Effect of Potassium 5,5′-Azotetrazolate on Phase Transformation and Thermal Decomposition of Ammonium Nitrate
2020-09-01WANGYiSONGXiaolanLIFengsheng
WANG Yi, SONG Xiao-lan, LI Feng-sheng
(1. School of Materials Science and Engineering, North University of China, Taiyuan 030051, China; 2. School of Environment and Safety Engineering, North University of China, Taiyuan 030051, China; 3. School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China)
Abstract:Potassium 5,5′-azotetrazolate (raw PZT) was synthesized using 5-amino-1H-tetrazol (5-ATZ) as the precursor. By dissolving raw PZT and ammonium nitrate (AN) in water, evaporating the water off, and then “AN+PZT” composites were prepared. IR and XRD analyses were employed to characterize raw PZT and “AN+PZT” composites. Thermal analyses were performed to probe the effect of raw PZT on phase transition and thermolysis of AN. The molecular structure of raw PZT was confirmed by IR spectrum, and results demonstrate that raw PZT is a kind of hydrate. XRD patterns of “AN+PZT” composites reveal that some eutectics generate during the preparation. DSC curves of “AN+PZT” composites disclose that the phase transformation of AN at ambient temperature is entirely inhibited by doping with raw PZT. Peak temperature for decomposition of AN also decrease obviously by doping with raw PZT. The final decomposition products of “0.8AN+0.2PZT” composite are N2O, HNO3 and H2O. By compared “0.8AN+0.2PZT”composite with pure AN, it is found that HNO3 disappear, while NH3 is detected as an intermediate product. It may be because that PZT react with HNO3 firstly after decomposition of AN, and NH3 is oxided by HNO3 at a higher temperature. This promotes thermal decomposition of AN in mechanism since the consumption of HNO3 dominates its thermolysis.
Keywords:physical chemistry; ammonium nitrate; AN; PZT; phase transition; thermal decomposition mechanism
Introduction
Ammonium nitrate (AN) is a low cost and easy available energetic material which is extensively used in industrial explosives[1-3]. In propellants, AN is also a promising oxidizer of which the combustion is of the feature such as free halogen, low temperature, and low signature. However, two factors block the application of AN. Firstly, both energy and combustion performances of AN are much poor. Thus, AN had not been applied as main oxidizer in the propellants of any type of rocket, and it were just slightly used in some formulation of gas generation agents[4-6]. Secondly, phase transformation at ambient temperature is the most fatal defect of AN. We all know that AN exists as five crystal forms, and the phase transformation occurs at 125, 84, 32, and -18℃, respectively. Phase transformation at 32℃ is accompanied by a significant volume expansion which results in crack formation in the propellant grain[7]. The mechanical strength of the AN pills is dependent on the phase transition behavior. Thus, before used, it is indispensable to introduce some phase stabilizer to make phase transformation of AN disappeared. At least, the phase transformation at ambient temperature must be inhibited.

1 Experiment
1.1 Materials
Ammonium nitrate (AN) were purchased from Tianjin Guangfu Chemical Co., Ltd. (Tianjin, China).
1.2 Fabrication
Raw PZT is prepared with the method mentioned in reference [14]. The next step is to prepare “AN+PZT” composites. Herein, please note that grinding the mixture of AN and PZT without any liquid medium is forbidden because this would cause an exothermic solid reaction between AN and PZT, which produces a lot of gas and it smells like ammonia. In this work, we dissolve both AN and PZT into deionized water together. Then put the aqueous solution into a water bath oven at 70℃. Several days later, the water evaporates off and the mixture is obtained. Several mixtures are prepared, in which the mass fraction of PZT in the mixture are 3%, 5%, 10%, 15%, 20%, and 30%. The obtained samples are marked as “0.97AN+0.03PZT”, “0.95AN+0.05PZT”, “0.9AN+0.1PZT”, “0.85AN+0.15PZT”, “0.8AN+0.2PZT”, and “0.7AN+0.3PZT”, respectively.
