基于二甲基苯并联咪唑的钌(Ⅱ)多吡啶配合物的合成、光谱和电化学性质
2020-09-10曹曼丽尚美洁刘文婷姚苏洋叶保辉
曹曼丽 尚美洁 刘文婷 尹 伟 姚苏洋*, 叶保辉*,
(1广东第二师范学院化学系,广州 510303)
(2广东省普通高校先进材料与节能减排工程技术开发中心,广州 510303)
(3中山大学化学学院,广州 510275)
Ruthenium(Ⅱ)polypyridyl complexes have been deeply researched because of their excellent redox and photophysical properties,particularly,high stability and absorption and emission spectra in visible range,promising applications as solar energy conversion,molecular electronics,and so on[1-4].Among them,ruthenium polypyridyl complexes based on biimidazole(H2biim)[5-10]or bibenzimidazole(H2bbim)[9-13]or their derivatives moieties[14-19]have received much attention.The presence of the acidic imidazole proton allows deprotonation of the complexes and this results in a rich chemistry where changes in pH can be used to affect the electrochemical and photophysical properties of the complexes[20].The ability of complexes containing the biimidazole or bibenzimidazole ligands to engage in hydrogen bonding via the externally-directed pair of N-H groups has been exploited extensively in crystal engineering and supramolecular chemistry.
As is well known,hydrogen bonding is one of the mostpopular tools in supramolecular chemistry because hydrogen bonds have relatively high strength and predictable directional nature compared to other non-covalentinteractions[21-23].There is a growing awareness of the important role that hydrogen-bonded complexes can play in complex electron-transfer mechanisms[24].The proton-coupled electron transfer(PCET)reactions can occur within hydrogen-bonded intermediates.With incorporation of reversible redox couples and proper designs,electron transfer can provide a convenient means both to detect the hydrogen-bonded complexes and control their assembly.
In this paper,we reported a family of ruthenium(Ⅱ)complexes,namely[(bpy)2Ru(DMBbimHx)]y+(DMB-bimH2=7,7′-dimethyl-2,2′-bibenzimidazole,1-A:x=2,y=2;1-B:x=1,y=1;1-C:x=0,y=0).Theun-deprotonated 1-A,mono-deprotonated 1-B and bi-deprotonated 1-C were synthesized,and their spectroscopic and electrochemical properties were studied.Interestingly,different electrochemical behaviors of the mono-deprotonated 1-B were observed in dichloromethane and the more polar acetonitrile.In dichloromethane,it undergoes a two-step oxidation,which indicates the existence of PCET between the[Ru(bpy)2(DMBbimH)]+cations.
The single crystals of[Ru(bpy)2(DMBbimH)]PF6·2CH2Cl2(2),hydrogen-bonded dimer of[Ru(bpy)2(DMBbimH)]+,were obtained from the solution of 1-B in dichloromethane.The[Ru(bpy)2(DMBbimH)]+cations exist as hydrogen-bonded dimers in dichloromethane,which is consistent with the result of electrochemical measurements of complex 1-B.
1 Experimental
1.1 Materials and methods
The reagents and solvents employed were commercially available and were used as received.1H NMR spectra were recorded on a Varian Mercury-Plus 300 NMR spectrometer with chemical shifts relative to tetramethylsilane(TMS).The C,H and N microanalyses were carried out with a Vario EL elemental analyzer.Electron spray ionization(ESI)mass spectra were obtained on an LCQ DECA XP quadrupole ion trap mass spectrometer with methanol as the carrier solvent.UV-Vis spectra were performed on a Shimadzu UV-315 UV-Vis spectrophotometer at room temperature,and the solvents used for spectral experiments were distilled from CaH2and kept over molecular sieves(type 4A).
The cyclic voltammetry(CV)and square wave voltammetry(SWV)measurements were made with a CHI-660 Electrochemical Workstation.The experi-ments were performed with a standard three-electrode arrangement:working electrode,glassy carbon electrode;quasi reference electrode,Ag-AgCl electrode;counterelectrode,Ptwire electrode.Background correction was accomplished by subtracting the current curves of the blank electrolyte(containing the same concentration of supporting electrolyte)from the experimental curve.The experiments were performed with 0.001 mol·L-1solutions of the complexes in dried acetonitrile or dried dichloromethane with 0.1 mol·L-1ammonium hexafluorophosphate as supporting electrolyte.
1.2 Syntheses
DMBbimH2[25]andcis-Ru(bpy)2Cl2·2H2O[26]were synthesized as reported in the literature procedures.
