericardial conditions[4,5]. Based on the anatomical location of the obstruction, primary BCS is classified as “classical BCS” if the obstruction involves the hepatic veins, usually presenting with more acute and severe symptomatology and the “hepatic vena cava BCS” if the intra- and/or suprahepatic portion of the IVC is obstructed, presenting with chronic evolution and more favorable prognosis[6]. The mean, age-standardized, incidence and prevalence rates of BCS has been estimated to be 0.8 per million per year and 1.4 per million inhabitants, respectively in Sweden, and 0.2 per million and 2.4 per million inhabitants respectively in Japan[7,8]. Although the incidence of BCS is consistent throughout the European countries, it varies significantly among the Asian populations[9]. Classical BCS is the most common type of primary BCS in the western countries, whereas hepatic vena cava BCS is more frequent within the East Asian population[10].
If left untreated, the natural course of the disease is extremely unfavorable with a mortality rate of 50% in 2 years, while the 3-year survival rate of untreated patients is < 10%, as the venous outflow obstruction leads to hepatic congestion and fulminant fibrosis, typically within 3 mo[11].
Management should be based on a step-by-step approach, with regard to clinical presentation, time of thrombosis and liver function reserve and includes anticoagulation therapy, orthotopic liver transplant (OLT), surgical shunts and percutaneous Interventional Radiology techniques, such as catheter-directed local thrombolysis combined with angioplasty and transjugular intrahepatic portosystemic shunt (TIPS)[12]. In the latter, shunt creation between the systemic and portal circulation leads to a reduction of portal vein pressure and therefore splanchnic congestion, allowing for a retrograde perfusion of the sinusoids of the periportal zone 1 and 2 of the liver acinus. As a result the hypoxic damage of the hepatocytes is reduced allowing the recovery of hepatic histology and function[13]. This review summarizes the clinical and pathophysiological implications of BCS, focuses on the available data regarding the safety and efficacy of TIPS for the treatment of BCS and discusses unresolved issues and future perspectives.
PATHOPHYSIOLOGY
BCS develops from a spectrum of diseases determining hepatic venous outflow obstruction, both thrombotic and non-thrombotic. The obstruction can occur at every point in the hepatic drainage system, from the small intrahepatic veins to the larger hepatic veins to the junction to inferior vena cava and right atrium. Usually, at least two hepatic veins must be obstructed for the disease to be clinically detectable. The obstruction of the hepatic veins results in increase of hepatic sinusoidal pressure, sinusoid dilation, and filtration of interstitial fluid, which leads to ascites; in addition, there is increase in the intrahepatic resistances and, therefore, decrease in portal venous flow, leading to hypoxic damage of hepatocytes[14].
Diseases causing thrombotic hepatic venous outflow obstruction (primary BCS) include: Hypercoagulability disorders (Figure 1) (factor V Leiden mutation[15,16], protein C or S and antithrombin-III deficiency), infections/sepsis, oral contraceptive therapy[17-19], pregnancy and post-partum, chronic inflammatory or autoimmune diseases (antiphospholipid syndrome, Behçet, systemic erythematous lupus), myeloproliferative disorders (polycythemia vera, essential thrombocythemia, myelofibrosis, and paroxysmal nocturnal hemoglobinuria[20,21]), dehydration, chemoradiotherapy, sickle cell disease, paraneoplastic syndromes or neoplastic thrombosis, leiomyosarcoma of the inferior vena cava, complication of liver transplantation[22,23], and total parenteral nutrition[24].
External, ab-estrinseco compression of hepatic veins (secondary BCS) is present in 25% of patients, with various etiology, ranging from neoplasm to pregnancy to hydatid cyst.
Membranous occlusion of the vena cava or hepatic veins, sustained by endoluminal fibrous webs, is a rare cause of BCS, more common in the Asian population, and can be both congenital and derived from a previous venous thrombosis[25-28].
Idiopathic causes account for 20%-30% 5 of cases of BCS and, among these, up to 87% can have occult myeloproliferative diseases[29].
Hepatic vein thrombosis may be associated with concurrent portal vein thrombosis (10%-20% of cases)[30], underlining a prothrombotic state of the patient.
CLINICAL DIAGNOSIS
The diagnostic work-up for BCS is based on physical examination, which shows a cohort of signs and symptoms, associated with altered laboratory exams and suggestive imaging.
