Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes
2020-09-18
Romina H Aspera-Werz,Department of Traumatology,BG Trauma Clinic,Siegfried Weller Institute for Trauma Research,Eberhard Karls Universität Tübingen,Tübingen 72076,Germany
Sabrina Ehnert,Monja Müller,Sheng Zhu,Tao Chen,Weidong Weng,Andreas K Nussler,Department of Traumatology,BG Trauma Clinic,Siegfried Weller Institute for Trauma Research,Eberhard Karls Universität Tübingen,Tübingen 71076,Germany
Johann Jacoby,Institute for Clinical Epidemiology and Applied Biometry,Eberhard Karls Universität Tübingen,Tübingen 71076,Germany
Abstract
Key words:Primary human osteoblast;Cigarette smoke;Tobacco heating system;Mesenchymal stem cells;Electronic nicotine delivery systems;Bone
INTRODUCTION
Cigarette smoking (CS) is the most popular way to consume tobacco,and is one of the leading causes of preventable death worldwide[1].Of the current estimated one billion smokers,6 million die per year due to harmful substances that arise when tobacco is burned and become distributed throughout the bodyviathe bloodstream,thereby affecting several organs[2,3].
Detrimental effects of CS also manifest in the musculoskeletal system[4,5].Recent evidence demonstrated that CS could lead to an imbalance in bone turnover mechanisms,leading to osteoporosis,osteoarthritis,and fracture[6-8].Moreover,CS increases the risk of delayed fracture healing[9],non-union[10],complication rate[11],and leads to more extended hospital stays[12-14].
Tobacco combusted at about 800 °C generates approximately 6500 molecular species,more than 150 of which have been identified as toxic compounds[3,15-17].However,it remains unknown which of these compounds are involved in the impaired bone homeostasis observed in smokers.Our previous results,as well as other publications,have demonstrated that the most pharmacologically active component,nicotine,and its first metabolite,cotinine are not the main factors responsible for the adverse effects observed in bone-forming cells[18-20].
Interestingly,it has been demonstrated that oxidative stress induced by compounds produced during conventional cigarette combustion may be one of the factors responsible for the impaired osteogenic differentiation of bone-forming cells and osteogenic precursors cells[18,21-25].
Quitting conventional CS is the most efficient way to significantly reduce the harmful effects of cigarette smoke on human health[11];unfortunately,quitting smoking is not always a viable alternative for many smokers (i.e.,those which cannot,wish not,or fail to quit)[26].Many attempts have been made to replace cigarettes with smoke-free nicotine replacement therapies (e.g.,nicotine patches,sprays,or chewing gums).However,these replacement products tend to fail in smokers because,although they deliver nicotine,the smoking ritual is absent.Therefore,new approaches have been on developing reduced-risk alternatives for smokers that maintain the smoking ritual,while providing the same levels of nicotine as conventional cigarettes with less harmful constituents.
For this purpose,electronic nicotine delivery systems (ENDS),including e-cigarettes or tobacco heating systems (THS),focus on heating rather than combustion to reduce the generation of harmful constituents.E-cigarettes heat liquids based on propylene glycol,glycerin,flavor,and selectively nicotine into an aerosol that is inhaled.Instead of burning tobacco,THS heat tobacco rolled up in a stick form up to 350 °C (avoiding combustion and formation of ashes).In contrast to E-cigarettes,THS contain tobacco and convey the feeling of smoking a conventional cigarette.
步骤1 已知方案评估指标为{c1,,cn,,cN},组织各决策专家{e1,,et,,eT}借助语言集SIL(如式(6))以CFGJ形式构造直接影响矩阵,并将CFGJ转化为PD-HFLTS,令其为
Several studies have demonstrated reduced levels of toxic and harmful compounds from ENDS[27-29].However,effects on cell toxicity and function have shown controversial results[30-34].To our knowledge,the effects of THS compared to conventional cigarettes on skeletal tissue and bone-forming cells has not previously been explored.
Therefore,the present study aimed to evaluate the effect of THS on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts,as well as to directly compare THS and conventional cigarette combustion on bone cells.
MATERIALS AND METHODS
Cell Culture Medium and supplements were purchased from Life Technologies(Darmstadt,Germany).Chemicals were obtained from Sigma (Munich,Germany).Tobacco heating system 2.4 "IQOS®" and sticks (three commercially available flavors;bronce,amber and yellow) were provided by Philip Morris (Germany).
Isolation of human pre-osteoblasts and osteogenic differentiation
Human osteoblasts (hOBs) were isolated from cancellous bone samples from BG Unfallklinik Tübingen.A consent form was obtained from all participants included in the study.hOBs isolation as well as all following experiments were performed in accordance with the 1964 Declaration of Helsinki and accordance with the ethical vote(538/2016BO2) approved by the ethics committee of the medical faculty of the Eberhard-Karls-Universität and University clinic Tübingen.The donors’ average age was 73.2 ± 4.3 years (1 male and 4 female).Bone fragments were collected from cancellous bone by mechanical disruption and washed with PBS to remove residual blood.Cancellous bone fragments were digested with 0.07%w/vCollagenase II (Serva,Heidelberg,Germany) in PBS at 37 °C for one hour.Following washing with PBS,released hOB were cultured in MEM/Ham’s F12,5%v/vFCS,100 U/mL penicillin and 100 mg/mL streptomycin,50 µmol/L L-ascorbate-2-phosphate,50 µmol/L β-glycerolphosphate in a water-saturated atmosphere of 5% CO2at 37 °C.Medium change was performed every 5 d.To induce osteogenic differentiation,cells in passage 2 were seeded at a density 1.3 × 105cells/cm2and treated with MEM/Ham’s F12,1%v/vFCS,2 mmol/L L-glutamine,200 µmol/L L-ascorbate-2-phosphate,10 mmol/L β-glycerolphosphate,25 mmol/L HEPES,1.5 mmol/L CaCl2,100 nmol/L dexamethasone.For experiments,several concentrations of AE of conventional cigarettes or THS were added to the differentiation medium.Twice a week,the medium was changed during osteogenic differentiation,which was sustained for 21 d[35].