1.2.1 Synthesisof[Ru(bpy)2(DMBbimH2)](PF6)2(1-A)[5]
[Ru(bpy)2Cl2]·2H2O(1 mmol),DMBbimH2(1.4 mmol)and ethylene glycol(14 mL)were added into a 50 mL three neck flask.The mixture was stirred and refluxed for 3 h under nitrogen protection,then cooled to room temperature and filtered.100 mL water was added to the filtrate,followed by the addition of 2 mL hydrochloric acid and 0.8 g KPF6.The mixture was stirred for a while then filtered,and the precipitate was rinsed with water several times then dried at 70℃.Yield:85% on the basis of[Ru(bpy)2Cl2]·2H2O.Anal.Calcd.for C36H30F12N8P2Ru(%):C,44.78;H,3.13;N,11.60.Found(%):C,45.04;H,3.32;N,11.78.ESI-MS(positive,CH3OH):m/z675.3([Ru(bpy)2(DMBbimH)]+);338.3([Ru(bpy)2(DMBbimH2)]2+).1H NMR(300 MHz,DMSO-d6):δ13.84(s,2H,N-H),8.83(d,J=8.2 Hz,2H,bpy-H),8.73(d,J=8.1 Hz,2H,bpy-H),8.21(td,J=7.8,1.5 Hz,2H,bpy-H),8.04(td,J=7.9,1.5 Hz,2H,bpy-H),7.98(dd,J=5.7,1.3 Hz,2H,bpy-H),7.88(d,J=5.5 Hz,2H,bpy-H),7.57(ddd,J=7.2,5.6,1.3 Hz,2H,bpy-H),7.46(ddd,J=7.0,5.6,1.3 Hz,2H,bpy-H),7.19(dd,J=7.7,4.4 Hz,2H,DMBbimH2-H6),6.97(t,J=7.7 Hz,2H,DMBbimH2-H5),5.45(d,J=8.3 Hz,2H,DMBbimH2-H4),2.60(s,6H,Me-H).
1.2.2 Synthesis of[Ru(bpy)2(DMBbimH)]PF6(1-B)
The complex 1-A was dissolved in the minimum volume of dichloromethane and subjected to column chromatography on neutral alumina(1 cm×10 cm).A red-violet band was eluted with dichloromethaneacetonitrile(3∶1,V/V).The solvent was evaporated to dryness under vacuum,then the complex 1-B was obtained with a yield of 75%.Anal.Calcd.for C36H29F6N8PRu(%):C,52.75;H,3.57;N,13.67.Found(%):C,52.53;H,3.63;N,13.42.ESI-MS(positive,CH3OH):m/z675.3 ([Ru(bpy)2(DMBbimH)]+);338.3([Ru(bpy)2(DMBbimH2)]2+).1H NMR(300 MHz,DMSO-d6):δ8.77(d,J=8.2 Hz,2H,bpy-H),8.68(d,J=8.1 Hz,2H,bpy-H),8.14(td,J=7.9,1.4 Hz,2H,bpy-H),8.06~7.88(m,4H,bpy-H),7.76(d,J=5.6 Hz,2H,bpy-H),7.52(ddd,J=7.2,5.6,1.2 Hz,2H,bpy-H),7.44(ddd,J=7.1,5.5,1.3 Hz,2H,bpy-H),6.85(d,J=7.2 Hz,2H,DMBbimH-H6),6.66(t,J=7.7 Hz,2H,DMBbimH-H5),5.31(d,J=8.1 Hz,2H,DMBbimH-H4),2.55(s,6H,Me-H).
1.2.3 Synthesis of Ru(bpy)2(DMBbim)(1-C)
The complex 1-A was dissolved in a small amount of methanol,and an excessive amount of ammonia gas was introduced[27],and the precipitate was filtered to obtain the complex 1-C.Yield:50%.Anal.Calcd.for C36H28N8Ru(%):C,64.18;H,4.19;N,16.63.Found(%):C,62.37;H,4.02;N,16.36.ESI-MS(positive,CH3OH):m/z675.3([Ru(bpy)2(DMBbimH)]+);338.3([Ru(bpy)2(DMBbimH2)]2+).1H NMR(300 MHz,DMSO-d6):δ8.69(d,J=8.2 Hz,2H,bpy-H),8.60(d,J=8.1 Hz,2H,bpy-H),8.04(t,J=7.8 Hz,2H,bpy-H),7.89(d,J=5.5 Hz,2H,bpy-H),7.83(t,J=7.8 Hz,2H,bpy-H),7.73(d,J=5.5 Hz,2H,bpy-H),7.45(t,J=6.6 Hz,2H,bpy-H),7.36(t,J=6.6 Hz,2H,bpy-H),6.52(d,J=7.0 Hz,2H,DMBbim-H6),6.32(t,J=7.5 Hz,2H,DMBbim-H5),5.16(d,J=7.9 Hz,2H,DMBbim-H4),2.49(s,6H,Me-H).
1.2.4 Synthesis of[Ru(bpy)2(DMBbimH)]PF6·2CH2Cl2(2)
Single crystals of 2 were grown at room temperature by slow evaporation of the solution of 1-B in dichloromethane.Dark red single crystals were obtained about one week later.Anal.Calcd.for C38H33Cl4F6N8PRu(%):C,46.12;H,3.36;N,11.32.Found(%):C,46.05;H,3.43;N,11.27.
1.3 Structure determination
The intensity data for compound 2 were collected on a Bruker Smart Apex CCD area detector,using graphite-monochromated MoKαradiation(λ=0.071 073 nm).The structure was solved by direct method and refined by full matrix least squares techniques againstF2(SHELXTL-2016 program package[28-29]).The hydrogen atoms of the“amine group”were located by Fourier difference synthesis and refined isotropically.All other hydrogen atoms were included at calculated positions with fixed thermal parameters.All non-hydrogen atoms were refined anisotropically. The solvents were squeezed by SQUEEZE/PLATON program.Crystal data,data collection parameters and refinement statistics are listed in Table 1.Selected bond lengths and angles are presented in Table 2.
CCDC:2005043,2.