The typical and most common form of clinical presentation of BCS is the chronic one, with a slow-onset pain in the right upper abdomen, jaundice (not always present in chronic form), hepatosplenomegaly, progressive abdominal swelling/stretching (due to ascites), haematemesis (due to esophageal varices caused by portal hypertension); 50% of patients can manifest renal impairment[31-33]. This form is usually caused by fibrosis of intraparenchymal veins, mostly due to chronic inflammation. The blockage of hepatic veins causes liver damage and can lead to cirrhosis and, on longterm, to hepatocellular carcinoma development; therefore, these serum alphafetoprotein levels must be monitored in these patients[34].
Acute/subacute/fulminant forms are also described, although less common, with rapid development of abdominal pain, ascites, hepatomegaly, jaundice, and renal failure; the fulminant form is characterized by development of hepatic encephalopathy within 8 wk from the onset of jaundice. These forms are caused by acute occlusion of the hepatic veins, mostly due to thrombosis, and there is no time to for the body to develop collateral venous channels[17,35]. Up to 15% of patients can be asymptomatic[36].
Laboratory diagnosis
Laboratory exams could be suggestive of liver damage, with abnormally high values of hepatic enzymes as transaminases (ALT and AST); serum bilirubin and alkaline phosphatase levels can also increase.
In addition, ascitic fluid examination can help provides useful clues to the diagnosis of Budd-Chiari syndrome, including the following: High protein concentrations (> 2 g/dL), although; it may not be seen in patients with acute disease. White blood cell (WBC) count usually < 500 g/µL. Serum/ascites albumin gradient > 1.1 g/dL, although it may not be seen in patients with acute disease[25].
Imaging
Diagnostic imaging plays a crucial role in diagnosis of BCS[37,38]. Ultrasound (US) examination and US-Doppler evaluation play a fundamental role in diagnosis, allowing hepatic vein obstruction assessment - usually with no flow signal inside -, intrahepatic and subcapsular collaterals visualization, and portal vein flow inversion (from hepatopetal to hepatofugal)[39,40].
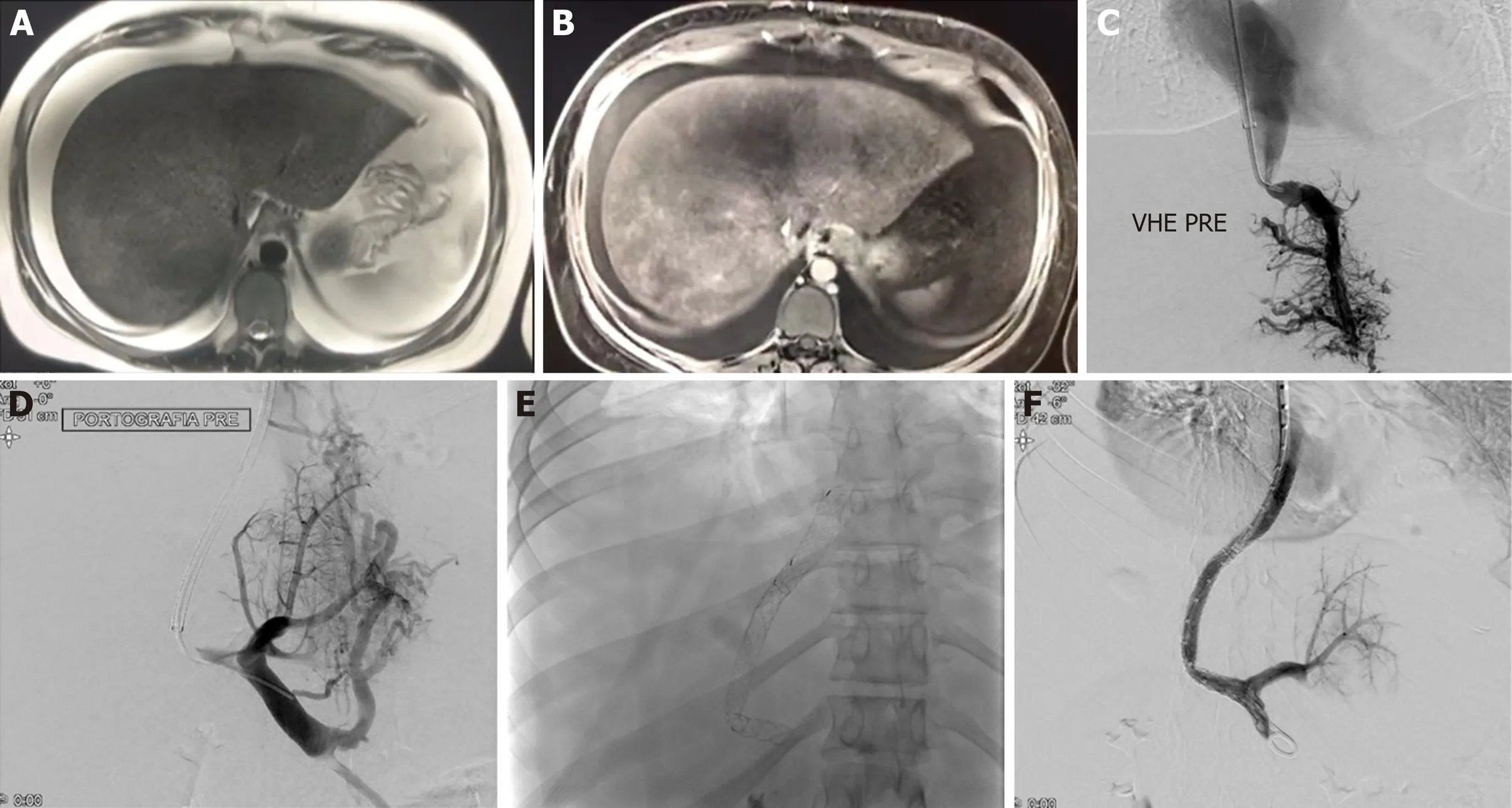
Figure 1 Twenty-nine years old girl with Budd-Chiari syndrome and leiden factor V positive for heterozygote. A: Gadolinium-enhanced T1-weighted magnetic resonance image (MRI) obtained during arterial phase. Hyperintense structures represent portal venules, which are visible because of postsinusoidal portal hypertension; B: T2-weighted MRI shows hyperintensity ascites and splenomegaly; C: Using a Rösch-Uchida transjugular liver access set, a small collateral hepatic vein branch was accessed, the portal vein was punctured (D), and wire access into the superior mesenteric vein was achieved. A 10-mm diameter, 6-cm long Viatorr stent (W. L. Gore and Associates, Flagstaff, AZ, United States) was deployed, extending from the right portal vein to the inferior vena cava (E, F).