1.3 Characterization and analyses
Morphology was observed with a field-emission scanning electron microscope (SEM, JEOL JSM-7500). IR analysis was performed on American thermofisher scientific Nicolet 6700 infrared spectrometer (iodine bromide tablet). The phases of the samples were investigated with an X-ray powder diffractometer (XRD, Bruker Advance D8) using Cu K_αradiation at 40kV and 30mA. Thermal analyses were performed on a differential scanning calorimeter (TA Model Q600) at heating rates of 20℃/min (N2atmosphere, sample mass of approximately 5mg, and Al2O3crucible). DSC-IR analysis was carried out at heating rates of 10℃/min by using a thermal analyzer system (DSC, Mettler Toledo) coupled with a Fourier transforms infrared spectrometer.
2 Results and Discussion
2.1 Characterization of raw PZT

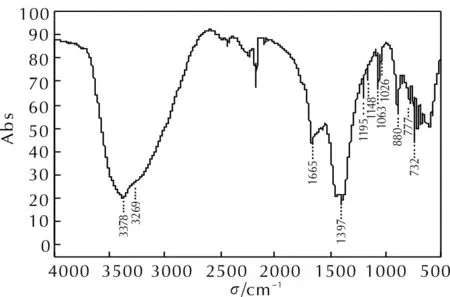
Fig.1 IR spectrum of raw PZT
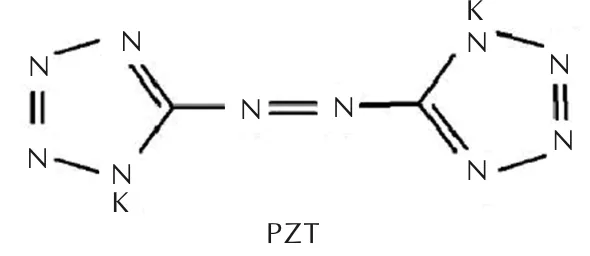
Fig.2 Molecular structure of PZT
2.2 Characterization of “AN+PZT” composites
SEM images of all “AN+PZT” composites are showed in Figure 3.Figure 3(a) presents the micron morphology of “0.97AN+ 0.03PZT”.Its particle size is about 300-500μm and its particle surface is very complicated. It seems that the particle surface of “0.97AN+0.03PZT” is etched, which may be resulted from the evaporation of the solvent. The micron morphologies of other composites are similar as that of “0.97AN+0.03PZT”. XRD patterns of raw PZT, pure AN, and all composites are illustrated in Figure 4. Raw PZT is a kind of crystal. Comparing patterns of composites with patterns of raw PZT and pure AN, we can find that some new phases form. The main diffraction peaks of raw PZT locate at 2θof 32.6° and 11.1°. In all patterns of “AN+PZT”, the diffraction peak at 2θof 32.6° still exists. The main diffraction peak of pure AN locate at 2θof 29.1° and 40.2°. In all patterns of “AN+PZT”, the diffraction peaks at 2θof 29.1° and 40.2° still exist. This means that all “AN+PZT” composites are polycrystalline of AN and PZT. However, the new peak locating at 2θof 34.3° also exist in the patterns of all composites samples. This implies that eutectic of PZT and AN forms when the solvent is evaporated off. Each composite is the crystal mixture of raw PZT, pure AN, and the co-crystal.