MSCs culture and osteogenic differentiation
Human immortalized bone marrow mesenchymal stem cells (SCP-1 cells,provided by Dr.Matthias Schieker[36]) were cultured in Minimum Essential Medium Eagle alpha(MEM α) supplemented with 5%v/vfetal bovine serum (FBS),100 U/mL penicillin and 100 mg/mL streptomycin,in a water-saturated atmosphere of 5% CO2at 37 °C.Medium change was performed every 5 d.Osteogenic differentiation of SCP-1 cells seeded at a density 1 × 105cells/cm2was induced with MEM α medium containing 1%v/vFCS,100 U/mL penicillin,100 mg/mL streptomycin,200 μmol/L L-ascorbate-2-phosphate,10 mmol/L β-glycerol-phosphate,25 mmol/L HEPES,1.5 mmol/L CaCl2,and 100 nmol/L dexamethasone.For experiments,several concentrations of AE of conventional cigarettes or THS were added to the differentiation medium.The medium was changed twice a week during osteogenic differentiation,which was sustained for 21 d[18].
Conventional cigarette and THS AE generation
AE were generated according to the standard of Health Canada smoking regime which better represent the human smoking behavior[37].For conventional cigarettes(Marlboro,Philip Morris,New Your City,NY,United States) AE preparation,a blocked filter cigarette was placed in a standard gas washing bottle,subjected to a negative pressure by using a peristaltic pump.One cigarette was bubbled through 25 mL of cell culture medium at a rate of 2 puff/min,each puff lasting for 2 s and puff volume 58.89 ± 4.54 mL.For THS AE preparation,a stick connected with the device THS 2.4 "IQOS®" was placed in a standard gas washing bottle and proceeded as described before.The freshly prepared AE was sterile filtered (0.22 μm filter) before use and freshly prepared for every exposure.The concentration of AE was determined and standardized by its optical density at 320 nm (OD320).An OD320of 0.61 ± 0.08 or 0.25 ± 0.01 was considered 4 × 10-1puff/mL AE of conventional cigarettes or THS,respectively.
Experimental setup
SCP-1 cells (n= 3) and hOBs (n= 5) were seeded in culture medium at a concentration of 1 × 105or 1.3 × 105cells/cm2,respectively.After attachment,cells were washed with PBS and stimulated with AE from conventional cigarettes or THS in concentrations between 4 × 10-1–4 × 10-5puffs/mL in differentiation medium.Untreated cells were considered as control.The medium was changed twice a week with fresh AE during osteogenic differentiation,which was sustained for 21 d.
Mitochondrial activity – resazurin conversion assay
Cell viability was indirectly measured by resazurin conversion assay (mitochondrial activity).Briefly,cells were incubated with 0.0025%w/vresazurin in PBS for 30 min at 37 °C.The resulting Resorufin fluorescence was measured (excitation = 544 nm/emission = 590 nm) with a plate reader and corrected to the background.Changes in resazurin conversion are displayed relative to untreated cells[18,25].The EC50was calculated using the EC50calculator tool of the AAT Bioquest webpage (www.aatbio.com/tools/ec50-calculator).
Live staining – calcein-AM staining
Cell viability was determined by intracellular esterase activity with Calcein-AM staining (permeable non-fluorescent dye which is converted to a green fluorescent dye by esterases).Cells stimulated with AE according to the experimental setup were washed with PBS and were incubated with calcein-AM (2 µmol/L),and Hoechst 33342(1:1000 in PBS) at 37 °C for 30 min.Cell images were taken (Epifluorescence:EVOS FL,life technologies,Darmstadt,Germany) after washing with PBS[25,38].
Osteoblast function - AP activity assay
Osteoblast function was evaluated by AP activity (early osteogenic marker).Cells were incubated with AP reaction buffer (0.2%w/v4-nitrophenyl-phosphate,50 mmol/L glycine,1 mmol/L MgCl2,100 mmol/L TRIS,pH = 10.5) for 40 min at 37 °C.Formed 4-nitrophenol was determined photometrically (λ = 405 nm) with a plate reader,corrected to the background and normalized to relative cell numbers.Changes in AP activity are displayed relative to untreated cells[18,25].
Total protein content - sulforhodamine B staining
The cell number was determined by sulforhodamine B (SRB) staining (total protein content).Cells were fixed with ice-cold ethanol for one hour at -20 °C.After washing with tap water,cells were stained with 0.4%w/vSRB (in 1% acetic acid) for 30 min at ambient temperature.Unbound SRB was removed by washing with 1% acetic acid.Bound SRB was resolved with 10 mmol/L unbuffered TRIS solution (pH = 10.5).Resulting SRB staining was quantified photometrically (λ = 565 nm) with a plate reader and corrected to the background[18].
Calcium deposition - alizarin red staining
Calcium deposition was measured by Alizarin red staining (late osteogenic marker).Cells were fixed with ice-cold ethanol for one hour at -20 °C.After washing with tap water,cells were stained with 0.5%w/vAlizarin Red solution (pH = 4.0) for 30 min at ambient temperature.Unbound Alizarin red was removed by washing with tap water.The resulting staining (red) was assessed microscopically.Bound Alizarin red was resolved with 10%w/vcetylpyridinium chloride.Resolved Alizarin Red was quantified photometrically (λ = 562 nm) with a plate reader and corrected to the background.Changes in matrix mineralization are displayed relative to untreated cells[18,25].
Free radical production – 2’,7’-dichlorofluorescein-diacetate assay
To measure the formation of reactive oxygen species (ROS),2′,7′-dichlorofluoresceindiacetate (DCFH-DA) fluorescent probe was used.Briefly,cells were washed with PBS and incubated with 10 μmol/L DCFH-DA in serum-free culture medium for 25 min at 37 °C.After washing twice with PBS,cells were stimulated with AE according to the experimental setup.As a positive control,0.01%v/v(882 μmol/L) H2O2was used.The fluorescence intensity,representing levels of O2−,H2O2,HO,and ONOO−,was quantified after 0,5,10,and 15 min of incubation using a plate reader (excitation = 485 nm/emission = 520 nm).The slope of the linear part of the curve,resembling the product formation rate,was calculated.Cellular localization of the fluorescence was confirmed by fluorescence microscopy[18,25].