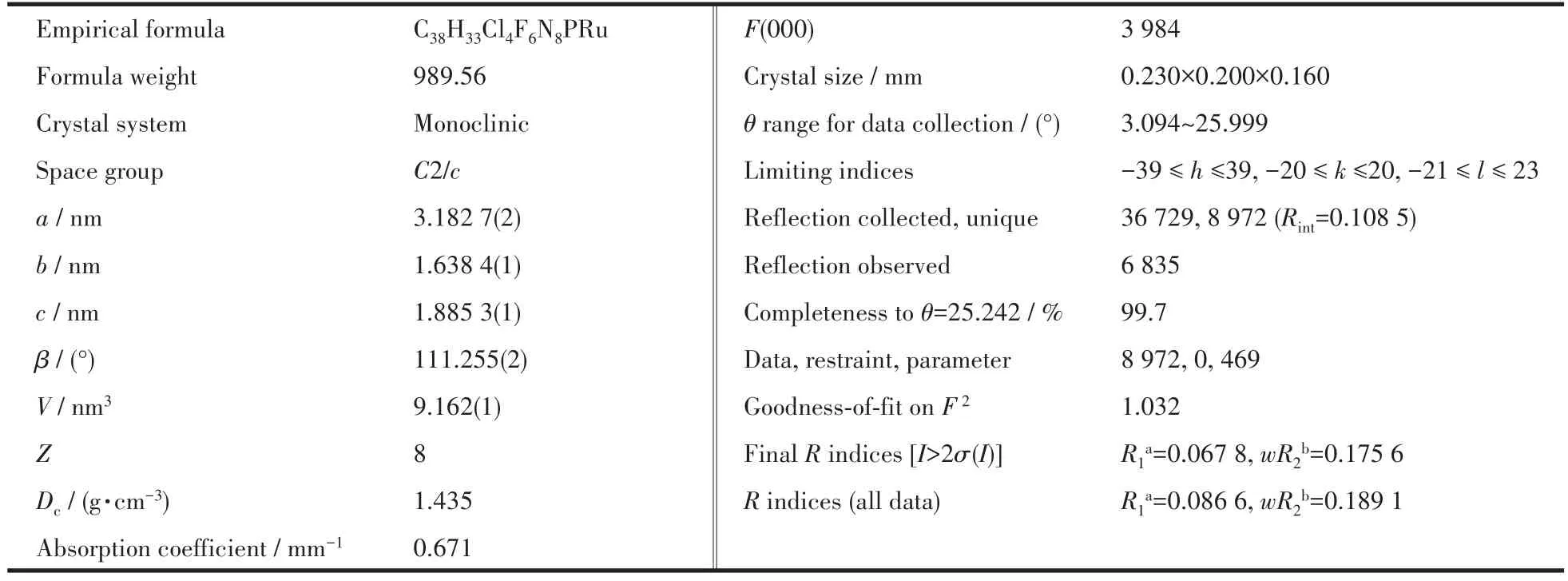
Table 1 Crystal data and structure parameters for complex 2

Table 2 Selected bond lengths(nm)and angles(°)for complex 2
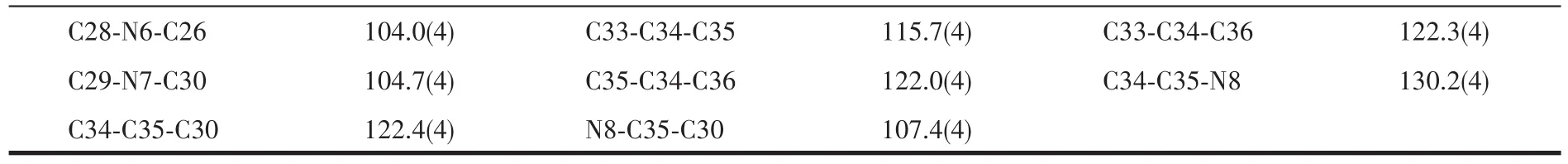
Continued Table 2
2 Results and discussion
2.1 Synthesis
Reaction betweencis-Ru(bpy)2Cl2·2H2O and DMBbimH2in refluxing ethylene glycol resulted in the formation of Ru(bpy)2(DMBbimH2)](PF6)2,1-A,which can be converted into mono-deprotonated 1-B and bideprotonated 1-C under suitable condition.The reactions in Fig.1 are reversible and each of the three species involved can be converted into any one of the other two simply by the adjustment of pH.We have been able to isolate all of the three species in their pure states.
The mass spectra of these complexes were nearly identical,where peaks at 675.3 and 338.3 can be assigned to [Ru(bpy)2(DMBbimH)]+and [Ru(bpy)2(DMBbimH2)]2+.This helps us to confirm the structures of the complexes to some extent.However,the1H NMR(section 2.2),UV-Vis spectra(section 2.3),and electrochemical properties(section 2.4)of the complexes 1-A,1-B,and 1-C showed obvious differences.Single crystals were grown from the dichloromethane solution of 1-B.The molecular structure of compound[Ru(bpy)2(DMBbimH)]PF6·2CH2Cl2(2)has been confirmed by X-ray crystallography(section 2.5).

Fig.1 Structures and reactions of complexes 1-A,1-B and 1-C
2.2 1H NMR
NMR spectroscopy has proved to be a useful tool in the structural characterization of ruthenium polypyridyl complexes[30].We confirm the structures of complexes 1-A,1-B and 1-C through their1H NMR spectra(Fig.2)which were recorded in DMSO-d6at room temperature.
In the spectrum of 1-A,the four doublets and four triplets betweenδ8.83 andδ7.46 belong to the aromatic protons of bpy groups.The relative intensities of these resonances were,as expected,1∶1∶1∶1∶1∶1∶1∶1.The doublet atδ7.19,triplet atδ6.97,doublet atδ5.45 can be respectively assigned to the aromatic protons H6,H5 and H4 of DMBbimH2,and a singlet atδ2.60 can be assigned to the methyl group of DMBbimH2.In the spectrum of 1-A,there was a broad signal atδ13.80.The broadness and extreme low-field shift suggest that it corresponds to the N-H protons of DMBbimH2.