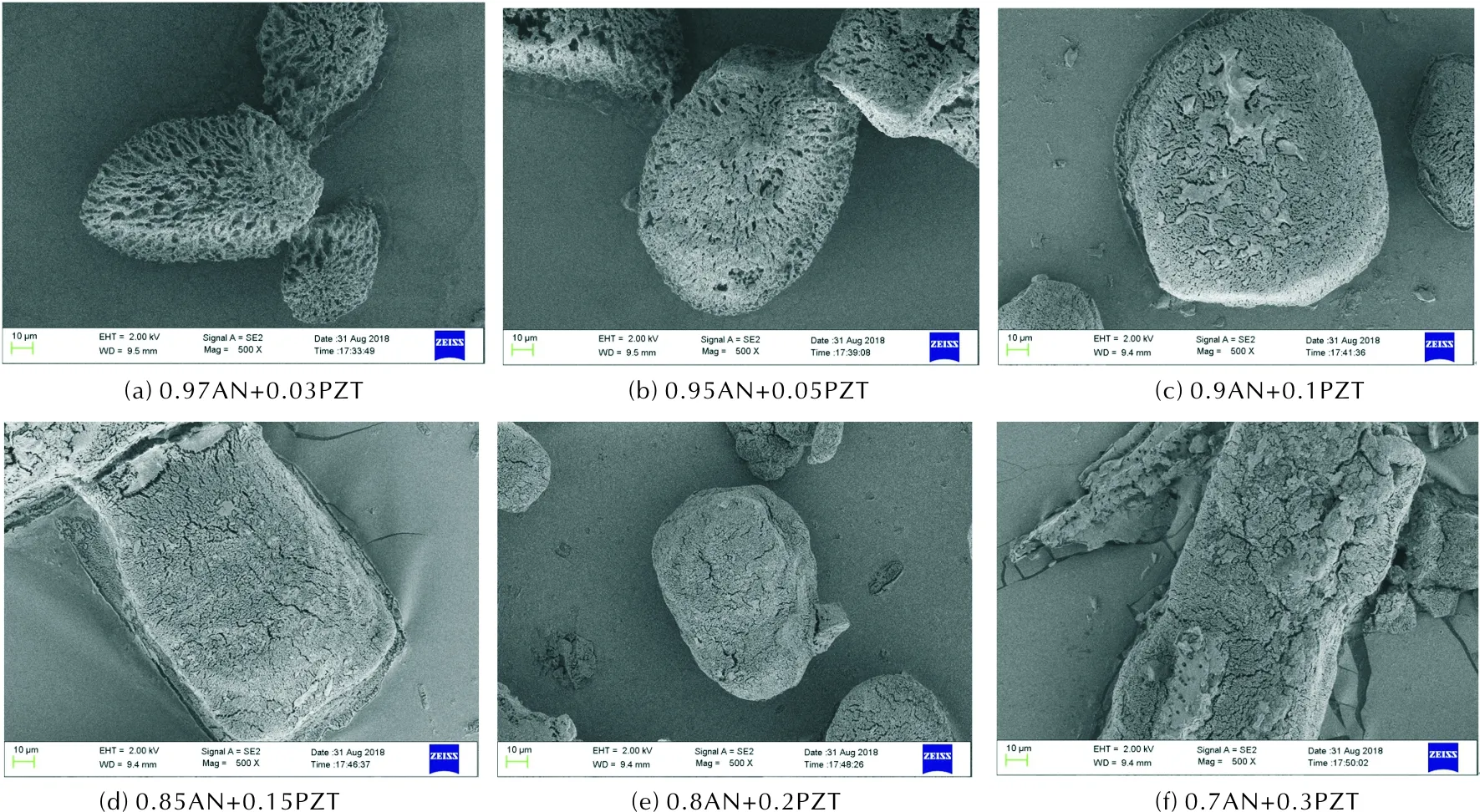
Fig. 3 SEM images of samples
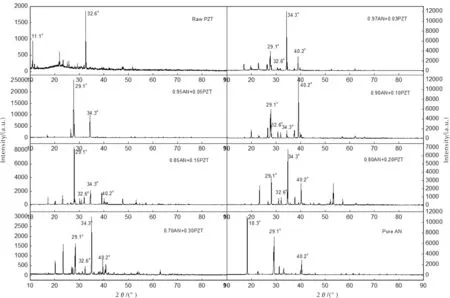
Fig. 4 XRD patterns of samples
2.3 Thermal analysis
Thermal decomposition of raw PZT is investigated by TG/DSC analysis and the result is showed in Figure 5. Figure 5(a) illustrates the DSC trace of raw PZT. A broad endothermic peak locates at 87.5-158.1℃, attributed to the sublimation of crystallized water in PZT molecules with heat absorption of +251.3J/g. A exothermic peak, relating to thermolysis of PZT, locates at 234.3-269.3℃ with heat release of -105.6J/g. Figure 5(b) and Figure 5(c) illustrate the TG/DTG curves of raw PZT. The TG curve indicates that the consumption of the decomposition contains two steps which correspond to the sublimation of crystallized water and decomposition of PZT. In the first consumption step, the mass loss is 15.3%. At elevated temperature, the consumption becomes very steep with mass loss of 48.5%. After that, as increasing of temperature, the mass loss does not change. This means there is still 36.2% material does not decompose and remains as residue. In DTG curve, it is revealed that the rate of the first consumption is very low and the rate of the second consumption is very high. This means the sublimation of crystallized water is a slow process and the decomposition of PZT is a fast process.