Primary cilia length - Immunofluorescent staining
Primary cilia length was determined by acetylated α-tubulin immunofluorescence staining.After washing with PBS,cells were fixed with 4%w/vparaformaldehyde for 10 min at room temperate.Briefly,cells were washed and permeabilised with 0.2%v/vTriton-X-100 solution for 20 min at room temperature,followed by treatment with 2%v/vparaformaldehyde for 10 min at room temperature.Unspecific binding sites were blocked (5%w/vBSA in PBS) for one hour at room temperature,followed by incubation with anti-acetylated α-tubulin antibody SC-23,950 (1:100 in PBS,Santa Cruz,Heidelberg,Germany) overnight at 4 °C.After washing,cells were incubated with ALEXA-488 labelled secondary antibody (1:2000 in DPBS,Life Technologies,Darmstadt,Germany) for 2 h at room temperature.Nuclei were stained with Hoechst 33342 (1:1000).Images were taken with an epifluorescence microscope (EVOS FL,life technologies,Darmstadt,Germany),and primary cilia length was analyzed using the ImageJ software (Version 1.5,NIH,Bethesda,MD,United States) (line tool) based on the maximum intensity projection method[39,40].
Statistical analysis
Results are presented as mean ± SE.Each experiment was performed three independent times for SCP-1 cells or with five donors for hOBs (biological replicates,n= 3;n= 5 respectively) measured as triplicate or more (technical replicates,n≥ 3).Statistical analyses were performed using the GraphPad Prism Software (Version 5,El Camino Real,United States).Data sets were compared by the nonparametric Mann Whitney testU-test (two single groups) or the Kruskal-WallisHtest (multiple groups)followed by Dunn’s multiple comparison test.α = 0.05 was set as the maximum type I error rate.aP<0.05,bP<0.01,cP<0.001 are used to classifyPvalues in comparisons with untreated cells within AE concentration;eP<0.05,fP<0.01,gP<0.001 are used to classifyPvalues for comparisons between AE from conventional cigarette and AE from THS system within AE concentration.The statistical methods of this study were reviewed by Johann Jacoby from Institute for Clinical Epidemiology and Applied Biometry at the University of Tübingen.
RESULTS
THS and conventional cigarette AE characterization
In order to compare the AE generated with THS and conventional cigarettes,the time to consume one unit,the pH,and the absorbance at 320 nm were measured after AE generation.The time to consume one unit was 1.6 minutes longer for THS than for a conventional cigarette,a difference that was significant (Figure1A).The pH of the AE varied:Kruskal-Wallisχ2df=2 = 8.9027,P= 0.0116.In particular,the pH of the AE generated from the THS (Median = 8.17) was higher than that generated from conventional cigarettes (Median = 7.70,Figure1B).Interestingly,the absorbance at 320 nm of the AE produced from conventional cigarettes was two-fold higher than the AE produced from THS (Figure1C),suggesting that the AE generated with regular cigarettes possessed significantly increased turbidity (presence of suspended particles)compared to AE generated using THS.AE generated with THS showed no significant differences in suspended particulates compared to the cell culture medium.This result was corroborated by increases in the amount of particles found in the gas washing bottle after conventional cigarette AE generation compared to THS AE generation(Figure1D).
THS has less effect on cell viability than conventional cigarettes following a single exposure
In order to evaluate the impact of THS on MSCs and the viability of bone-forming cells,SCP-1 cells and hOBs were exposed to fresh AE generated using either conventional cigarettes or THS.After 48 h,cell viability was evaluated by resazurin conversion and esterase activity.The use of THS produced a significant reduction in SCP-1 cells' metabolic activity with concentrations of 2 × 10-1and 4 × 10-1puff/mL when compared to control cells (Figure2A).However,for conventional cigarettes AE,similar adverse effects on SCP-1 cell viability were observed with concentrations of 2 ×10-1,4 × 10-1and 4 × 10-2puff/mL (Figure2A).We detected a significant decrease of SCP-1 cell viability by approximately 30% for THS and conventional cigarettes compared to control,however concentrations from THS were five times higher than the conventional cigarettes (Figure2A).Esterase activity (measured with calcein-AM)confirmed the previous