In the spectrum of 1-B,two of resonances of the bpy group were overlapped atδ8.06~7.88.With the removal of the first proton,the signal of N-H proton disappeared,signals of H4,H5 and H6 of complex 1-B shifted towards the up-field region by 0.14,0.31 and 0.34,respectively.The chemical shift of the methyl group protons is atδ2.55.
For 1-C,the varieties of chemical shifts of bpy group and methyl group(δ2.49)protons were smaller,while the aromatic protons of DMBbim ligand exhibited a larger shift.With the removal of the second proton,signals of H4,H5 and H6 of complex 1-C further shifted towards the up-field region by 0.15,0.34 and 0.33,respectively.This is because that as the bibenzimidazole ligand is gradually deprotonated,the overall electron cloud density of the ligand increases.H4,H5 and H6 are getting closer and closer to the dehydrogenated N atoms,so they are gradually affected by dehydrogenation.
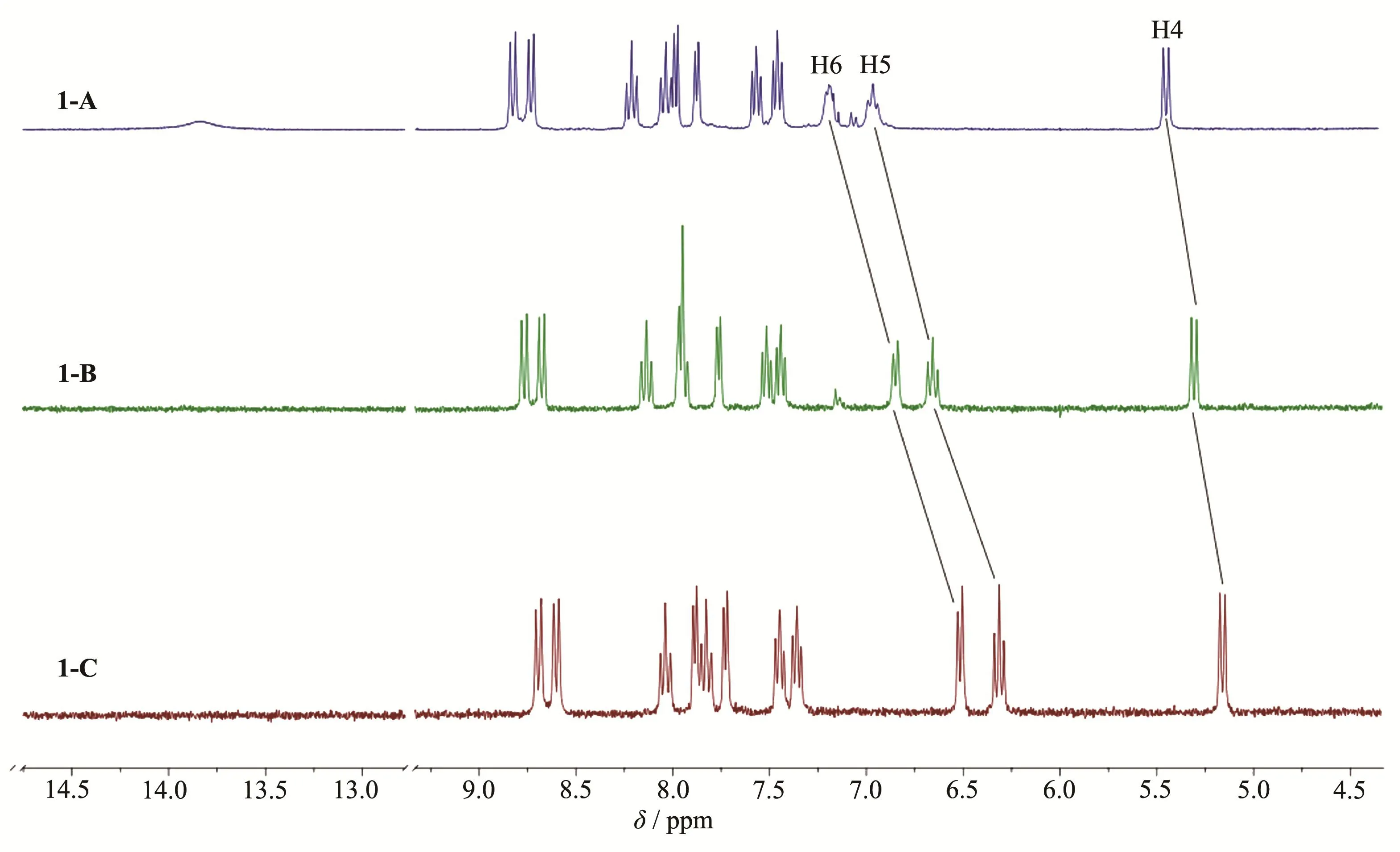
Fig.2 1H NMR spectra of complexes 1-A,1-B and 1-C in DMSO-d6
2.3 Absorption spectral studies.
Absorption spectral profiles of the complexes in acetonitrile are presented in Fig.3,and the related data are summarized in Table 3.In order to compare the relative intensity of the absorption bands,iso-molar solutions(60 μmol·L-1)of the complexes were used.
