Annual Occurrence of Calanus sinicus Carcasses in the Jiaozhou Bay and the Adjacent Waters
2020-09-27WANGShiweiWANAiyongZHANGGuangtaoandSUNSong
WANG Shiwei, WAN Aiyong, ZHANG Guangtao,and SUN Song
Annual Occurrence ofCarcasses in the Jiaozhou Bay and the Adjacent Waters
WANG Shiwei1), 2), 3), #, WAN Aiyong1), 2), 3), #, ZHANG Guangtao1), 2), 3), 4),*,and SUN Song1), 2), 3), 5)
1),,,266071,2),,266071,3),,266237,4),100049,5),,,266071,
The dead proportion of a calanoid copepodpopulation was investigated monthly with neutral red staining method at 12 stations in the Jiaozhou Bay and the adjacent waters from December 2008 to November 2009.could be observed through the whole year, with an evident numerical peak from February to April and an extremely low abundance in summer. Annual variation of the dead proportion differed greatly between adult females and copepodites. Dead females were observed only in the southern part and outside the bay sporadically in five months, when the egg production was the most active. Dead proportion of females was usually less than 5%. Dead copepodites could be observed in all but three months in summer in the whole study area, and the proportion varied between 3.8%–18.2%. Death percentage of copepodites increased significantly in the northern part in January and June, when water temperature exceeded it’s favorable limits; however, the fluctuated salinity had no significant effect on the survival of both females and copepodites. Thus the dead proportion ofwas relatively low in the Jiaozhou Bay, while difference could be observed between females and copepodites. Dead copepodites were common in all samples, but dead females were presented mainly as ‘reproduction cost’ during generation alternation.
dead proportion;; Jiaozhou Bay; natural mortality; population dynamics
1 Introduction
Planktonic copepods, which might be the most numerous metazoans on the earth, play an important role in transferring the organic matters fixed by primary producers to higher trophic levels (Ohman and Hirche, 2001). Understanding the factors that regulate its population dynamics is very important in biological oceanography. Sea- sonal and spatial patterns of copepods abundance are shaped by the accumulation of growth and egg reproduction aswell as losses through mortality. Over the last 30 years many efforts have been put in examining the growth and fecundity, while only a few investigations have quantified the rates of mortality (Hirst and Kiørboe, 2002). Further- more, current mortality estimation method that follows natural populations over time does not distinguish dead and live individuals in field samples. Though no overall estimation exists on global scale, several studies have al- ready shown that zooplankton carcasses might be far beyond negligible but sometimes even prevalent in both ma- rine and freshwater environments (Tang., 2009).
Neutral red staining is a simple and reliable method for separating live and dead zooplankton in natural samples(Dressel., 1972; Elliott and Tang, 2009), with which substantial numbers of carcasses have been documented in marine environment(Tang., 2009). We suggest that live/dead proportion may also include valuable information for advancing our understanding in population dyna- mics. Firstly, the abundance of natural zooplankton popu- lation could be overestimated if dead animals are treated as alive ones, which would consequently bias a series of ecological rates estimated with population abundance or biomass. Secondly, naturally occurring intact carcasses like- ly represent instances of non-predatory mortality (Elliott., 2010). Currently, most studies focus on predatory mortality, but a modeling study suggested that predation cannot account for all zooplankton mortality in the field(Hirst and Kiørboe, 2002). As non-predatory mortality could result from pollution, parasitism, harmful algal blooms,.(Elliott., 2010), which can be further applied into investigations focusing on how unfavorable environments will influence copepod population dynamics. Thus, the abi-lity to quantify zooplankton carcasses in field samples will also make it feasible to study how unfavorable conditions influence onmortalities. However, live/dead inspection is seldom included in field sampling methodo- logy, and no year-round observation is available until now.