results,as 4 × 10-2puff/mL AE from conventional cigarettes produced detrimental effects on SCP-1 cell viability compared to AE generated from THS (Figure2C).Cytotoxicity from THS was decreased in hOBs when compared to MSCs.Interestingly,AE from conventional cigarettes produced a stronger effect on the viability of hOBs compared to MSCs (Figure2A,B).A significant reduction to 35%viable cells was observed with AE from conventional cigarettes compared to untreated hOBs (Figure2B).THS only showed a significant decrease to 67% viable cells for the highest concentration evaluated (4 × 10-1puff/mL) (Figure2B).From these results,we concluded that THS AE was significantly less toxic to bone-forming cells and osteoprogenitor cells than AE from conventional cigarettes following a single exposure.
THS is less toxic to bone cells than conventional cigarettes following chronic exposure
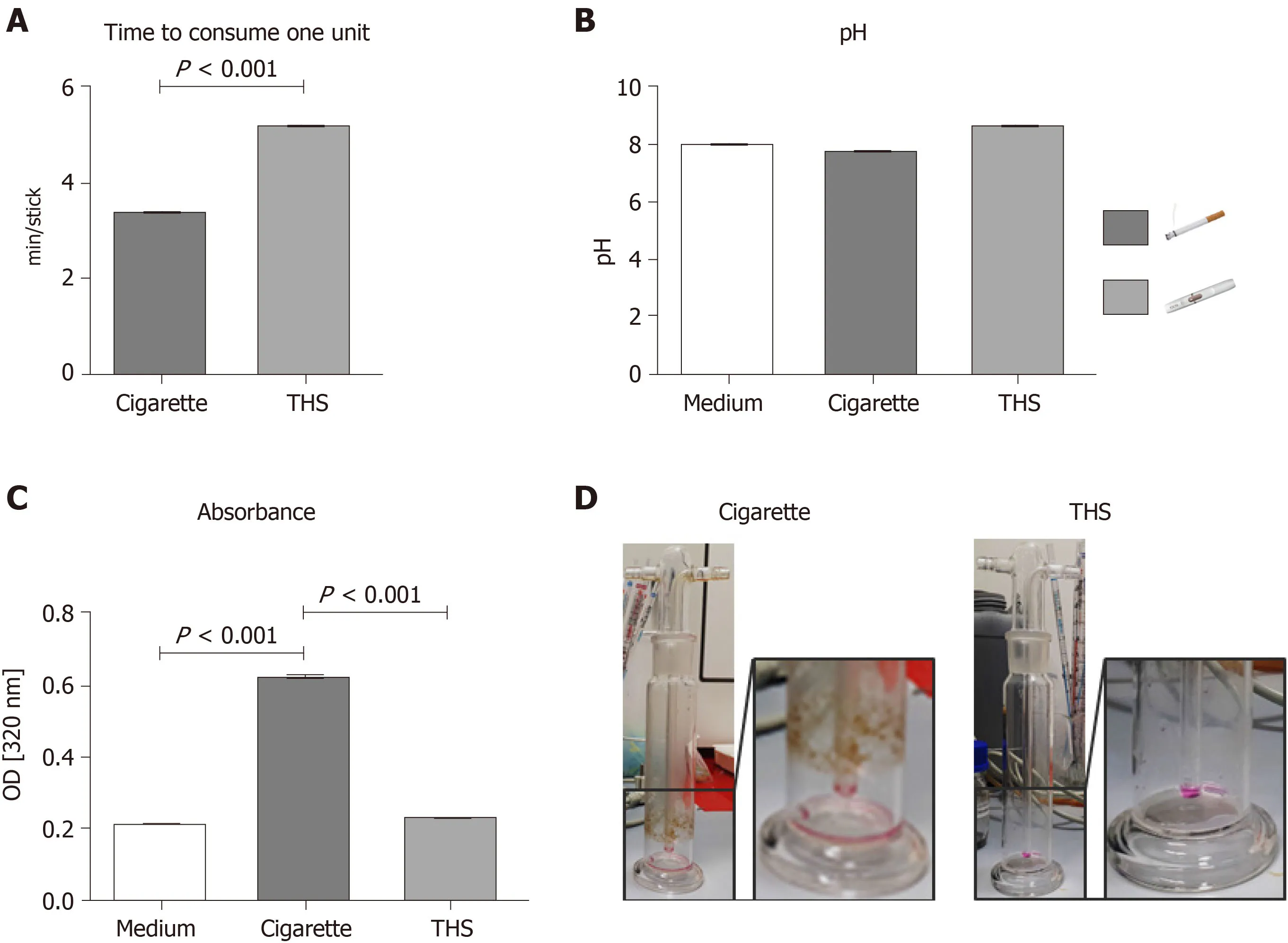
Figure1 Tobacco heating systems and conventional cigarette aqueous extract characterization.A:Time to consume one unit [cigarette or tobacco heating systems (THS) stick] measured in minutes;B:pH from the aqueous extract (AE) generated with conventional cigarettes or THS;C:Absorbance at 320 nm from the different fractions produced from conventional cigarette or THS;D:Representative picture of gas washing bottle after conventional cigarette or THS AE generation,respectively.Each measure was conducted with six independent AE for each condition in triplicates.Data were analyzed using the nonparametric Mann Whitney test or the Kruskal-Wallis H test followed by Dunn’s post-hoc tests.Data are presented as mean ± SE,and P <0.001 for the comparison.THS:Tobacco heating systems.
Chronic smokers consume cigarettes on a regular basis over long periods of time.Therefore,smokers are in continuous and repeated contact with the toxic compounds present in cigarette smoke.The toxic components of cigarette smoke are distributed throughout the bloodstream to the entire body,producing adverse effects on several different tissues and cells,including the bone-forming cells.To better understand the cytotoxicity of tobacco alternative products on MSCs and hOBs,we evaluated the effects of chronic exposure.SCP-1 cells and hOBs were osteogenically differentiated with AE from conventional cigarettes and THS.The concentrations of AE ranged from 4 × 10-5puff/mL to 4 × 10-2puff/mL.These concentrations were chosen based on previous results obtained from the single exposure experiment (Figure2).The concentrations 2 × 10-1puff/mL and 4 × 10-1puff/mL were not used for further experiments due to the cytotoxic effects observed from the single exposure (Figure2).Following either 14 or 21 d of osteogenic differentiation,the metabolic status of the cells was evaluated using resazurin conversion assay (Figure3).Chronic exposure to THS AE resulted in no significant changes in SCP-1 cell viability after either 14 or 21 days of osteogenic differentiation when compared to untreated cells (Figure3A,B).As expected,conventional cigarette AE significantly reduced cell viability by 50% using a concentration of 2 × 10-2puff/mL after 14 and 21 d of exposure compared to control cells (Figure3A,B).Conventional cigarette AE significantly increased cytotoxicity using concentrations of 4 × 10-2puff/mL and 2 × 10-2puff/mL on SCP-1 cells after 14 and 21 d of chronic exposure,respectively (Figure3A,B).Similarly,THS AE did not show cytotoxic effects in hOBs exposed to concentrations up to 4 × 10-2puff/mL after either 14 or 21 d of chronic exposure (Figure3C,D).However,conventional cigarette AE significantly decreased hOBs viability following exposure to a concentration of 4 ×10-2puff/mL (Figure3C,D).Chronic exposure to 4 × 10-2puff/mL conventional cigarette AE resulted in a significant decrease in hOBs mitochondrial activity in comparison to THS exposed to the same concentration (Figure3C,D).These results were confirmed by the EC50calculated for THS and conventional cigarette AE.In the case of THS AE,an EC50of 0.19 puff/mL and >0.04 puff/mL were calculated for SCP-1 cells and hOBs,respectively.The EC50of conventional cigarettes was lower in SCP-1 cells (0.02 puff/mL) and hOBs (0.03 puff/mL).Thus,THS showed less cytotoxic effects than conventional cigarettes on bone cells chronically exposed to AE.Aditionally,MSCs were more sensitive to AE adverse effects that hOBs.