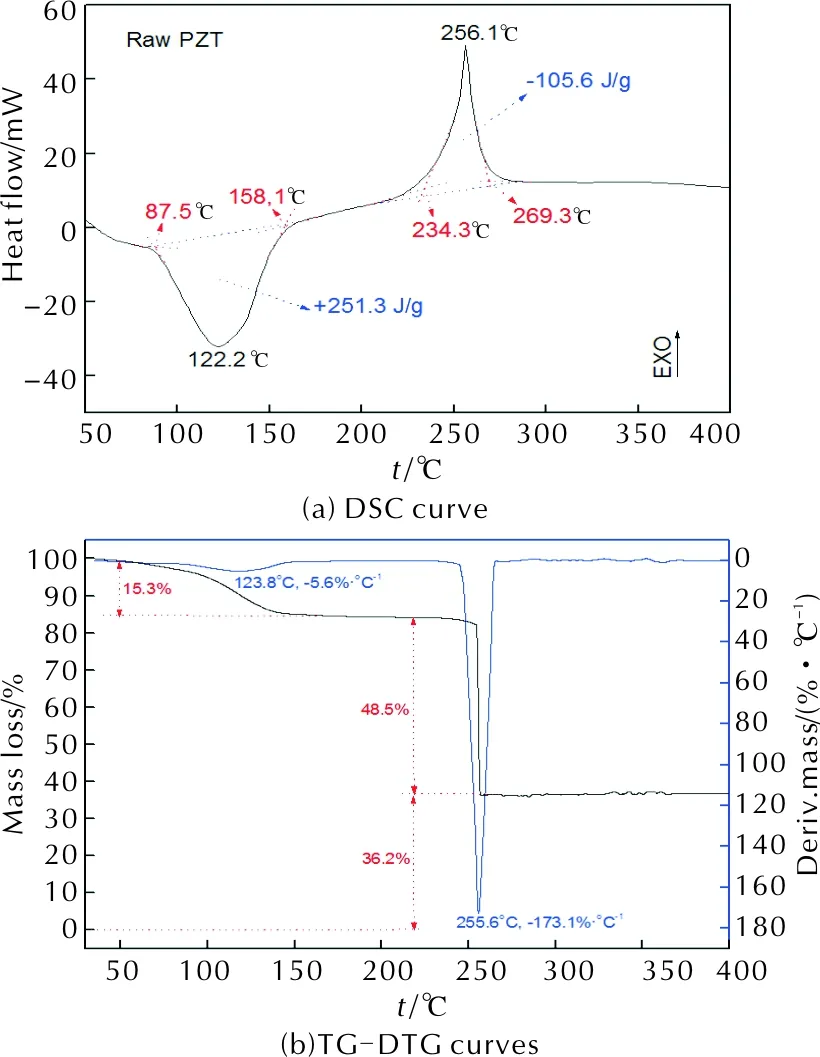
Fig. 5 Thermal analysis of raw PZT
After understood thermolysis of raw PZT, we investigate the effect of raw PZT on phase transformation and thermal decomposition of ammonium nitrate, and the results are showed in Figure 6. The data extracted from DSC traces are listed in Table 1.