The Jiaozhou Bay, a semi-enclosed bay adjacent to the southern Yellow Sea with an average depth of 7m, is a long- term ecological site in the Chinese Ecosystem Research Network. We applied neutral red staining method in this area to investigate annual carcass occurrence of, an ecologically important copepod species found in shelf waters around China, Japan and Korea (Uye, 2000). In previous studies, temperature was suggested to be the most important limiting factor of its geographical distribution and reproduction rate (Hwang and Wong, 2005; Zhang., 2005), but temperature’s influences on survival of adults copepodites were still uncertain. Our specific objectives include illustrating the importance of carcasses in the natural population, understanding the temporal and geographical variation in adults and juveniles, and explainingpopulation dynamics in different seasons.
2 Materials and Methods
2.1 Determination of Environmental Factors
Twelve stations were investigated monthly from Decem- ber 2008 to November 2009 (Fig.1). Temperature and sa- linity were recorded with a CTD (Sea Bird Electronics, SBE-19). Seawater samples (500mL) for the measurementof chlorophyll(Chl) concentration were collect- ed from surface layer (0–1m) and filtered through GF/F glass-fiber filters. The filters were then extracted with 90% aqueous acetone for 24h at 0℃, and the Chlconcentration was measured with a Turner Designs Model 7200 fluorometer.

Fig.1 Map of sampling sites in the Jiaozhou Bay and the adjacent waters with isobaths. Stations were divided into three groups according to the locations, i.e., NP (A5, A3, B2 and C1), SP (C4, C3 D1, D3 and D5) and OS (D7, D8 and D6).
2.2 Neutral Red Staining
Zooplankton samples were collected from each station with conical plankton nets (mouth opening: 0.08m2; mesh size: 160mm) towed vertically from 1m above the bottom to surface at a slow speed (≤0.3ms−1). Before every tow, the net was rinsed thoroughly to minimize accidental carry- over of dead animals from earlier tows. Staining procedures described by Tang. (2006) were followed. Zoo- plankton samples in the cod end were transferred carefully to a graduated staining jar with 200mL pre-screened natural seawater. Neutral Red stock solution (0.01gmL−1) was added according to a ratio of 1.5mL to every 1000mL. The sample was kept in a light-shading incubator for about 15min, then gently concentrated onto a 100-mm nylon mesh and rinsed repeatedly with filtered natural seawater. The rinsed sample was then transferred carefully to a glass sample jar with alkaline filtered seawater (pH=9). After adding formaldehyde to a final concentration of 4%, the samples were kept at 4℃ in refrigerator for less than 4d. In the laboratory, the preserved samples were acidified to a final pH<7 with diluted hydrochloric acid and counted under a dark field dissecting microscopy. Due to the low total abundance of males, only adult females and copepodites (including all developmental stages) were counted.
2.3 Data Analysis
Since the death percentage could be misleading when sample size was low, all the stations were divided into three groups according to geographic locations: four shallow stations in the northern part (NP), five stations in the sou- thern part (SP) and three stations outside the entrance (OS), as shown in Fig.1. The densities of total and dead females and copepodites were calculated at each station, respectively. Death percentage was calculated with sums of car- casses and the total abundance from all stations within each group, and samples with less than 10 live individuals were excluded.
3 Results
3.1 Environmental Characteristics
Annual variation of surface water temperature showed an obvious inshore/offshore trend (Fig.2). Surface temperature varied between 0.6–26.1℃ in NP, 3.9–25.6℃ in SP and 4.7–24.6℃ in OS. It was similar among all stations only in February and September, when the cooling and warming periods shifted. Surface temperature was low- er in NP during the cooling period but much higher than the other two parts during the warming period. From March to July, surface temperature increased more rapidly and varied more significantly among stations in NP because of tidal mixing. The largest difference between NP and OS was 3.6℃ in June. After October, temperature decreased rapidly in NP. Average temperature in NP wasabout 4–5℃ lower than in SP and OS, and the biggest dif- ference was 5.2℃ in December.

Fig.2 Annual variations of temperature, salinity and Chl a concentration in the northern part (NP), southern part (SP), and outside area (OS) of the Jiaozhou Bay.
Average surface salinity was always the lowest in NP and increased gradually outwards. It ranged 27.8–31.1 in NP, 30.0–31.4 in SP, and 30.7–31.4 in OS. Salinity de- creased significantly in December and July because of the heavy snow and rain falls.
Monthly average Chlconcentration ranged between 0.06–7.39mgL−1in NP, which was much higher than 0.16–1.68mgL−1in OS and 0.14–4.47mgL−1in SP. Annual va- riations of Chlconcentration were different in the three domains. In OS, the highest concentration was 1.68mgL−1in February, followed by 1.59mgL−1in August and 1.37mgL−1in June. Similar bimodal patterns were found in NP and SP, with a major peak of 7.39 and 4.47mgL−1in July and a minor peak of 3.72mgL−1and 2.10mgL−1in April. The highest concentration in April was observed at station A5 as 6.68mgL−1, and in July the highest concentration was at station C1 as 14.19mgL−1.
3.2 Death Percentage of Females
Females ofwere captured in all cruises except in July (Fig.3). Average density increased from December to April and decreased abruptly in May. In Decem- ber and January, females were distributed mainly in SP and OS areas with an average density varying between 9.0 and 14.2indm−3, but very scarce in NP. It expanded into the whole investigated area from February to April, and average density in each part increased from 6.8–13.3indm−3in February to 21.2–40.5indm−3in March and 14.1–51.1indm−3in April. In May, though females were ob- served in all three locations, the average density decreased to 3.9–8.0indm−3. Females were absent from the whole in- vestigation area in July, while they could not be observed until October in NP. In August and September, females could be observed only in SP with extremely low density (1.4 and 1.7indm−3respectively). After the re-occurrence in October, females were 8.1indm−3on average in OS, but they were still lower than 1.5indm−3in the bay area.
Dead females were observed only in five months in SP and OS areas. In March and April, female carcasses were most abundant. The highest density observed outside the bay in April was only 1.8indm−3, but inter-station variation was much greater in SP. Death percentage in these two months varied from 2.35%–4.66%. Though total female abundance decreased evidently in May, no carcass was col- lected in the whole investigated area. Female carcasses appeared again in June, with percentages of 2.56% and 1.06% in SP and OS, respectively. In October, carcasses were observed only in OS, and in November it appeared only in SP. Death percentage was 3.03% and 4.65%, re- spectively.

Fig.3 Calanus sinicus. Annual variation of total density (indm−3), carcass density (indm−3) and death percentage (%) of females in the three domains: the northern part (NP), southern part (SP), and outside area (OS) of the bay. Error bars in top and middle plots represent standard deviations among stations in each group. In the bottom plot, numbers above each column indicate total carcasses counted.
3.3 Death Percentage of Copepodites
The annual variation ofcopepodites was similar to that of females (Fig.4), but the density was nearly three times higher than females. Though average density of copepodites was also the lowest in NP in December and January, it increased rapidly and outnumbered the other two parts in February and March. Average density in three locations increased from 18.0–44.7indm−3in December to 130.1–213.2indm−3in March. Unlike females, density of copepodites decreased gradually after March and was 9.2–67.6indm−3in July. Copepodites were also very scarce in sum- mer. From July to October, it disappeared completely in NP, but was present continuously in SP with an average density between 2.1 and 8.6indm−3. In OS, copepoditeswere absent only in August. When total copepodites spread into the whole investigation area again in November, the quantity also increased outwards from NP to OS; however, the average density was 6.0–21.5indm−3and was much lower than that in the spring peak.
Copepodite carcasses were absent from the whole area only in July, August and September. From December to June, however, copepodite carcasses were observed in all of the areas. Its density was quite similar from December to February (0.8–3.3indm−3), but increased abruptly to 9.1–11.7indm−3in March and then decreased gradually to 1.7–3.4indm−3in June. In October and November, co- pepodite carcasses appeared again in SP and OS areas, and the average density varied between 6.1 and 9.3 indm−3. Death percentage varied between 1.02% and 9.30% in most cases, but in NP it was as high as 14.29% and 15.38% in January and June respectively.
4 Discussion
Dead proportion estimated with this method showed the true degree to which the traditional microscope-counting results can be biased with carcasses neglected. In the Jiao- zhou Bay, carcasses usually account for a small portion in naturalpopulation, especially for adult females. However, dead proportion differed significantly among different months and between adults and juveniles. Carcasses of females presented mainly during and after the spawning peak period, while those of copepodites appear- ed continuously except in summer. Our results also have implications for the understanding of population dynamics of, particularly in generation alternation and thermal limitations.