THS has a lower impact on MSCs and human osteoblast function after chronic exposure
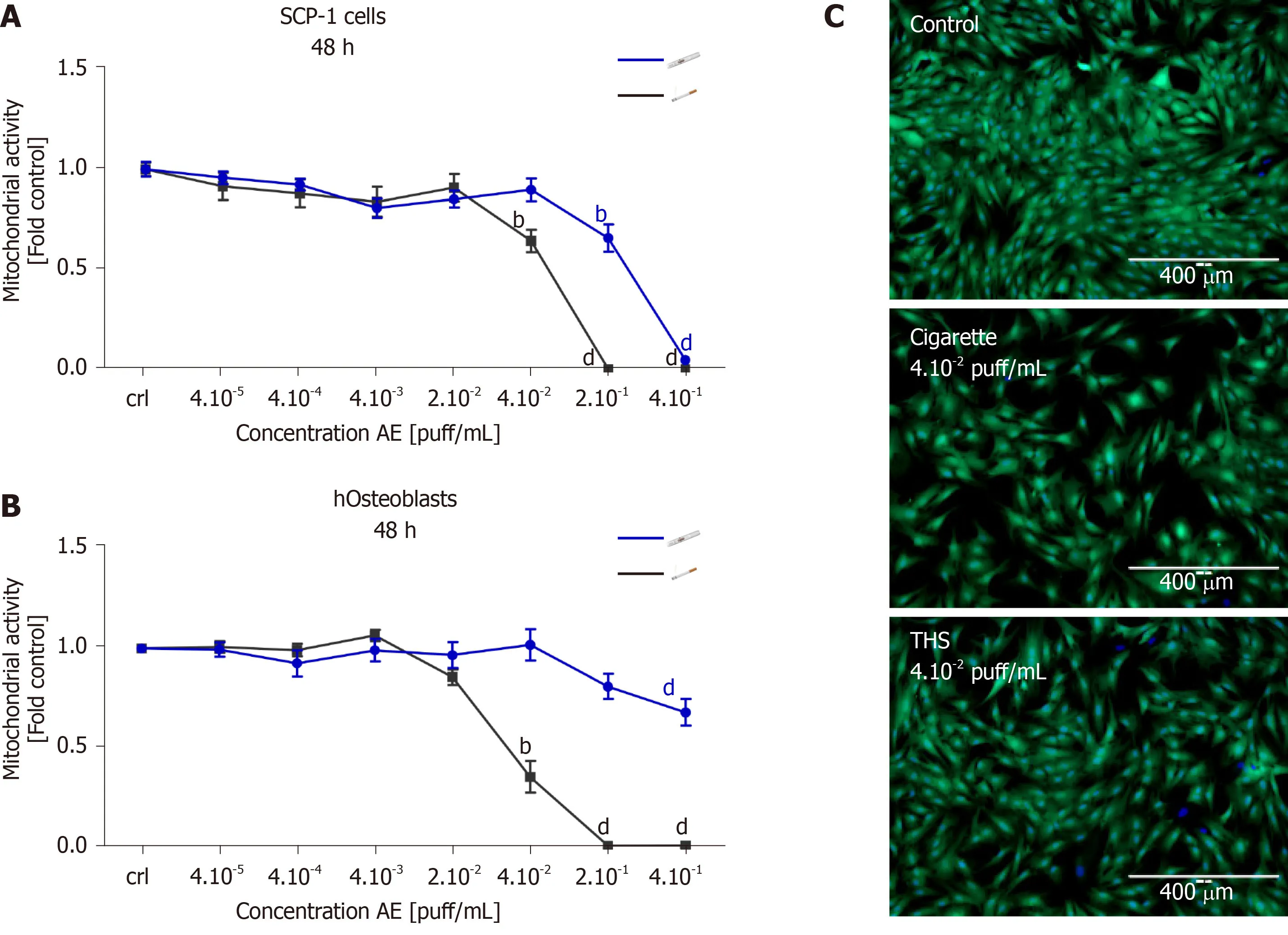
Figure2 High concentrations of aqueous extract from tobacco heating systems has minor effects on bone cells viability after a single exposure than aqueous extract from conventional cigarettes.SCP-1 cells and primary human osteoblasts were treated with increasing concentrations of aqueous extract (AE) from conventional cigarettes and tobacco heating systems.Cell viability was evaluated by resazurin conversion (mitochondrial activity) in SCP-1 cells (A) and primary osteoblast (B) after 48 h of treatment;C:Representative live staining pictures from SCP-1 cells using calcein-AM (green) and nuclear staining using Hoechst 33342 (blue) was shown after 48 h of exposure to AE (scale bar 400 µm).Each measure was conducted at least three independent times in triplicates.Data were analyzed using the Kruskal-Wallis H test followed by Dunn’s post-hoc tests.Data are presented as mean ± SE,and P values indicated as bP <0.01 and dP<0.001 for comparisons with untreated cells within the same AE type.AE:Aqueous extract.
Since AE from THS produced less cytotoxic effects than AE from conventional cigarettes,we were interested in whether THS would affect osteogenic differentiation of MSCs and hOBs function.The potential of SCP-1 cells to differentiate into osteoblasts and hOBs function were evaluated using AP activity (an early marker of osteogenic differentiation[41]) and calcium deposition (a late marker of osteogenic differentiation[41]) after 14 and 21 d,respectively.As expected,2 × 10-2puff/mL conventional cigarette AE significantly decreased AP activity three-fold in SCP-1 cells when compared to untreated cells following 14 d (Figure4A).In contrast,increased AP activity was observed in SCP-1 cells treated with 4 × 10-2puff/mL AE from conventional cigarettes (Figure4A).This increase in AP activity could be due to the detrimental effects on SCP-1 cell viability observed after treatment with 4 × 10-2puff/mL AE from conventional cigarettes (Figure2A).Nevertheless,THS AE produced significant reductions in the AP activity of SCP-1 cells by 1.6 times that of untreated cells,however the concentration of THS AE was twice as high as that of conventional cigarette AE (Figure4A).Regarding matrix production,THS decreased calcium deposition in a dose-independent manner up to 20% (Figure4B).However,conventional cigarette AE significantly decreased calcium deposition at concentrations of 2 × 10-2and 4 × 10-2puff/mL (Figure4B).Due to the role of AP and matrix formation as early and late markers of osteogenic differentiation respectively,these results indicate that THS had less impact on osteogenic differentiation in SCP-1 cells compared to conventional cigarettes.Repeated exposure of hOBs to THS AE did not affect AP activity (Figure4C).Conventional cigarettes AE significantly decreased AP activity in hOBs in a dose-dependent manner using concentrations higher than 4 × 10-3puff/mL (Figure4C).THS did not significantly affect calcium deposition in hOBs using any of the concentrations evaluated (Figure4D).Only conventional cigarette AE significantly decreased calcium deposition in hOBs using concentrations of 2 × 10-2and 4 × 10-2puff/mL in comparison to THS (Figure4D),suggesting that THS has less impact on hOBs function compared to conventional cigarettes.