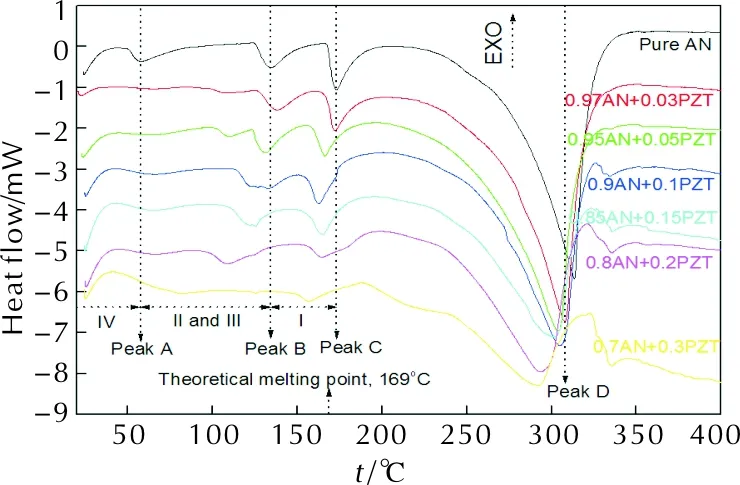
Fig. 6 DSC curves of AN+PZT composites

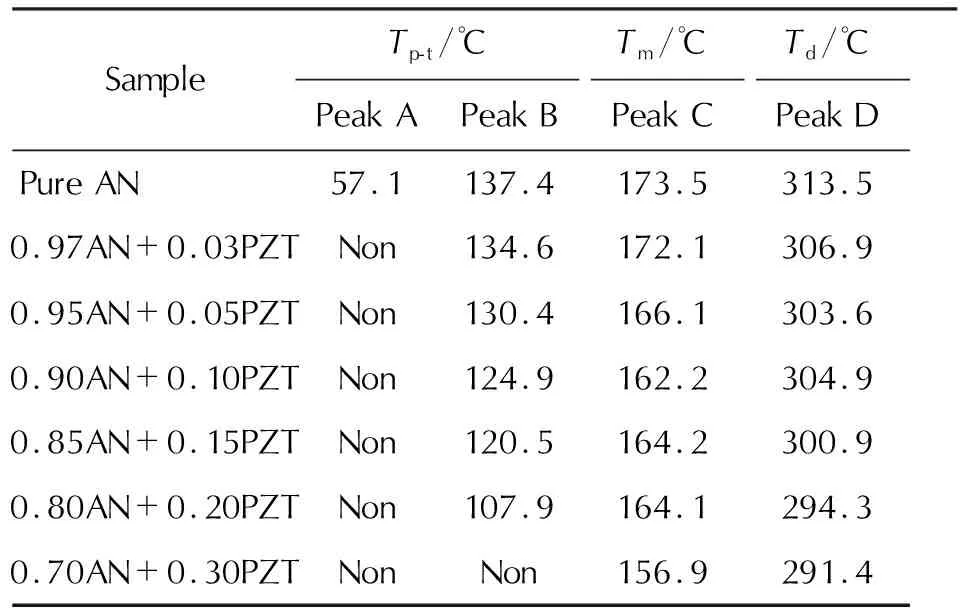
Table 1 Characteristic parameters of phase transformation, melting, and decomposition of samples
For further investigating the effect of PZT on thermal decomposition of AN, DSC-IR analyses are performed and the results are illustrated in Figure 7. Figure 7(a) indicates that much gas produce in time lapse of 1182-1629s. The detected signal is very strong. We extract the IR spectra of the gas products at 1182, 1343, 1466, 1532, and 1629s, and show them in Figure 7(b). It indicates that the main decomposition products of pure AN are N2O, HNO3, and H2O. This is accordance with the common mechanism of AN[15]. Firstly, AN dissociates to NH3and HNO3; and the HNO3decomposes to ·OH and ·NO2radicals; and ·OH reacts with NH3to form ·NH2and H2O; and then ·NH2reacts with ·NO2to produce N2O and H2O (see Eqs.1-4).