Dead proportion observed in this study was slightly high- er than that for freshwater calanoid copepods (0–3.5%, Bickel., 2009; 0.5%, Gries and Güde, 1999), but lower than most results from marine environments (Elliot andTang, 2009). Extremely low mortality (near zero) had been observed for early copepodite stages ofspp. in a Norwegian fjord Lurefjorden (Eiane., 2002). Based on collection of dead zooplankton in sediment traps, estimates of annual variation in non-predatory mortality were obtained for freshwater mesozooplankton. Copepods other thanshowed sedimentation losses of only 0.5% of the standing stock per day (Gries and Güde, 1999). Hence, carcass occurrences in field samples unlikely can induce significant overestimation of abundance and biomass in, comparing with those possible errors during sampling and counting processes. However, its in- fluences on mortality estimation are still non-negligible. In population dynamic models, field data before and after removal of carcasses from abundance values can result in significantly different mortality rates (Elliott and Tang, 2011a). In the lower Chesapeake Bay, where about 10% to 30% ofindividuals are carcasses (Tang., 2006; Elliott and Tang, 2011b), non-predatory mortality comprised an average of 25% of the total mortality for nauplii, and 12% of total mortality for copepodites, respectively. We applied the same methods toin the northern Yellow Sea, where the dead proportion was 1%–2.8% for the whole developmental stages, and was generally lower than the results in the Jiaozhou Bay. How- ever, in the Yangtze River Estuary area, dead proportion showed similar patterns in different developmental stages but with much larger variations. Further studies are required to fully understand the regional differences.
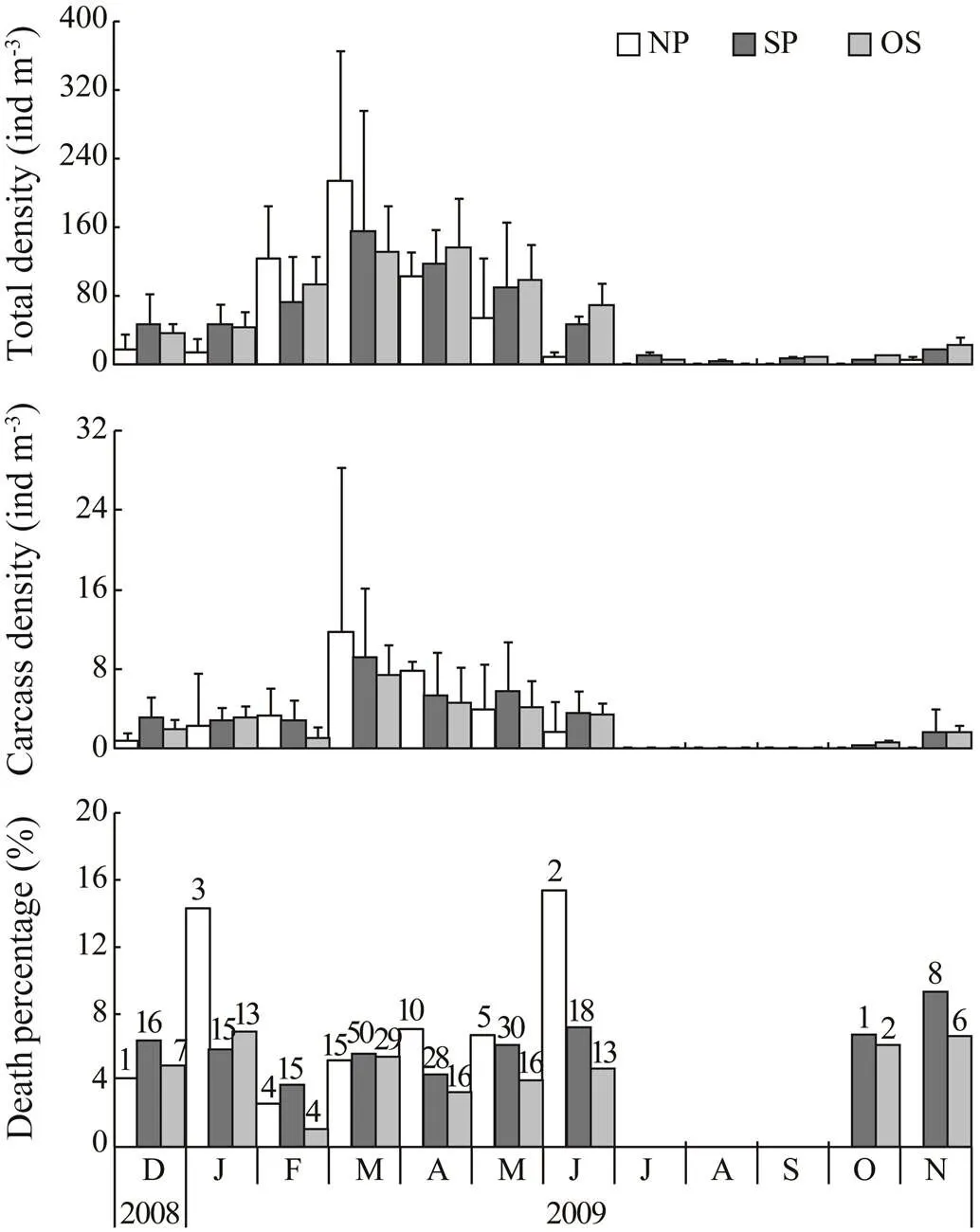
Fig.4 Calanus sinicus. Annual variation of total density (indm−3), carcass density (indm−3) and death percentage (%) of copepodites in different areas. The areas are the same as in Fig.2. Error bars in top and middle plots re- present standard deviations among stations in each group. In the bottom plot, numbers above each column indicate total carcasses counted.
Stage-specific differences and monthly variation of dead/ live proportion were seldom included in previous studies with visual discrimination or staining methods. At deep stations in the Japan Sea, dead females were observed in higher proportion than copepodite stage IV, V and males, which might be the results of long time accumulation (Te- razaki and Wada, 1988). In the lower Chesapeake Bay, themean dead proportions forwere 30% for NI–NIII nauplii, 12%–15% for NIV–NVI nauplii and both CI– CV and CVI copepodites, and 9% for females, respective- ly (Elliott and Tang, 2011b). It was suggested that cope- podites suffer higher natural mortality in natural environ- ments than females.
Year-round sampling also made it possible to investigate the ecological adaptation ofin natural environments. In this study, water temperature expanded be- yond the reported thermal range of 5 to 22℃ forfrom January to February and July to September. Death percentage of copepodites increased significantly both at extremely low temperature in January and at the highest temperature in June. Death percentage of copepodites was the lowest in February, when average water temperature varied between 4.44 and 4.76℃ in the three areas. In January, when the temperature was 0.6℃ in NP, 3.9℃ in SP, and 4.7℃ in OS, copepodites showed lower abundance and higher death rate in NP than in the other two areas. As only one live female was sampled in NP in January, influences of extreme temperature on the dead proportion of females cannot be quantified. In the other two areas, however, no dead female was observed from the 252 spe- cimens enumerated in January and February. Thus both females and copepodites ofcan tolerate temperature as low as 4℃, while temperature as low as 0.6℃ was deleterious to the survival of copepodites. In June, when surface temperature increased to higher than 20℃ in NP but still lower than 20℃ in the other two domains, copepodites also showed lower abundance and higher death percentage in NP than in the other two areas. Females disappeared completely in NP, while in the other two areas only two dead individuals were observed from 131specimens enumerated in this month. It indicates that tem- perature higher than 20℃ is deleterious to the survival of both females and copepodites. The above results also sug- gested the temperature difference might partly contributes to the spatial changes of death percentage and carcasses densities.
However, only three carcasses were observed in 126copepodites from July to September, when temperature in- creased above 22℃ at all stations. Since females had dis- appeared completely in July, these individuals should be advected from off-shore waters. The Yellow Sea Cold Wa- ter Mass (YSCWM) was suggested to be an over-summer- ing site for, and the influence of the YSCWM can reach to some coastal areas next to Qingdao (Zou., 2001; Zhang., 2007). The changing physical environments during the advection made it difficult to compare this dead proportion with those observed locally in the Jiaozhou Bay. Relative low salinity is unlikely detrimental tosurvival as observed in this study. Salinity decreased significantly in December and July be- cause of heavy snow or rain falls. In NP, it was as low as 27.8 and 28.8, respectively. However, only one dead cope- podite was observed from a total of 23 individuals, while no female was observed.
Dead females could be observed mainly during late and post-spawning months, which showed coincidence with generation alternation. It was suggested in previous studies that reproduction was costly, as unfed and non-repro- ducing females lived longer than fed and reproducing ones(Hopp., 1997). Additionally, survival of mated females were lower than pre-mated females and decreased more rapidly under stressful conditions (Feifarek, 1983). Natural death following reproduction has been suggested as a possible reason for copepods carcasses in the Atlantic Ocean (Wheeler, 1967) and the Japan Sea (Terazaki and Wada, 1988). In the Jiaozhou Bay, spawning ofpeaked mainly from February to May. Although adult fe- males were quite scarce in November, egg reproduction rate increased slightly (Wang, 2009). Generation alternation was observed in the Jiaozhou Bay from April to May. Average prosome length of females in the Jiaozhou Bay decreased from 2.70mm in March and April to 2.25mm in May (Wang, 2009). In the southern Yellow Sea, the ma- ximum prosome length observed in May was only 2.38mm (Zhang., 2005). Female abundance also decreased significantly from April to May in this study. It indicates that females mature in early spring almost die out before May in the Jiaozhou Bay. According to our results, the generation alternation was likely the reason for elevated dead proportion in March and April. Though no evident change was observed in either abundance or prosome length of females, generation might alternate in October and No- vember as pointed by Wang (2009). The maximum longevity ofunder laboratory conditions was 142d at 13℃ (Uye, 1988). Thusrecruited in Novem- ber can live till next April, while those recruited in June can only live till October.
To sum up, by a year-round survey on the presence of both live and deadin the Jiaozhou Bay, distinct seasonal and inter-stage differences were observed in dif- ferent developmental stages. Dead proportions of copepo- dites were generally higher than those of females. Copepodite carcasses increased significantly when water temperature exceeded its favorable limits in January and June, especially in the north part of the Jiaozhou Bay. However, female carcasses were generally related with spawning activities and the seasonal generation alternation in spring. In addition to the natural life cycle, temperature might be the primary reason to explain the above spatial and temporal differences rather than salinity that had no negative influence on survival of both females and copepodites.
Acknowledgements
Our investigation is supported by the National Key Re- search and Development Program of China (No. 2017YF E111100), the Aoshan Science and Technology Innovation Project (No. 2016ASKJ0202), and the National Natural Sci- ence Foundation of China (Nos. 41406148, 40830854).
Bickel, S. L., Tang, K. W., and Grossart, H. P., 2009. Use of ani- line blue to distinguish live and dead crustacean zooplankton composition in freshwaters., 54: 971-981.
Dressel, D. M., Heinle, D. R., and Grote, M. C., 1972. Vital stain- ing to sort dead and live copepods., 13: 156-159.
Eiane, K., Aksnes, D. L., Ohman, M. D., Wood, S., and Marti- nussen, M. B., 2002. Stage-specific mortality ofsppunder different predation regimes., 47: 636-645.
Elliott, D. T., and Tang, K. W., 2009. Simple staining method for differentiating live and dead marine zooplankton in field samples.:, 7: 585-594.
Elliott, D. T., and Tang, K. W., 2011a. Influence of carcass abun- dance on estimates of mortality and assessment of population dynamics in.,427: 1-12.
Elliott, D. T., and Tang, K. W., 2011b. Spatial and temporal dis- tributions of live and dead copepods in the lower Chesapeake Bay (Virginia, USA)., 34: 1039-1048.
Elliott, D. T., Harris, C. K., and Tang, K. W., 2010. Dead in the water: The fate of copepod carcasses in the York River Estuary, Virginia., 55: 1821-1834.
彩票背面的图案上,歪歪扭扭写着几行字,开头“雪萤”两个字吸引了范坚强,他小心将折叠的彩票展开。一些字被汗水洇得模糊了,一些字处于折痕处已经磨损无存,还有一些被涂抹掉了。他连猜带蒙地辨出几句话:雪萤:
Feifarek, B. P., Wyngaard, G. A., and Allan, J. D., 1983. The cost of reproduction in a freshwater copepod., 56: 166-168.
Gries, T., and Gude, H., 1999. Estimates of the non-consump- tion mortality of mesozooplantton by measurement of sedimen- tation losses., 44: 459-465.
Hirst, A. G., and Kiørboe, T., 2002. Mortality of marine planktonic copepods: Global rates and patterns., 230: 195-209.
Hopp, U., Maier, G., and Bleher, R., 1997. Reproduction and adult longevity of five species of planktonic cyclopoid copepods reared on different diets: A comparative study., 38: 289-300.
Hwang, J. S., and Wong, C. K., 2005. The China coastal current as a driving force for transporting(Copepoda: Calanoida) from its population centers to waters off Taiwan and Hong Kong during the winter northeast monsoon period., 27: 205-210.
Ohman, M. D., and Hirche,H.J., 2001. Density-dependent mor- tality in an oceanic copepod population., 412: 638- 641.
Tang, K. W., Bickel, S. L., Dziallas, C., and Grossart, H. P., 2009. Microbial activities accompanying decomposition of cladoceran and copepod carcasses under different environmental conditions., 57: 89-100.
Terazaki, M., and Wada, M., 1988. Occurrence of large numbers of carcasses of the large, grazing copepods from the Japan Sea., 97: 177-183.
Uye, S. I., 1988. Temperature-dependent development and growth of(Copepoda: Calanoida) in the laboratory., 167/168: 285-293.
Uye, S. I., 2000. Why doesprosper in the shelf ecosystem of the Northwest Pacific Ocean?, 57: 1850-1855.
Wang, S. W., 2009. Reproduction, population recruitment and life history ofin the Yellow Sea. PhD thesis. Institute of Oceanology, Chinese Academy of Sciences (in Chi- nese with English abstract).
Wheeler, E. H., 1967. Copepod detritus in the deep sea.,12: 697-701.
Zhang, G. T., Sun, S., andYang, B., 2007. Summer reproduction of the planktonic copepodin the Yellow Sea: Influences of high surface temperature and cold bottom water., 29: 179-186.
Zhang, G. T., Sun,S.,and Zhang,F., 2005. Seasonal variation of reproduction rates and body size ofin the sou- thern Yellow Sea, China., 27: 135-143.
Zou, E. M., Guo, B. H., Tang, Y. X., Jae, H. L., and Heung, J. L., 2001. An analysis of summer hydrographic features and circulation in the southern Yellow Sea and the northern East China Sea., 32: 340-348 (in Chinese with English abstract).
#The two authors contributed equally to this study.
. E-mail: gtzhang@qdio.ac.cn
November 4, 2019;
January 6, 2020;
April 28, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Phaeocystis globosa Bloom Monitoring: Based on P. globosa Induced Seawater Viscosity Modification Adjacent to a Nuclear Power Plant in Qinzhou Bay, China
- Effect of pH, Temperature, and CO2 Concentration on Growth and Lipid Accumulation of Nannochloropsis sp. MASCC 11
- Fuzzy Sliding Mode Active Disturbance Rejection Control of an Autonomous Underwater Vehicle-Manipulator System
- The 9–11 November 2013 Explosive Cyclone over the Japan Sea- Okhotsk Sea: Observations and WRF Modeling Analyses
- Real-Time Position and Attitude Estimation for Homing and Docking of an Autonomous Underwater Vehicle Based on Bionic Polarized Optical Guidance
- Characterization of the Complete Mitochondrial Genome of Arius dispar (Siluriformes: Ariidae) and Phylogenetic Analysis Among Sea Catfishes