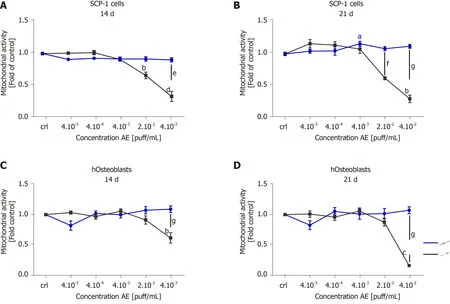
Figure3 Tobacco heating systems is less toxic than conventional cigarettes after chronic exposure.SCP-1 cells and primary human osteoblasts were osteogenically differentiated with increasing concentrations of aqueous extract (AE) from conventional cigarette and tobacco heating systems (THS)for 21 d.Cell viability was evaluated by resazurin conversion (mitochondrial activity) in SCP-1 cells (A,B) and primary human osteoblast (C,D) after 14 (A,C) and 21 d (B,D).Each measurement was conducted at least three independent times in triplicates.Data were analyzed using the Kruskal-Wallis H test followed by Dunn’s post-hoc test.Data are presented as mean ± SE.P values are classified as aP <0.05,bP <0.01,cP <0.001 for comparisons with untreated cells within AE type and as eP <0.05,fP <0.01,gP <0.001 for comparisons of conventional cigarette with THS within the same concentration.AE:Aqueous extract.
THS induces less oxidative stress and causes less damage to primary cilia structures than conventional cigarettes in MSCs.
Since it has previously been demonstrated that oxidative stress induced by the compounds contained in CS causes primary cilia structure disruption,which in turn impairs osteogenic differentiation of SCP-1 cells[18,25];we were interested in evaluating the levels of ROS produced by SCP-1 cells as well as primary cilia structure integrity following THS exposure.As expected,conventional cigarettes AE significantly increased ROS levels by ten-fold in SCP-1 cells using a concentration of 2 × 10-1puff/mL (Figure5A).This increase in oxidative stress seemed to be dose-dependent.Surprisingly,THS showed no significant increase in ROS levels in SCP-1 cells(Figure5A).Only a slight (not significant) increase in ROS levels was observed in SCP-1 cells treated with the highest concentration of THS evaluated (Figure5A).Additionally,THS did not affect the length of the microtubule-based organelles,primary cilia (involved in the initiation and maintenance of MSCs osteogenic differentiation[42,43]) in SCP-1 cells after 14 d of chronic exposure for any of the concentrations evaluated (Figure5B,C).However,primary cilia structure of SCP-1 cells exposed to conventional cigarettes showed significant reductions in length by 50% using a concentration of 4 × 10-1puff/mL (Figure5B,C).Representative immunofluorescence staining pictures of acetylated α-tubulin in SCP-1 cells differentiated with either conventional cigarette AE or THS AE at a concentration of 4× 10-1puff/mL,or untreated,are shown in Figure5C.In summary,THS induced less oxidative stress due to reduced levels of harmful constituents,and did not impair primary cilia structure,thereby producing less impact on MSCs osteogenic differentiation than conventional cigarettes.
No differential variation in cytotoxicity was observed between different THS flavors in MSCs
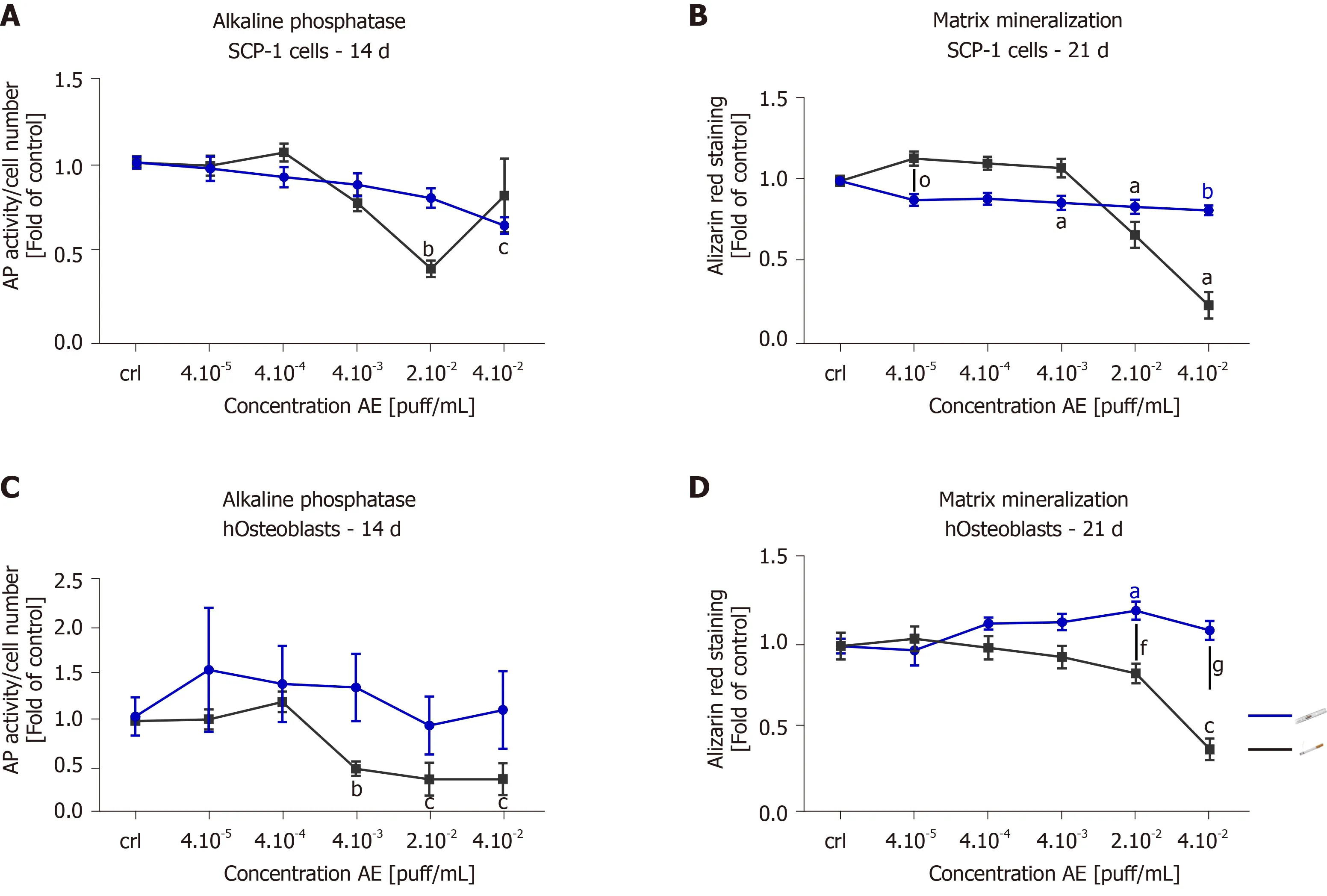
Figure4 Tobacco heating systems has a lower impact on bone cells function than conventional cigarettes.SCP-1 cells and primary human osteoblasts were osteogenically differentiated with increasing concentrations of aqueous extract (AE) from conventional cigarettes and tobacco heating systems(THS) for 21 d.Cell function was evaluated by alkaline phosphatase activity (early differentiation marker) (A,C) after 14 d.Calcium deposition (late marker of differentiation) was evaluated by Alizarin red staining (B,D) after 21 d (B,D).Each measurement was conducted at least three independent times in triplicates.Data were analyzed using the Kruskal-Wallis H test followed by Dunn’s post-hoc test.Data are represented as mean ± SE,and P values are classified as aP <0.05,bP <0.01,cP <0.001 for comparisons with untreated cells within the same AE type and fP <0.01,gP <0.001 for comparisons conventional cigarette with THS within the same concentration.AE:Aqueous extract.