Computed tomography (CT) and magnetic resonance imaging (MRI) are secondlevel imaging modalities that can show the cause of the obstruction of hepatic vein flow, as well as helping assess the activation of the portal venous collateral system, and evaluate liver vascular anatomy for the planning of a TIPS. Compensatory hypertrophy of the caudate lobe of the liver is frequently seen, due to its autonomous drainage in the inferior vena cava, and can lead to inferior vena cava compression.
Hepatic venography is mandatory to assess hepatic veins anatomy, the extent of thrombosis and, most important, to measure venous pressure and gradients. Hepatic venography in BCS usually shows a “spider-web” pattern of hepatic veins.
Percutaneous liver biopsy can be useful in determining the cause of the obstruction: pathologic findings are represented by high-grade venous congestion with sinusoidal dilation, centrilobular liver cell atrophy and fibrosis, and thrombosis of the terminal hepatic venules.
Differential diagnosis
BCS must not be mistaken with toxin-induced venous-occlusive disease (VOD) or sinusoidal obstruction syndrome (SOS), usually seen in patients undergoing high-dose chemotherapy before bone marrow transplantation: in fact, SOS is characterized by sinusoidal and small hepatic veins’ narrowing and occlusion, due to endothelial damage and necrosis, predominantly occurring as a complication of high doses of chemotherapy with alkylating agents for hematopoietic stem cell transplantation; ultrasound examination plays a critical role in the differential diagnosis, as the large hepatic veins are patent in SOS, whereas must be occluded on BCS[41].
CONSERVATIVE TREATMENT AND TRANSPLANT
The management of BCS depends on the severity of disease. Nowadays, a step-wise approach has been proposed[12,33]. The major treatment options include anticoagulation, thrombolysis, TIPS and OLT.
All patients should receive anticoagulant therapy, even if they are asymptomatic[42,43], because in BSC prothrombotic states are frequently observed, thus the potential risk of increase and recurrence of venous thrombosis; moreover, use of anticoagulation therapy improve the prognosis of BCS[44]. In presence of symptoms, diuretics and paracentesis for ascites and in combination with pharmacological and endoscopic therapy for the management of portal hypertension-related bleeding should be added.
OLT is indicated as a rescue therapy and should be considered after the failure of conventional treatment, in patients with fulminant BCS, as well as in patients with chronic forms of BCS accompanied by established cirrhosis and hepatic decompensation[12,45].
The outcome of transplantation has remarkably improved over the years[46,47]. One of the largest series of patients reported in literature includes 248 patients[47]. They reported 1-, 5-, and 10-year survival rates of 76%, 71%, and 68%. In these patients, most deaths were caused by infection, multiorgan failure and graft failure, or hepatic artery thrombosis, and occurred in the first 3 mo after transplantation. Late mortality resulted from recurrent BCS in 9 patients (13%).
Prior TIPS does not compromise the results of liver transplantation. After OLT, given that BCS often has a prothrombotic state, long-term anticoagulation after liver transplantation should be considered as the most important strategy for preventing recurrent BCS[48].
TIPS IN BUDD-CHIARI SYNDROME: LITERATURE DATA
We looked to emulate the example of Qiet al[49]in 2013 who conducted an initial review of the literature examining the available studies which heralded the start of the use of TIPS as a therapeutic option for BCS aiming to inform a more contemporary of attitudes based on studies conducted in this past decade. All major studies that specifically included a treatment arm examining the use of TIPS in BCS were searched from the principle databases extending as far back as 2010 so as to not replicate the seminal efforts of Qiet al[49]. As this was not strictly a systematic and largely a descriptive review to seek expert consensus, as are most of the guidelines surrounding the use of TIPS in BCS, there was no strict inclusion/exclusion criteria however studies with less than 10 patients and individual case reports were not included.
The principle study data was summarised to inform a discussion about the pertinent facets of this intervention from the indications, technique and stents used to the outcome and survival data. There was significant heterogeneity in study design and aims, patient numbers, treatment arms and study end-points (Supplementary material Tables 1 and 2)[11,50-64]and as with most guidelines on the subject of the management of BCS, conclusions were largely on the basis of expert opinion. All studies were retrospective, bar one, in nature owing to the paucity of cases of BCS in general and no large scale prospective studies were found. Of the 17 studies identified, study size varied from 13 to 91 patients with largely a predominance toward female patients (M:F ratio of up to 3:11) amongst studies. A wide distribution of geographical location and patient population was noted in keeping with global use of TIPS in BCS and reflects similar previous findings[49]. Follow up ranged markedly between studies with mean study follow up varying between 22 and 82 mo. Outcome measures focused on which were reported in nearly all the studies were technical success rates, patency/dysfunction rates, requirement for reintervention, development of postprocedural encephalopathy, mortality and procedural complication rates as well as improvement in portosystemic gradient.
Etiology, indications and timing
In primary BCS where the occlusion is intrinsic to the vessel[65](as opposed to secondary BCS caused by external compression of a hepatic vein by a lesion) the underlying aetiology generally varies geographically with western populations susceptible to hepatic vein thrombosis secondary to underlying thrombophilic disorders and asian populations at greater risk of hepatic vein thrombosis due to a membranous obstruction. The former generally causes hepatic vein obstruction alone while the latter has a tendency to cause hepatic vein and vena cava obstruction[6]. Anecdotally, it was interesting to note this divide as most papers originating in Asia did not describe underlying haematological disorders as the underlying aetiology, however this was almost universally the case amongst papers from the western hemisphere. This has implications on the treatment options as is the case in China where most BCS cases are treated with balloon angioplasty and stenting alone to account for this difference in underlying disease pathophysiology[64]. In the western population the underlying cause is most frequently multifactorial involving a combination of various prothrombotic conditions[9]including but not exclusive to myeloproliferative disorders, Factor V Leiden mutation, prothrombin gene mutation, protein C deficiency, antiphospholipid syndrome, antithrombin-III deficiency as well as other inflammatory conditions including Sarcoidosis, Churg-Strauss and Behcet’s disease. One final risk factor identified amongst multiple studies[50,55]was the use of the oral contraceptive pill.