The highly intense absorption bands observed in UV region arise mainly due to theπ-π*transitions within the bpy and DMBbimHxunits[15,31].The strong absorption band at 290~300 nm can be attributed toππ*transition of bpy ligand.The 335 nm absorption band of 1-A,341 nm band of 1-B,338 nm band of 1-C,are assigned toπ-π*transition of the DMBbimHxligands,respectively.The moderately intense broad bands in visible region can be attributed to M(dπ)→bpy(π*)and M(dπ)→DMBbimHx(π*)MLCT transitions.
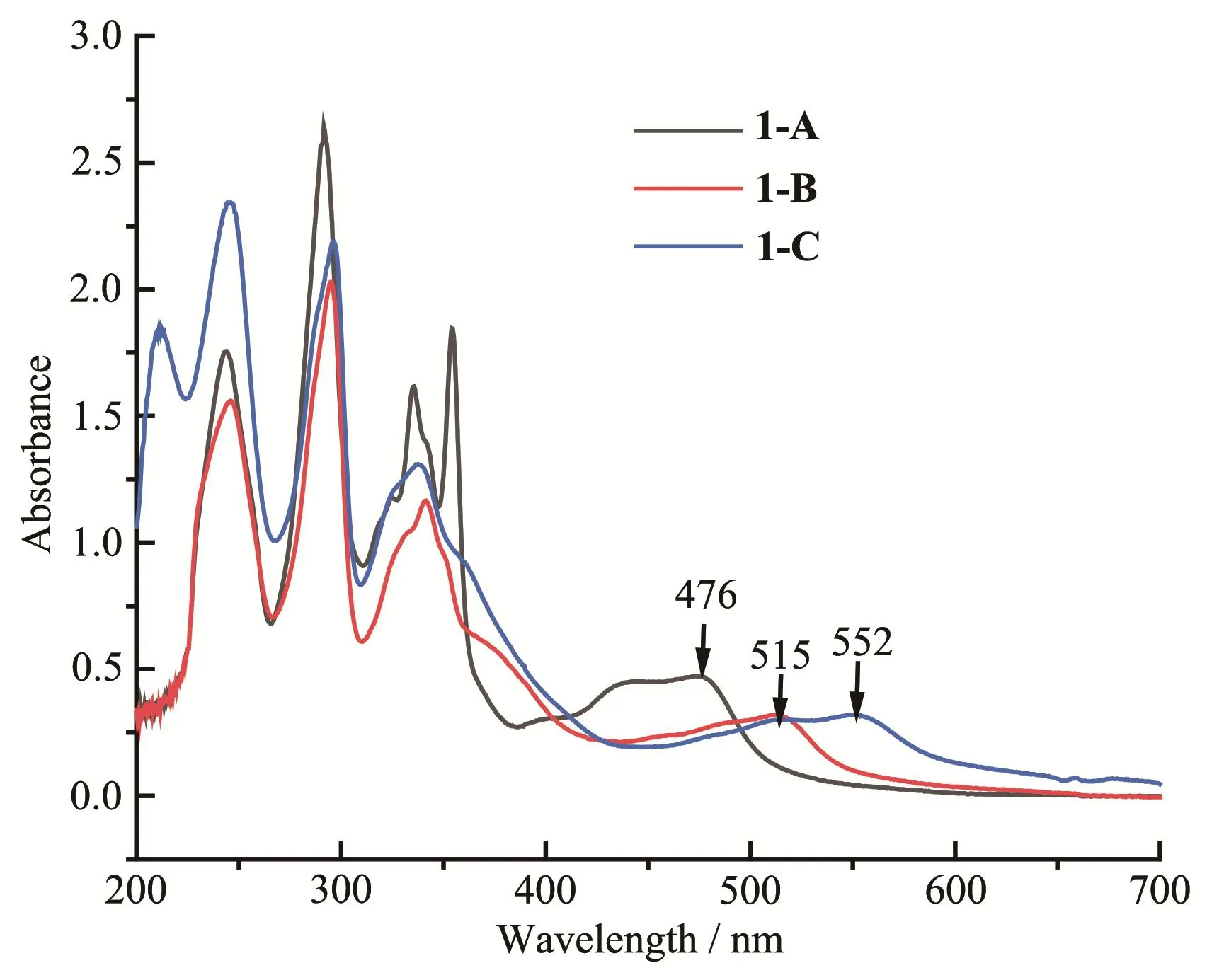
Fig.3 UV-Vis absorption spectra of iso-molar acetonitrile solutions of complexes 1-A,1-B and 1-C
In the absorption spectral profiles,it was observed that the MLCT bands in the complexes underwent gradual red-shifts.With the removal of protons,the absorption peak was red-shifted from 476 nm(1-A)to 515 nm(1-B)and finally to 552 nm(1-C).This is because the interaction(mono-deprotonation or bi-deprotonation in DMBbimHxligand)greatly increases the electron density at the Ru(Ⅱ)center and leads to a less-positive oxida-tion potential(section 2.4),resulting in the decrease of MLCT band energy.

Table 3 Absorption spectroscopic data for complexes in acetonitrile
2.4 Electrochemistry
Electrochemical behaviors of the complexes in dichloromethane and acetonitrile were studied through CV and SWV methods using Ag/AgCl reference electrode and ammonium hexafluorophosphate as supporting electrolyte.E1/2values were obtained from SWV using glassy carbon electrode.