Our previous results showed that THS AE was less harmful than conventional cigarettes on MSCs osteogenic differentiation,as well as hOBs function.Three THSflavored sticks (bronze - mocha and dried fruit flavor,amber - wood and nut flavor,and yellow - herbal flavor) were selected for further cytotoxicity screening.Doseresponses of mitochondrial activity in SCP-1 cells exposed to selected THS-flavored AE are shown in Figure6.No significant differences in cell viability were detected after a 48 h exposure to all AE tested with a concentration up to 4 × 10-2puff/mL compared to untreated cells.It is essential to highlight that a significant decrease in cell viability was observed with concentrations higher or equal to 2 × 10-1puff/mL with all flavored THS AE evaluated (Figure6),thus confirming the results shown in Figure2A.These results suggest that the cytotoxicity observed following THS treatment may be associated with exposure to very high,non-physiological concentrations,and was not associated with flavor.
DISCUSSION
While the detrimental effects of conventional cigarettes on the skeletal system have been extensively reported[4,5],the effects of THS on bone health remain unknown.Smoking conventional cigarettes disturbs bone homeostasis,resulting in an imbalance of bone-forming and bone-resorbing cells,resulting in loss of bone mass and an increased risk of osteoporosis and fracture[6,7,9-11].The detrimental impact of conventional cigarettes on bone function raises the need to develop new alternatives for smokers.ENDS,including e-cigarettes or THS,focus on heating rather than combustion,in order to reduce the generation of harmful constituents.Therefore,ENDS provide the same levels of nicotine as conventional cigarettes and maintain the smoking ritual.E-cigarettes heat liquids based on propylene glycol,glycerin,flavor and (optionally) nicotine into an aerosol that is then inhaled.THS,in contrast,heat tobacco rolled up in a stick form up to 350 °C instead of burning it.

Figure5 Tobacco heating systems induces less oxidative stress and causes less damage to primary cilia structures on bone cells precursor than conventional cigarettes.SCP-1 cells were treated with increasing concentrations of aqueous extract (AE) from conventional cigarettes and tobacco heating systems (THS).ROS production was evaluated by DCFH-DA assay in SCP-1 cells.0.01%v/v H2O2 was used as a positive control (A).Primary cilium length was quantified on day 14 by acetylated α-tubulin (green),and nuclei (blue) immunostaining (B).Representative immunostaining images of SCP-1 cells primary cilia after 14 days of chronic exposure to THS or conventional cigarettes AE (C) (scale bar 100 µm).Each measurement was conducted at least three independent times in triplicates.Data were analyzed using the Kruskal-Wallis H test followed by Dunn’s post-hoc tests.Data are represented as mean ± SE,and P values are classified as aP <0.05,bP <0.01,cP <0.001 for comparisons with untreated cells within AE type.AE:Aqueous extract.
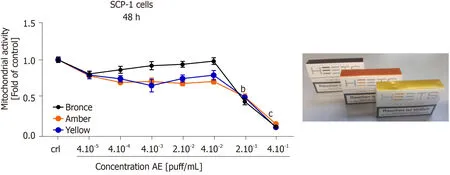
Figure6 No differential variation in cytotoxicity between different tobacco heating systems flavors on bone cells precursor.SCP-1 cells were treated with increasing concentrations of aqueous extract (AE) from tobacco heating systems bronce,amber and yellow flavor.Cell cytotoxicity was evaluated by resazurin conversion (mitochondrial activity) in SCP-1 cells after 48 h of exposure.Each measurement was conducted at least three independent times in triplicates.Data were analyzed using the Kruskal-Wallis H test followed by Dunn’s post-hoc tests.Data are presented as mean ± SE,and P values are classified as b P <0.01,cP <0.001 for comparisons with untreated cells within AE type.AE:Aqueous extract.
A better understanding of the effects of THS on the skeletal system is required.Previousin vitrostudies demonstrated that the detrimental effects of cigarette smoke on bone homeostasis could take place independently of nicotine[18-20].The effects appear to be dependent on oxidative stress induced by constituents present in the cigarette smoke[18,21,23,25,44].
The results presented in this study have demonstrated that the time to consume one unit was significantly longer for THS than conventional cigarettes,thus the smoking experience was longer for THS.Additionally,pH did not significantly change between the different AE fractions and the cell culture medium,validating that this was not the reason for the stronger cytotoxicity observed from conventional cigarette AE.Interestingly,AE produced from THS showed no substantial differences in suspended particulates compared to the cell culture medium.Conversely,AE generated with conventional cigarettes had a significantly increased turbidity.This result was in line with previous studies that have demonstrated that THS delivers less harmful constituents than conventional cigarettes[27,28].
Our analysis demonstrated a reduced impact on MSCs and hOBs viability following treatment with THS AE compared to conventional cigarettes after a single exposure.Moreover,different THS flavors were not related to the cytotoxic effects on bone cells.More sustained adverse effects were observed in response to chronic exposure for 21 d to conventional cigarette AE than to THS AE.This result was supported by reduced cell viability,reduced AP activity,and less mineralized matrix formation measured in bone-forming cells and bone precursor cells.This demonstrated a significantly lower influence by THS AE on the differentiation of MSCs and hOBs function,supporting previous publications regarding decreases in detrimental effects by THS in lung tissue and the cardiovascular system evaluatedin vivoandin vitrowhen compared to 3R4F cigarette combustion[30,31,45,46].
