Practical choice for robust and efficient differentiation of human pluripotent stem cells
2020-09-18
Mei Fang,Li-Ping Liu,Hang Zhou,Yu-Mei Li,Yun-Wen Zheng,Institute of Regenerative Medicine,Affiliated Hospital of Jiangsu University,Jiangsu University,Zhenjiang 212001,Jiangsu Province,China
Yun-Wen Zheng,School of Biotechnology and Heath Sciences,Wuyi University,Jiangmen 529020,Guangdong Province,China
Yun-Wen Zheng,Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery,University of Tsukuba Faculty of Medicine,Tsukuba,Ibaraki 305-8575,Japan
Yun-Wen Zheng,Yokohama City University School of Medicine,Yokohama,Kanagawa 234-0006,Japan
Yun-Wen Zheng,Division of Regenerative Medicine,Center for Stem Cell Biology and Regenerative Medicine,The Institute of Medical Science,the University of Tokyo,Tokyo 108-8639,Japan
Abstract
Key words:Human pluripotent stem cells;Three dimensional;Embryoid body;Differentiation;Efficient;Three germ layers
INTRODUCTION
The first five lines of human embryonic stem cells (hESCs) were obtained in 1998 from the inner cell mass of a 3- to 5-day-old fertilized embryo[1].Subsequently,induced pluripotent stem cells (iPSCs) were created by reprogramming fibroblasts[2].Human pluripotent stem cells (hPSCs),including hESCs and human (h)iPSCs,have the ability to self-renew and differentiate into any cell type from all germ layers[3],driving the development of regenerative medicine.The cells and organoids derived from hPSCs have various potential applications including complex diseases studies,cell-based drug screening,and limitless transplantation treatments[4,5].With the rapid development of regenerative medicine technology,many differentiation approaches based on hPSCs have been explored.However,some challenges remain.To meet the needs of clinical application and basic research,high efficiency and stability are the key objectives during hPSC differentiation into high-quality target cells.Thus,it is important to identify an efficient and robust differentiation approach that can increase the differentiation ratio of target cells,produce stronger functions in cells,generate more complete structural organoids,or be reproduced in different cell lines or in other laboratories.Currently,there are great differences in these experimental schemes.Differentiation efficiency[6]and stability are impacted by whether the method is based on an embryoid body (EB) or a two-dimensional (2D) or 3D system,or if single or multiple cell co-cultures are used.
In this review article,we combine the experiences of our laboratory with a summary of existing mainstream approaches involving hPSC differentiation,with the goal of providing a reference and time-saving guide for future experimental design.
DIFFERENTIATION INDUCTION FROM HPSCS
EB-based differentiation system
EB has been a very common model ofin vitrohPSC differentiation for more than 50 years[7].The EB-based method is widely used to differentiate majority of cell lineages from the three germ layers (Table1) and has an obvious advantage in improving the differentiation efficiency of some cells[8-10],such as hematopoietic progenitors[11]and melanocytes[12].Combined with suspension bioreactor technology,this advantage can be further amplified for large-scale production[13].Additionally,EB formation provides an excellent way to assess and manipulate developmental potential[7].Differentiation predictions can be made in the early stage of EB to predict which germ layer hPSC is likely to differentiate into,which can save on the cost for subsequent differentiation and indirectly improve differentiation efficiency.For example,Spalt like transcription factor 3 (SALL3)expression in EB indicates a high probability of differentiating into the ectoderm and a low chance of differentiating into the mesoderm/endoderm[14].Our study also confirmed these findings,and we found that iPSC lines that expressedhigher levels ofSALL3on day 7 of EB formation showed greater potential for melanocyte differentiation[15].Additionally,miR-371-3 plays both a predictive and functional role in neurogenic differentiation[16],and the low expression of fibroblast growth factor 1 (commonly known asFGF-1),ras homolog family member U(commonly known asRHOU),and thymidine phosphorylase (commonly known asTYMP)genes are associated with low hepatic differentiation[17],which can be used to predict the differentiation potential in early EB.Therefore,EB-based differentiation systems not only help to increase the percentage of target cells,but also contribute to the prediction of differentiation potentials in the early stage,which improves efficiency directly and indirectly,respectively.