As shown in Fig.4,in dichloromethane,the mononuclear complex 1-A underwent oxidation irreversibly at 1.35 V.CV and SWV measurements of 1-B in dichloromethane revealed characteristic phenomena for cooperative proton electron transfer.Two successive electrode oxidation couples(E1/2,1=0.97 V andE1/2,2=1.33 V(vs Ag/AgCl))were observed,revealing that the complex undergoes two successive one-electron reversible oxidation processes due to Ru(Ⅱ)-Ru(Ⅱ)/Ru(Ⅲ)-Ru(Ⅱ) and Ru(Ⅲ)-Ru(Ⅱ)/Ru(Ⅲ)-Ru(Ⅲ) processes,respectively.This is due to the presence of hydrogen-bonded dimers in 1-B.In 1-B,there is only one N-H bond on the DMBbimH ligand.Two[Ru(bpy)2(DMBbimH)]+cations are easily bonded by hydrogen bonds to form a dimer structure,which can not only exist in solid state,but also exist stably in weak polar solvents.The redox of two Ru centers in the dimer is not completely symmetrical and simultaneous.One of the metal centers Ru(Ⅱ) is oxidized to Ru(Ⅲ) first(Fig.5),and then the other metal center is oxidized.The extent of electronic interaction between two metal centers in homo-bimetallic complexes can be estimated from the differences between two oxidation potentials(ΔE1/2=|E1/2,2-E1/2,1|)[15].ΔE1/2value obtained in this way was 0.36 V.
The doubly deprotonated complex 1-C underwent oxidation irreversibly at 0.71 V in dichloromethane.As expected,deprotonation of the bi-benzimidazole ligand leads to shifts towards more negative potentials for the oxidation of the ruthenium center.This indicates an enhanced electron density at the metal center induced by deprotonation[11].
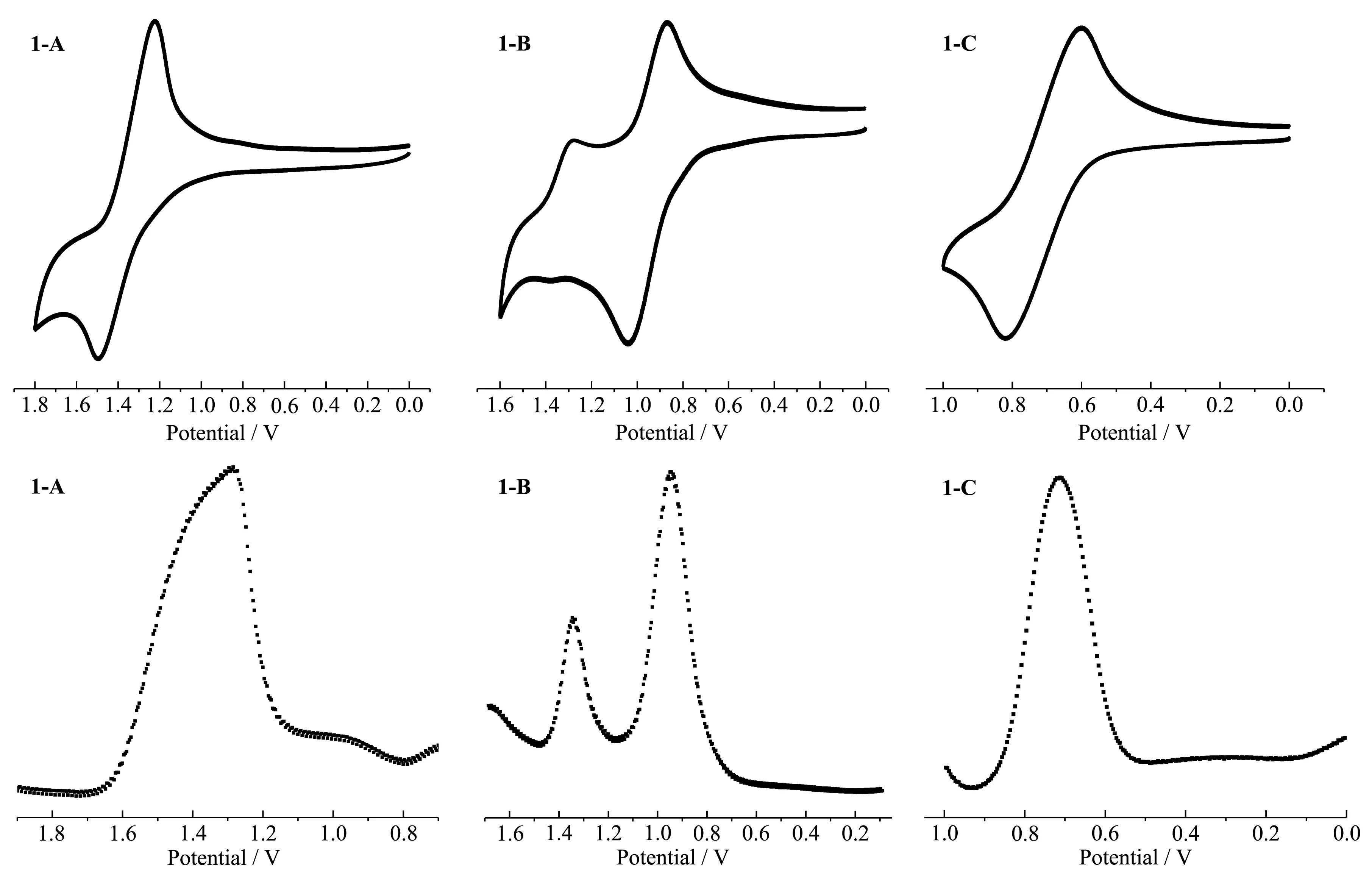
Fig.4 CV and SWV of 1-A,1-B,1-C in dichloromethane
Fig.5 Redox reactions of hydrogen bonding dimer in 1-B
As we know,the polarity of solvent has a great effect on the formation of hydrogen-bonded species and the electrochemical properties of complexes.Therefore,the electrochemical behaviors of the complexes in acetonitrile,whose polarity is much stronger than dichloromethane,were explored.The CV and SWV of the complexes are shown in Fig.6.In the curves we can easily find that each complex exhibited a reversible wave corresponding to Ru(Ⅱ)/Ru(Ⅲ) oxidation process in acetonitrile.Ru(Ⅱ)/Ru(Ⅲ) oxidation potential gradually shifted towards lower potential as the complex was changed from 1-A(E1/2=1.22 V)to 1-B(E1/2=0.88)and finally to 1-C(E1/2=0.57 V)and this is line with the observed absorption spectral trends.
Comparing Fig.4 and Fig.6,we can easily find out that the electrochemical properties of complex 1-B in dichloromethane and acetonitrile differ greatly,which is due to the different polarities of solvents.In dichloromethane,mononuclear complex 1-B undergoes a twostep oxidation,which indicates the existence of PCET between[Ru(bpy)2(DMBbimH)]+cations.The[Ru(bpy)2(DMBbimH)]+cations exist as hydrogen-bonded dimers in dichloromethane,and the existence of hydrogenbonded dimers is proved by single crystal structure(section 2.5).However,the dimers cannot exist stably in more polar acetonitrile,and the cations exist as monomers,therefore,only one peak can be observed on CV and SWV curves.
In conclusion,for 1-B,dimers are formed preferentially over the corresponding monomers in weakly polar solvents,such as dichloromethane.CV measurements show that 1-B reversibly generates stable mixedvalence states RuⅡRuⅢupon oxidation.As a result,it is reasonable that the mixed-valence states are stabilized by proton transfer of complementary hydrogen bonds[27].
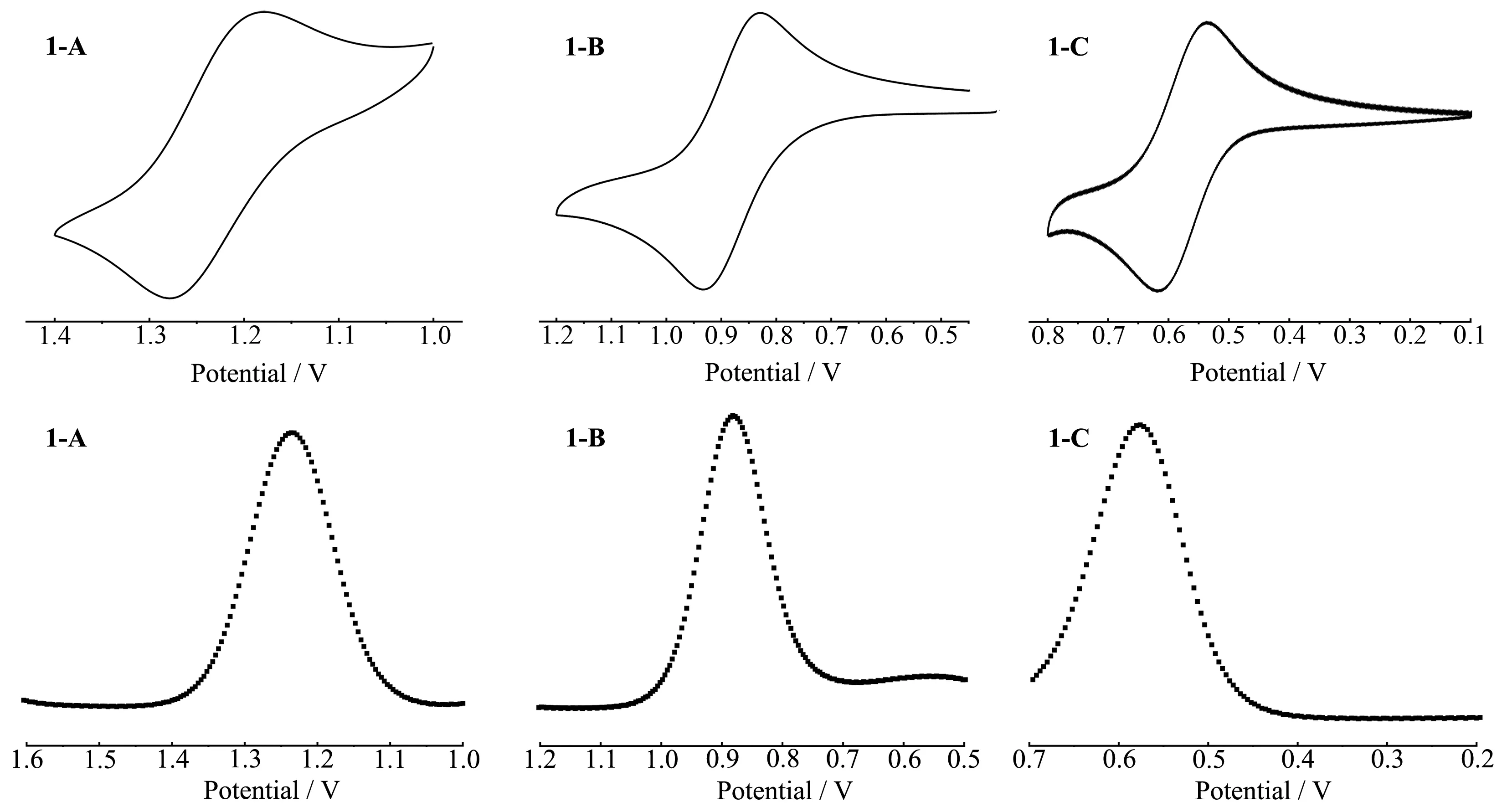
Fig.6 CV and SWV of 1-A,1-B and 1-C in acetonitrile
2.5 Single-crystal structure of 2
Dark red single crystals of complex[Ru(bpy)2(DMBbimH)]PF6·2CH2Cl2(2)were obtained from dichloromethane solution of 1-B.The single crystal structure of 1-B indicates that it belongs toC2/cspace group of monoclinic system.Each asymmetric unit contains a[Ru(bpy)2(DMBbimH)]+cation,a PF6-anion and two dichloromethane molecules.The components are supported by elemental analysis data.The solvents were squeezed by SQUEEZE/PLATON program because the dichloromethane molecules were disordered.
As shown in Fig.7a,each Ru(Ⅱ)ion is coordinated with six N atoms from two bpy ligands and one DMB-bimH-ligand to form an octahedral coordination geometry.The Ru-N bond length between Ru(Ⅱ)ion and bpy is between 0.204 3(4)and 0.205 4(4)nm,and the Ru-N bond length with DMBbimH-ligand is slightly longer(0.208 9(4)~0.210 8(4)nm),but agrees with literature values[5-19].The N-Ru-N bite angles vary from 78.3°to 78.8°,while thetransangles lie in a range of 171.7(2)°~179.2(2)°.The DMBbimH-ligand coordinates in a bidentated manner with N5 and N7,remaining the other two N atoms(N6 and N8)uncoordinated.The hydrogen atoms on N6 and N8 are disordered,and H6A and H8A are both half-occupied due to the symmetry.Each[Ru(bpy)2(DMBbimH)]+cation pairs up with a symmetry-equivalent cation to generate a dimeric unit[Ru(bpy)2(DMBbimH]22+containing a self-complementary double hydrogen-bonded bridge(Fig.7a).The N6…N6A separation is 0.271 5 nm and the N6-H6A-N6A angle is 166.5°,and the N8…N8A separation is 0.278 5 nm and the N8-H8A-N8A angle is 172.9°.Only half of hydrogen atoms(H6A and H8A)are presented in Fig.7a.The Ru…Ru distance defined by this supramolecular arrangement is 1.011 9 nm.
The dihedral angle between the planes of two benzimidazole in a DMBbimH-ligand is 6.2°,indicating an almost planar structure of the DMBbimH-moiety in 2.It is worth mentioning that the angle between two DMBbimH-ligands in a dimer is twisted to about 57.2°(Fig.7b),which should be attributed to the side-by-side steric repulsion between the methyl groups at the 7 and 7′positions.
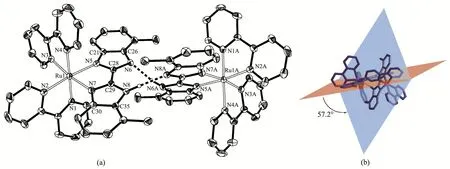
Fig.7 Structure as an ORTEP drawing with 30% probability thermal ellipsoids(a)and dihedral angle(b)of hydrogen-bonded dimer in 2

Table 4 Selected hydrogen bond parameters in 2
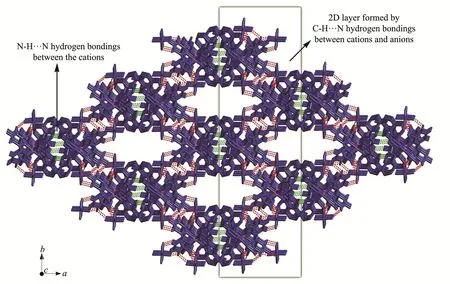
Fig.8 Three-dimensional network formed by hydrogen bonds in 2
There are abundant weak intermolecular forces in the structure,as shown in Fig.8[32].The cations and anions are connected by C-H…F weak hydrogen bonds(Table 4)to form two-dimensional layers,which are linked into three-dimensional network by N-H…N hydrogen bonds between cations.The solvent molecules fill in one-dimensional channels along thec-axis direction formed by the cations and anions.
3 Conclusions
In summary,three ruthenium(Ⅱ)polypyridyl complexes containing 7,7′-dimethyl-2,2′-bibenzimidazole ligand(DMBbimHx)were synthesized and determined by1H NMR,UV-Vis absorption and electrochemical measurements.From 1-A to 1-B and then to 1-C,N-H in DMBbimHxligands gradually deprotonated,resulting in evident differences of the spectroscopic and electrochemical properties of complexes.Interestingly,for mono-deprotonated 1-B,the electrochemical properties behaved very differently in dichloromethane and acetonitrile,which is due to different polarities of solvents.In acetonitrile,there was only one redox peak in CV curve,while in dichloromethane,it underwent a two-step oxidation,which indicates the existence of PCET in hydrogen-bonded dimers formed by[Ru(bpy)2(DMBbimH)]+cations.The single crystal structure of 2 indicates that[Ru(bpy)2(DMBbimH)]+cations exist as hydrogen-bonded dimersin weakly polarsolvent dichloromethane.This is consistent with the result of electrochemical measurements of complex1-B in dichloromethane.