It was demonstrated that oxidative stress induced by the compounds contained in conventional cigarette combustion could be one of the responsible factors for the impaired osteogenic differentiation of bone-forming cells and precursors cells,due to an imbalance in the anti-oxidative system that negatively affects the function of the anti-oxidative enzymes[18,21-25].Interesting,Munakataet al[32]demonstrated that THS induced less oxidative stress than conventional cigarettes in bronchial epithelial cells.Our results also show that THS AE did not increase the level of ROS production in MSCs.Nevertheless,previous results demonstrated that nicotine and cotinine inhibit catalase and glutathione reductase enzymatic activity,affecting the function of the cells' antioxidant system[18].This fact alludes that using ENDS may be a less harmful alternative for smokers that are orthopedic patients with increased oxidative stress levels (due to the trauma and associated surgery).
In our study,we observed no detrimental effect of THS AE on MSCs primary cilia structure.Since primary cilia play an essential role in the initiation of osteogenic differentiation in MSCs,as well as in the maintenance of the differentiated status of the cells[42,47],we conclude that the minor effect of THS on bone-forming cells and progenitor cells is due to decreased oxidative stress,which conserved the primary cilia structure,in contrast to the previous results reported with conventional cigarettes[18,25,39].
A major limitation in this study that could be addressed in future research is that this study focused on the effect of THS AE only in MSCs and hOBs.As several cell types are involved in bone homeostasis,THS could potentially influence the function of other cells,such as immune cells,osteoclasts,or osteocytes.Co-culture systems may provide a better alternative for screening the effects of ENDS on bone metabolism and predicting cytotoxicity in bone tissue.Furthermore,the addition of cytokines,normally increased after fracture,to a co-culture system could better represent the fracture healing process.By combining a more complex setup with different cells types and an inflammatory environment expected after fracture,disturbance in factors that influence the healing could be analyzed under ENDS exposure (e.g.,TGF-β1,MSCs chemoattractant affected conventional cigarette AE[39])
Additionally,our system could be improved by including other cell types,e.g.,endothelial cells or immune cells,to represent better the molecular species that finally cross the bloodstream.Additionally,multiple interconnected cell culture systems representing functionalities of different organs,could represent exposure of boneforming cells closer to humans.In this system,the paracrine interaction among different organs could be instigated as well as the potential toxicity of or side effects of the additional metabolites of ENDS coming from different tissues (e.g.,lung,liver,etc.)could be evaluated.However,it should be consider that increasing the complexity of the model also decreases the analytical methods available for characterization.
This research serves as one of the firstin vitrostudies to demonstrate reductions in the harmful effects on bone-forming cells and bone progenitor cells treated with THS compared to conventional cigarettes.THS could be a potential alternative for smokers to maintain appropriate bone homeostasis and delay development of secondary osteoporosis,which consequently would reduce health system costs.However,more studies are required to confirm if THS could be a viable alternative for smokers to maintain appropriate bone homeostasis and improve delays in bone healing.
ARTICLE HIGHLIGHTS
Research background
Cigarette smoking (CS) is the most common method of consuming tobacco.Deleterious effects on bone integrity,increased incidence of fractures,and delayed fracture healing are all associated with CS.Tobacco combusted at about 800 °C generates approximately 6500 molecular species,more than 150 of which have been identified as toxic compounds.New approaches have been on developing reducedrisk alternatives for smokers that maintain the smoking ritual,while providing the same levels of nicotine as conventional cigarettes with less harmful constituents.
Research motivation
New technologies designed to develop a reduced-risk alternative for smokers are based on electronic nicotine delivery systems,such as e-cigarettes and tobacco heating systems (THS).Instead of burning tobacco,THS heat tobacco rolled up in a stick form up to 350 °C (avoiding combustion and formation of ashes).THS contain tobacco and convey the feeling of smoking a conventional cigarette.Several studies have demonstrated reduced levels of toxic and harmful compounds from electronic nicotine delivery systems.
Research objectives
The present study aim to examine the effects of THS on osteoprogenitor cell viability and function compared to conventional CS.
Research methods
Human immortalized mesenchymal stem cells and primary human pre-osteoblasts isolated from cancellous bone samples were osteogenically differentiated with aqueous extracts generated from either the THS 2.4 “IQOS” or conventional“Marlboro” cigarettes for up to 21 d.Cell viability was analyzed using resazurin conversion assay (mitochondrial activity) and calcein-AM staining (esterase activity).Osteogenic differentiation and bone cell function were evaluated using alkaline phosphatase (AP) activity,while matrix formation was analyzed through alizarin red staining.Primary cilia structure was examined by acetylated α-tubulin immunofluorescent staining.Free radical production was evaluated with 2′,7′-dichlorofluorescein-diacetate assay.
Research results
THS is significantly less toxic to bone cells than CS when analyzed by mitochondrial and esterase activity (P <0.001).No significant differences in cytotoxicity between the diverse flavors of THS were observed.Harmful effects from THS on bone cell function were observed only at non-physiological concentrations.In contrast,conventional cigarettes significantly reduced the AP activity (by two-fold) and matrix mineralization (four-fold) at low concentrations.Moreover,morphologic analysis of primary cilia revealed no significant changes in the length of the organelle involved in osteogenesis of osteoprogenitor cells,nor in the number of ciliated cells following THS treatment.Assessment of free radical production demonstrated that THS induced significantly less oxidative stress than conventional CS in osteoprogenitor cells.
Research conclusions
The present study demonstrate reductions in the harmful effects on bone-forming cells and bone progenitor cells treated with THS compared to conventional cigarettes.
Research perspectives
THS could be a potential alternative for smokers to maintain appropriate bone homeostasis and delay development of secondary osteoporosis,which consequently would reduce health system costs.
ACKNOWLEDGEMENTS
We would like to Dr.Alexander Nussbaum for providing the THS 2.4 system "IQOS®"device and sticks,Bianca Braun for their excellent technical assistance,Dr.Julia Hoeng,Dr.Bjorn Titz,Dr.Anita Iskandar and Shoaib Majeed (M.Eng.) for the interesting discussions.
杂志排行
World Journal of Stem Cells的其它文章
- Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection
- Practical choice for robust and efficient differentiation of human pluripotent stem cells
- Stem cell therapy for Alzheimer's disease
- Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration
- Off-the-shelf mesenchymal stem cells from human umbilical cord tissue can significantly improve symptoms in COVID-19 patients:An analysis of evidential relations
- Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes