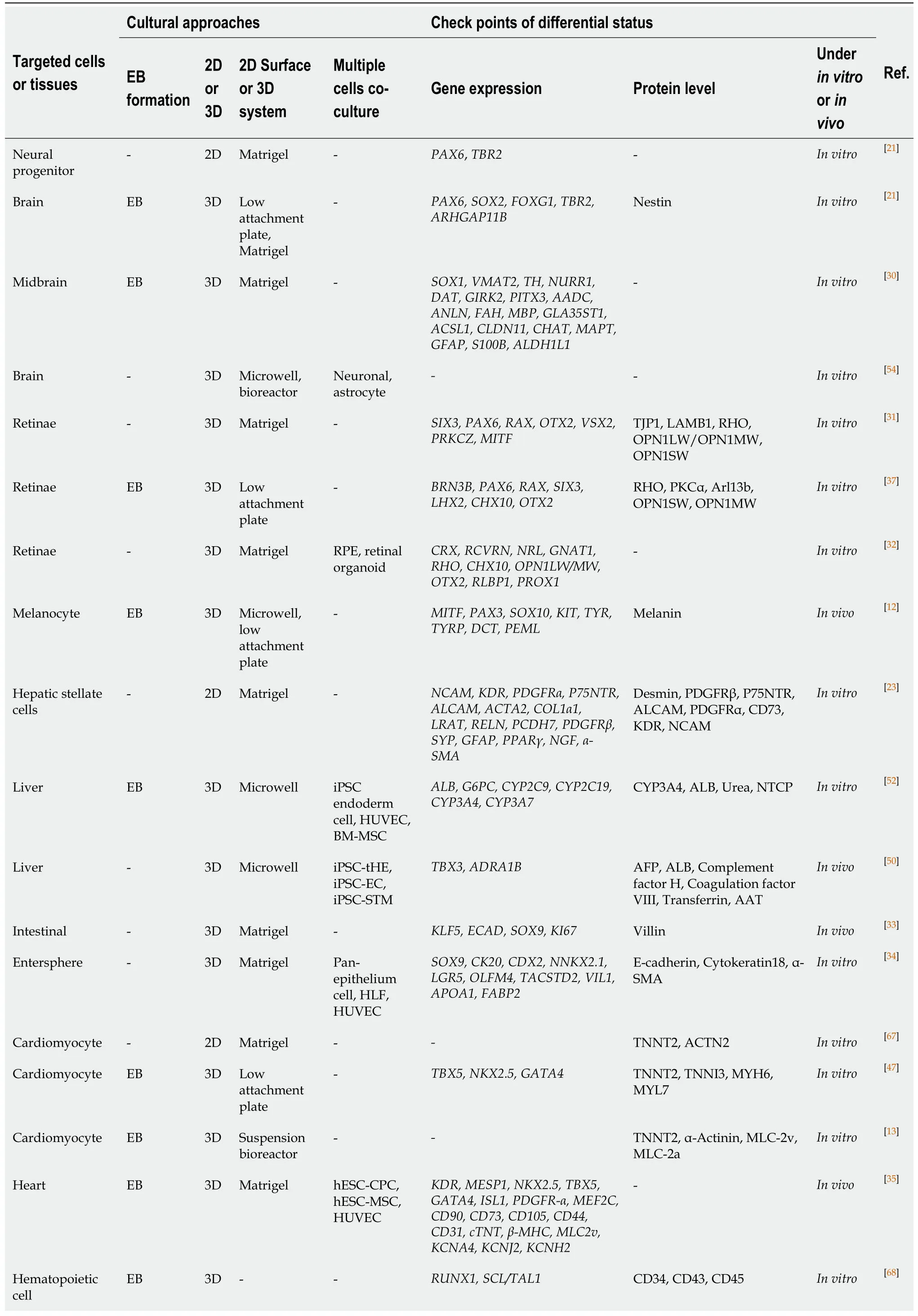
Table1 Summary of current approaches for human pluripotent stem cells differential direction into targeted cells or tissues

-:None;EB:Embryoid body;2D:Two-dimensional;3D:Three-dimensional;RPE:Retinal pigment epithelium;iPSCs:Induced pluripotent stem cells;HUVECs:Human umbilical cord vein endothelial cells;BM-MSC:Bone marrow mesenchymal stem cell.
Matrigel-mediated system
Matrigel,a natural extracellular matrix,is widely used in hPSC maintenance and can also be used in 2D and 3D hPSC differentiation (Table1).During 2D differentiation,the culture vessels are first coated with Matrigel,followed by single cell or cell cluster inoculation.The role of Matrigel in 2D is adherence of cells or cell clumps to a culture vessel.Furthermore,the major component of Matrigel is laminin,which promotes the formation of a rigid neuroepithelial structure[18].Laminin-positive basement membranes are crucial for continuous epithelial integrity[19].A massive volume increase of the human neocortex results from expansion of the cortical area and the related emergence of extensive cortical folding[20],which is thought to be due to the increase of the proliferative potential of neural progenitors (NPs)[21].As this study shows,two human ESC lines were differentiated into NPs in Matrigel-coated 2D adherent culture.Jaenisch and his colleagues constituted human cerebral organoids in an EB-based 3D system,which displayed markedly increased outgrowth of neuroepithelial tissue surrounding ventricle-like structures[21].Other desired cells can also be differentiated in a Matrigel-coated 2D culture system such as hepatocytes[22],hepatic stellate cells[23],intestinal epithelium[24,25],mesenchymal cells[26],cardiomyocytes(CMs)[27],monocytes,and macrophages[28].Thus,the Matrigel-based 2D culture approach is a basic method for the directed induced differentiation of hPSCs.
Matrigel can also be used for 3D differentiation of hPSCs.In addition to coating the substrate,the Matrigel-based 3D construct is formed by adding mixed Matrigel and special differentiation medium[29]in the hPSC differentiation process,resulting in differentiation in the solution of a suspended system.A 3D differentiation system provides enough space for establishing an organoid,and promotes cellular communication and interaction among cells compared to a 2D approach.Currently,many target cell lineages or tissues can be differentiated in this way including the brain[30],retinae[31,32],intestinal organoids[33,34],and heart[35].Interestingly,after adding Matrigel,retinal induction cells increase by up to 30%-70% of the total cells in the low cell adhesion plate[18].Because this gel promotes the epithelialization of hPSCs toward retinal differentiation,researchers have tried to use 3D Matrigel methods for differentiating hPSCs.Epithelialized cysts are obtained by floating culture clumps of Matrigel/hESCs and the subsequent floating culture results in self-formation of retinal organoids[31].This includes patterned neuroretina,ciliary margin,and retinal pigment epithelium,which autonomously generates stratified retinal tissues,comprising photoreceptors with ultrastructure of outer segments in long-term culture.This system has been validated in two lines of human hPSCs[31].Clearly,the use of Matrigel is common in 2D or 3D differentiation of hPSCs into target cells.However,the Matrigelembedded 3D differentiation system has distinct advantages in self-organizing and generating organoids with a more complete structure when compared to a 2D culture.
3D suspension system
During hPSC differentiation,there are many decisions in creating a 3D floating state such as a non- or ultra-low attachment plate,microwell plate,and suspended bioreactors.At present,a variety of cell lineages have been generated by using 3D suspension system such as eye[36-38],skin[39],brain[40-43],liver[44,45],heart[46,47]and blood[11].For example,during the 3D differentiation process,the authors generated iPSCderived fully functional hepatocyte-like organoids in gene expression,protein secretion,and biotransformation[48].Likewise,iPSC-derived platelets can be harvested by using a 3D differentiation system,and it is very similar to human platelets in terms of both ultrastructural features andin vivoandin vitrofunctional characterizations[49].Thus,the 3D differentiation system can produce cells with ideal functions.The yield of differentiated cells is also important.The omni-well-array culture platform can produce massive and miniaturized iPSC-derived liver buds on a clinically relevant large scale (>108)[50].Hamaet al[13]designed a protocol that generated >90% hiPSCderived CMs that yielded on average 72 million cells per 100 mL in a 3D bioreactor.These results show that the yield from the 3D suspension system is remarkable in contrast to the 2D system.To test the reproducibility of the CM 3D differentiation protocol,a previous study compared biologically independent experiments with various passage numbers of iPSCs,and found minor inter-experimental variations[13].Overall,the 3D differentiation culture appears to have advantages in differentiation efficiency and stability over the 2D system.This indicates that the 3D differentiation method is optimal when hPSCs differentiation experiments are conducted.
Multiple cells co-culture system
Each organ has a variety of cell components with a certain structure and its own specific functions.Because of the communication and interaction among cells,coculturing with different supportive and tissue-constructive cells has been become attractive.The benefits of co-culturing multiple cells are that they can facilitate communication and interaction among different cells,enhance the hPSCs differentiation efficiency[51],and better simulate the environmentin vivo.It can bring surprises when used in a co-culture system to self-organize and generate an organoid.For example,to recapitulate hepatitis B virus-host interactions in liver organoids,Nieet al[52]co-cultured iPSC endoderm cells,human umbilical cord vein endothelial cells(HUVECs),and human bone marrow mesenchymal stem cells to form liver organoids in a 3D microwell plate,which exhibits stronger hepatic functions than iPSC-derived hepatic like cells.Furthermore,the co-culture pattern also has a higher differentiation yield[48]and organoids with more complex functions[53].There are co-culture combinations in other studies such as co-culturing hPSC-derived neurons and astrocytes[54];co-culturing iPSC-derived hepatic parenchymal and non-parenchymal cells[55];co-culturing hiPSC-derived retinal pigment epithelium and retinal organoids[32];and co-culturing HUVECs,hESC-derived MSCs,and hESC-derived cardiac progenitor cells[35].Human PSC-derived organoids with multiple cell components have a complete structure and sturdy function similar to a human organ,which may provide an alternative source for organ transplantation.Therefore,the 3D culture method is a better choice for organoid generation.
Transcription factor-directed differentiation
Transcription factors (TFs) play an important role in pluripotent stem cell induction and transdifferentiation[56].Recently,they have been used to differentiate hPSCs into desired cells or tissues such as neural[57],liver[58,59],and cardiac muscle[60,61].A growing body of TF-directed differentiation method of hPSCs has demonstrated that efficient cell fate is reprogrammedviaforced expression of single or multiple TFs[62].Sunet al[63]used the technique to design a single-step protocol for forebrain GABAergic neuron differentiation,which could generate cells similar to rodent cortical interneurons with>80% efficiency,and the target cells showed mature functional properties within 6-8 wk.By contrast,other process takes as long as 30 wk[64].The TF-mediated method can differentiate hPSCs into terminal cells directly,and the experimental procedure is relatively brief.
LIMITATION AND CHOICE
Current methods for hPSC differentiation described above have various limitations.2D differentiation culturing is performed on the surface of the culture vessel and the limited contact area limits the yield of the target cells.Furthermore,all structural components of organoids cannot be generated[37,65].Without 3D contact with Matrigel,Loweet al[31]reported that most cells died and the few surviving cells formed solid cell masses on 2D culturing.Most 3D culture methods involve various intermediate stages requiring varying combinations of recombinant factors and small molecules[63],thus rendering the method cumbersome to repeat.Although TF-mediated methods improve the differentiation efficiency of hPSCs,numerous tools for TF transfection,including plasmids and viruses,have led to the integration of exogenetic genes[66]into the target cells,thus presenting a remote prospects for their clinical application[56].In this situation,EB-based 3D culture systems allow for large-scale directional differentiation of hPSCs,and the co-culture method seems to constitute highly functional organoidsin vitroto compensate for organ transplantation insufficiency.
CONCLUSION
Although only a few articles have compared the differences between 2D and 3D differentiation,it can be concluded that 3D system with EB has obvious advantages for hPSC differentiation compared to 2D culture.The details of the differentiation approaches are shown in the “cultural approaches” of Table1.Regarding future studies,there are some key recommendations.First,the ability of EB not only can scale up culture systems and differentiation,but also predict the fate of hPSCs differentiation for reducing unnecessary waste.Second,the 3D differentiation system also has significant improvement in differentiation efficiency,and 3D space is necessary for organoid formation.Finally,it is a promising and challenging task that co-cultures multiple kinds of cells,supportive,structured,vascularized and further neurovascularized for organoid organization in 3D suspension system.Simply put,an EB-based 3D differentiation culture system is an efficient and powerful choice for hPSCs to meet the demand in clinical applications and basic research.
ACKNOWLEDGEMENTS
We thank the kind support and help suggestions from Drs.Ning-Ning Guo and Qian Zhang,and other Lab members.
杂志排行
World Journal of Stem Cells的其它文章
- Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection
- Stem cell therapy for Alzheimer's disease
- Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration
- Off-the-shelf mesenchymal stem cells from human umbilical cord tissue can significantly improve symptoms in COVID-19 patients:An analysis of evidential relations
- Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes
- Autophagy in fate determination of mesenchymal stem cells and bone remodeling