Presentation is classified according to onset with acute, subacute and chronic subtypes. Over two decades of the use of TIPS in BCS have led to a largely standardized pathway of care[52], based on the Baveno IV consensus, for these patients with initial consideration for medical therapy with anticoagulation in all patients without contraindications. TIPS more specifically is to be considered in patients with acute liver failure, Rotterdam class III or those that have failed medical therapy, previous hepatic venous stenting or diffuse hepatic vein thrombosis due to technical difficulty in maintaining venous patency in the latter group[9]over the longer term. The most common symptomatic indication is generally ascites followed by gastrointestinal/variceal haemorrhage with rates of ascites up to 100% and variceal bleeding of up to 30.9% amongst the studies reviewed. Prior hepatic encephalopathy should not be considered a contra-indication to TIPS in BCS as it has not been identified as a risk factor for the development of post-TIPS hepatic encephalopathy[55]. In addition pre-procedure jaundice is also not considered a contraindication for TIPS in BCS although this is the case in end-stage liver disease owing to the higher mortality in the latter cohort with the difference postulated to be due to the lack of liver cell death and necrosis in BCS patients[53]. No unifying consensus can be drawn amongst the studies as to the best time to perform TIPS, however this should be made immediately available to patients presenting with hepatic failure, refractory ascites or gastrointestinal haemorrhage[49].
Techniques, efficacy and complications
All studies reviewed used largely a standard TIPS technique to form the shunt (Figure 2). Complete occlusion of the hepatic vein or lack of a stump required a “shotgun” or modified technique for patients[53,56]. The standard technique involves the use of a Rosch-Uchida Transjugular liver access set to gain access generally from a right hepatic vein into a right portal vein with the modified technique for involving a direct puncture at the hepatic vein stump. Over the two decades of TIPS use for BCS, there has been a progressive trend from uncovered stents to Polytetrafluoroethane (PTFE) covered stent grafts. The most common grafts used amongst all the studies were BARD E-Luminexx/Boston Scientific WALLSTENT uncovered stents and Gore VIATORR/BARD Fluency/Boston Scientific WALLGRAFT covered stent-grafts. 3 studies did not specify if stents were covered or uncovered. Patency rates largely favour the use of covered stents with Tripathiet al[55]reporting significant reduced reintervention rates (22vs100,P< 0.001) (7) and Neumannet al[51]describing a doubling of patency rates (33% to 63%). Overall technical success rates were as high as 98 – 100% amongst all the studies, serving as a testament to the feasibility of consideration of TIPS procedures for BCS patients. Even amongst patients with initial failure, subsequent repeat attempts enjoyed a high rate of eventual success[59].
Portosystemic gradient improvement was noted in most studies as reduction to < 12 mmHg and commensurate shunt dysfunction if this was subsequently exceeded. There was marked heterogeneity in how portosystemic gradient improvement was accounted for either pre-procedurally as an end-point and post-procedural measuring and could focus an area that should be standardized reporting for studies involving cohorts undergoing TIPS though this may not always be possible as noted by Shalimaret al[62]who used symptom improvement as a proxy for clinical efficacy and TIPS success though this strategy has its limitations and precludes objective assessment of TIPS function, however the rest of the studies all reported an improvement in either mean portal vein pressure or the portosystemic gradient (Supplementary material Table 2).
Post-procedural complications varied vastly in frequency between studies. Hayeket al[59]reported the highest rate of complications of up to 74% however this reflected complete and accurate reporting of all minor periprocedural complications, whereas their rate of more commonly noted complications such as bleeding and malposition of stent were closer to those described in other studies at 14% and 6% respectively. The most common complications reported were post-procedure encephalopathy and postprocedure bleeding/hemoperitoneum with the latter noted as high as 21.4% of cases[63]. While overall hepatic encephalopathy was encountered almost universally amongst all studies, rates remained between 2%-3% likely reflecting the underlying relatively preserved hepatic function in most cases of BCS as compared to TIPS performed for liver failure on a background of cirrhosis/chronic dysfunction. A wide variety of inadvertent punctures and injury to surrounding structures was noted including puncture of the right atrium, hemopericardium, splenic rupture and pseudoaneurysm though overall these were isolated cases with no overall pattern emerging across studies.

Figure 2 Access technique. A: Initial inferior vein cava (IVC) venography depicting the origin of the obstructed right hepatic vein (arrowhead); B: Colapinto stylet positioning prior direct puncture of the IVC at the level of the origin of the thrombosed right hepatic vein (arrowhead), just below the diaphragm. Note the tip of the sheath within the right atrium (arrow); C: The Colapinto needle is turned anteriorly, parallel to the spine and access obtained at the main right portal branch; D: Final result after the deployment of 2 stent grafts.
A wide variation in shunt dysfunction was noted between 13.8%-85% across the 17 studies with marked heterogeneity partially accounted for by the difference in types of stents and length of follow up period, a problem also noted by Qiet al[49]. The highest rate of stent dysfunction was noted by Zahnet al[50]. in the oldest study included in this review in 2010, however overall numbers were low and period of follow up was long at mean 4 years (6 mo–12 years) with a high rate of an average of 2.5 ± 2.2 reinterventions per patient likely reflecting older techniques and the use of bare-metal stents however most patients were managed with re-angioplasty alone. More contemporary papers report dysfunction rate from 13.8% to 50%, the latter in the most recent study by Biet al[64]. Among 27 patients however demonstrated secondary patency rates after re-intervention of up to 91% with an overall dysfunction rate of only 9%. The role of anticoagulation post-TIPS for hepatic vein occlusion remains controversial despite recommendation for this amongst British and American guidelines[56,57]and apart from one study[56], no significant anticoagulation-related haemorrhage was observed.
Prognosis and survival
As Qiet al[49]reported nearly a decade ago, prognosis of BCS patients treated with TIPS remains good. Mortality reported in studies over the last decade varies from 0% to 26.2%. As with other outcome measures, cumulative survival is measured heterogeneously amongst all studies however most commonly reported, five year survival is noted to be between 56.1% to 88%. The most common reported causes of death were acute liver failure, variceal haemorrhage or intracerebral haemorrhage and hepatocellular carcinoma.
There is considerable controversy in the literature about the use of prognostic indicator scores in determining those that would benefit the most from a TIPS procedure and might enjoy the longest intervention-free survival. The most common of those used being the BCS-TIPS Prognostic Index proposed originally by Garcia-Paganet al[66]in 2008 which is calculated by way of a product of age (years) × 0.08 + Bilirubin (mg/dL) × 0.16 + INR × 0.63 with a score of > associated with poorer survival. Seijoet al[52]validated this score finding it to have better predictive capacity than the Rotterdam score and additionally validated a further Budd-Chiari Intervention free survival score finding similar discriminatory capacity as the Rotterdam score and obviating the need for an INR which may be inaccurate due to concomitant anticoagulant use in this cohort of patients. These findings were further confirmed by Qiet al[54]. These results are at odds with those described by Tripathiet al[55]who assessed the Rotterdam, BCIS and BCS-TIPS PI scores finding that only the the latter independently predicted mortality with BCS TIPS PI significantly higher in those who died compared with survivors (5.80 ± 1.45vs4.40 ± 1.33,P< 0.01) and this too was disproven when 39 patients included in a previous study were not considered. Hayeket al[59]found numbers far too few to consider meaningful analysis of this relationship and Sonavaneet al[63]were not able to demonstrate a predictive value of BCS-TIPS PI, Rotterdam class or MELD Score. Additional factors deemed to be independent predictors of survival on multivariate analysis by Qiet al[54]were age (HR = 1.0711, 95%CI: 1.0260–1.1181,P= 0.0017) and absence of IVC thrombosis (HR = 0.1375, 95%CI: 0.0259-0.7307,P= 0.0199).
FOLLOW-UP AND TIPS REDUCTION
Nowadays, color-doppler sonography (CDUS) is a commonly accepted screening modality for TIPS patients, both as a routine follow-up in asymptomatic patients and in those cases with clinically suspected TIPS malfunction[67]. A number of studies reported a variety of CDUS criteria with very high sensitivity and specificity to detect TIPS dysfunction[68].
In a routine US follow-up, a TIPS patient is scheduled for a control 24 h after the procedure, and then after one week, 1 mo, 3 mo, and at 3-mo intervals thereafter.
In selected cases, US contrast media can be used to improve the assessment of TIPS patency[69], if conventional Doppler-US fails in the so called “difficult patients”, due to bowel gas or obesity.
Venography is at present performed solely on the basis of a suspected shunt dysfunction during the sonographic examination. Portography is performed either by a right jugular or by a common femoral venous access; portosystemic pressure gradient (PPG) measurement is always recorded. In some instances, CT is also required for a better depiction of the liver and vascular anatomy and of the stent-graft position and patency. In cases of complications, further percutaneous treatments are required (Figure 3).
Ideally, the increased patency of covered stents would allow reduction of invasive follow-up and therefore reduce costs. Moreover, the longer durability of the stent-graft seems to improve the survival[70].
Compared to other cirrhotic patients undergoing TIPS, BCS patients present an higher shunt dysfunction rate (approximately 50%vs80% within 1 year)[71], probably due to the high prevalence of underlying thrombophilia condition[33]. The advent of polytetrafluoroethylene (PTFE) covered stents in TIPS seems even more crucial for BCS patients by more than doubling the shunt patency[33,72], and consequently, decreases re-intervention[73]and improves patient outcome after TIPS[74,75].
Hepatic encephalopathy (HE), the most concerned complication of TIPS, occurs in about 20% of BCS patients, suggesting that BCS patients may better tolerate TIPS. Routine treatment of post-TIPS HE can be applied, including correction of precipitating factors, medical treatment and shunt reduction or occlusion[55,75]. 5-year survival of BCS patients receiving TIPS could reach 78%[75]. Within the stepwise strategy, TIPS contributes to about 30% increase in survival in previous reports[66]. However, the different indications for TIPS indicated different patient subgroups and thus comparison based on similar patient characteristics is required. Age, bilirubin, and INR have been identified as independent predictors of survival in patients undergoing TIPS, and a prognostic index (BCS-TIPS PI) has thus been suggested for evaluating prognosis in this particular population: Age (years) 9, 0.08 + bilirubin (mg/dL) 9, 0.16 +INR 9, 0.63. A score higher than 7 is considered to be associated with poor prognosis[75].
Despite a large number of patients can be managed medically, up to 7% of them develop refractory encephalopathy following TIPS insertion[76]. Moreover, the HE is often associated with progressive liver failure. In these individuals, the only alternatives are OLT or reduction/occlusion of the TIPS. TIPS reduction or occlusion may be mandated in the acute post-procedural period because of accelerated liver failure, but more often it is weeks to months later after medical management has been maximized. It should also be mentioned that, in these patients, liver function progressively declines because of their underlying hepatic disease, and that the TIPS may accelerate this process through ischemia or other mechanisms.
These factors must be balanced against the risks of reducing a needed portosystemic shunt. Whether variceal bleeding or refractory ascites was the indication for creating the TIPS, the patient would again be at increased risk for recurrent hemorrhage or reaccumulation of ascites if the TIPS shunt is occluded or reduced.
Historically, three basic methods have been used to reduce flow through the shunt as follows: (1) TIPS occlusion, infrequently utilized because of reported fatal outcomes[77]; (2) TIPS reduction with bare metal stents; and (3) TIPS reduction with covered stents.
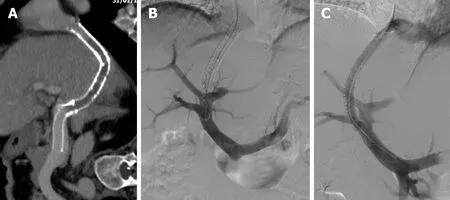
Figure 3 Transjugular intrahepatic portosystemic shunt revision. Shunt created 9 years before, using a dedicated stent graft (VIATORR® TIPS Endoprosthesis; GORE®, United States), in a patient with Budd-Chiari syndrome due to primary thrombocythemia. A: Computed tomography image demonstrating a complete occlusion of the intra-parenchymal segment of the stent graft; B: Digital subtraction angiography (DSA) following lesion crossing, confirming the occlusion; C: Final DSA demonstrating flow restoration following deployment of a 10 mm × 80 mm stent graft (Fluency™; BD, United States).
Before the availability of current stent grafts, multiple attempts were made to reduce the portosystemic flow in patients after TIPS positionin using smaller diameter bare metal stents. Haskal and Middlebrook[78]described a technique to insert a Wallstent (Boston Scientific, Middletown, MA, United States) with a silk suture in its mid-portion. Subsequently, the insertion of bare metal Palmaz stents (Johnson and Johnson, New Brunswick, NJ, United States) within the TIPS was described[79]. In this procedure, the stent was partially dilated at the portal extremity and completely dilated at the hepatic end. Both techniques produce flow reduction because of turbulent flow, but the degree of reduction and the elevation of portal pressure is difficult to predict.
The advent of commercial stent grafts from a number of vendors has considerably improved the precision and efficiency of the reduction.
Basically, 3 methods have been described[80]as follows: (1) Insertion of a balloonexpandable bare metal stent parallel to a new stent graft within the previously placed TIPS; (2) Insertion of a constrained self-expandable stent graft or incompletely dilated balloon-expandable stent graft within the TIPS; and (3) Insertion of a commercially available tapered stent graft within the TIPS.
UNRESOLVED ISSUES
Although TIPS is recommended as a safe and effective treatment option for BCS, some issues that remain to be determined. A main issue is the influence of TIPS on future transplant. Several authors acknowledge the contribution of TIPS in the overall survival of patients with cirrhosis awaiting transplant[81]. On the other hand, technical difficulties have been reported during transplantation in patients with previous TIPS and complications have been reported due to stent migration, especially in cases of migration to the supradiaphragmatic IVC and the atrium. Specifically, Tiveneret al[82,83]reported a case of atrial laceration during stent removal. However, according to large registries TIPS was did not seem to negatively affect OLT, while technical difficulties during transplantation mainly created by proximal stent migration and embedment can be overcome by specific surgical techniques[46,84,85]. Ungeret al[86]reported that TIPS was not correlated with an increased intraoperative complication rate- including bleeding events- and did not increase the duration of the operation. Although the authors acknowledge the fact that stent dislocation can create difficulties and modifications of the technique may be required (the piggyback OLT technique was not utilized), they noted that IVC clamping was possible in all the procedures. The only independent predictors of poor patient survival include > 12 h cold ischemic time, preoperative life support and re-transplantation. Moreover, a trend towards less blood transfusions was noted, a fact that could be attributed to the improvement of liver function and portal hypertension achieved following TIPS. Therefore, the authors recommend TIPS as a safe and effective bridging option recommended before OLT[86]. Therefore, currently available data demonstrate that prior TIPS does not compromise OLT results of liver transplantation and has no negative impact on patient prognosis. As a result, the recent 2016 EASL guidelines recommend pre-transplant TIPS in patient with BCS not responding to anticoagulation therapy and not amenable to catheterdirected thrombolysis and angioplasty[38]. Nevertheless, correct stent deployment is imperative in BCS patients and requires special training and attention, as it should balance between the risk of short-term shunt occlusion if deployed to deep within the hepatic vein and technical difficulties during surgery if deployed to proximal within the IVC. Additionally, stent migration is always possibility due to liver remodeling noted following TIPS.
Another major issue requiring further investigation is the timing of TIPS, as studies on the optimal time for creating the shunt are extremely limited. Recent data challenge current guidelines that recommend TIPS after failure of anticoagulation therapy, as early TIPS seems to reduce the long-term effects of microvascular ischemia which eventually lead to liver failure. Several authors suggest early TIPS in adjunct to anticoagulation, as the safety and effectiveness of the procedure justifies a more aggressive approach in order to reduce the development of hepatic fibrosis and liver failure, caused by chronic venous conjunction and portal hypertension[87]. Other authors propose decompressive procedures including early TIPS only in patients with signs of portal hypertension and recommend that medical therapy as sole treatment should be reserved only for patients without any signs of portal hypertension[9]. Nevertheless, good quality evidence to establish the possible superiority of early interventionvsstepwise treatment strategy are required.
Finally, as the long-term prognosis of patients with BCS is continuously improving due to the excellent results provided by minimal invasive interventions, long-term patency is a requisite in order to reduce re-interventions, avoid clinical relapse and improve the quality of life of the specific population. Stent grafts have demonstrated superior patency outcomes compared to bare stents, but there is certainly room for improvement especially in view of the continuously evolving endovascular technology.
CONCLUSION
To conclude, according to currently available data, TIPS is a safe and highly effective treatment option for patients with BCS and should be recommended for BCS patients, including those awaiting OLT. Issues such as early TIPS timing, the effect of TIPS in OLT and shunt patency improvement should be investigated in the ambit of multicenter controlled trials.To conclude, according to currently available data, TIPS is a safe and highly effective treatment option for patients with BCS and should be recommended for BCS patients, including those awaiting OLT. Issues such as early TIPS timing, the effect of TIPS in OLT and shunt patency improvement should be investigated in the ambit of multicenter controlled trials.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of succinate dehydrogenase deficiency and oncometabolites in gastrointestinal stromal tumors
- Acupuncture improved lipid metabolism by regulating intestinal absorption in mice
- Experimental model standardizing polyvinyl alcohol hydrogel to simulate endoscopic ultrasound and endoscopic ultrasound-elastography
- Efficacy of pancreatoscopy for pancreatic duct stones: A systematic review and meta-analysis
- Construction of a convolutional neural network classifier developed by computed tomography images for pancreatic cancer diagnosis
- Mixed epithelial endocrine neoplasms of the colon and rectum – An evolution over time: A systematic review