NH4NO3→NH3+HNO3+2.18kJ/g
(1)
HNO3→·OH+NO2
(2)
NH3+·OH→·NH2+H2O
(3)
NH2+NO2→[NH2NO2]→N2O+H2O
(4)
This is the radical mechanism for thermal decomposition of AN. Please note that, herein, no NH3gas exists in gas products of pure AN, but many HNO3gas are detected. This means that the oxidization of NH3by decomposition products of HNO3is not the rate-limiting step for thermal decomposition of AN. Thermolysis of AN is dominated by decomposition of HNO3, which has been confirmed in Izato′s and Sinditskii′s works[16-17]. In this work, the detection of HNO3also reveals that decomposition of HNO3is the rate-limiting step for thermolysis of AN. Now, please see Figure 7(c) and 7(d), they illustrate the result of AN doped with 20% raw PZT, i.e. the sample “0.8AN+0.2PZT”. The final decomposition products of “0.8AN+0.2PZT” are N2O and H2O. Comparing with the result of pure AN, we can find no HNO3exists in decomposition products of “0.8AN+0.2PZT”. This confirms that HNO3has reacted with something without decomposition. In Figure 7(d), please note that there are many NH3detected as intermediate product. The NH3may be produced from dissociation of AN, or from decomposition of raw PZT. In order to disclose this mechanism, the DSC-IR analysis of raw PZT is also performed and the result is showed in Figure 7(e) and 7(f). Figure 7(e) indicates that the decomposition of raw PZT is divided into two steps. Figure 7(f) reveals that the first step corresponds to sublimation of crystallized water and the second step relates to the decomposition of PZT. It is obvious that no NH3exists in decomposition products of raw PZT. Thus, in Figure 7(f), we speculate that the products (N2O, NO2, CO) are resulted from the reaction of N and C atoms (in PZT) with H2O steam, since there are no oxidizing groups existing in molecules of raw PZT. Herein, the H2O steam acts as the oxidizer. Now, let′s go back to decomposition of “0.8AN+0.2PZT” in Figure 7(d). The produced NH3should be the reminder of dissociation of AN. As abovementioned, AN dissociates to NH3and HNO3firstly. It is speculated that the PZT skeletons directly react with HNO3(prior to NH3) to produce N2O and H2O. Hence, the NH3is detected as an intermediate product. Of course, all NH3gas eventually reacts with decomposition products of HNO3, since no NH3is detected in final products of “0.8AN+0.2PZT”. We have known that the rate-limiting step for decomposition of AN is the decomposition of HNO3. Here, it confirms that addition of raw PZT improves the consumption of HNO3, i.e. thermal decomposition of AN is promoted in mechanism.
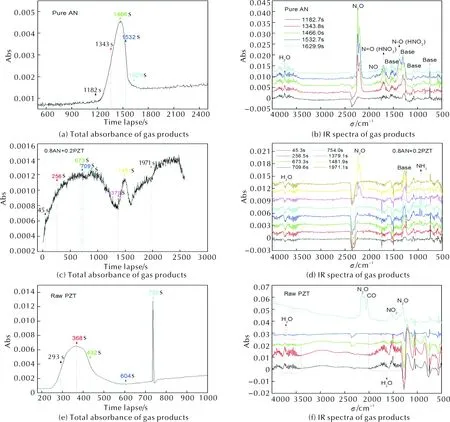
Fig. 7 DSC-IR spectra of samples
3 Conclusions
(1) Raw PZT is prepared and its molecular structure is confirmed by IR analysis. Six “AN+PZT” composites are prepared and their micron morphology and crystal phase are probed. Their particle surface is very complicated and some eutectic generate from preparation of the composites. The composites are not single crystal but rather polycrystalline.
(2) Thermal decomposition of raw PZT is investigated by TG/DSC analysis. An endothermic peak and an exothermic peak locate at 122.2℃ and 156.1℃, respectively, which relate to the sublimation of the crystallized water and decomposition of PZT. DSC traces of “AN+PZT” composites reveal that the phase transformation of AN at ambient temperature is entirely inhibited when 3.0% (or more) raw PZT is added into. The decomposition peak of AN also advances by adding raw PZT. DSC-IR analysis discloses that the decomposition products of pure AN are N2O, HNO3, and H2O. The final decomposition products of “0.8AN+0.2PZT” are N2O and H2O; meanwhile, some NH3gas is detected as intermediate product. It is speculated that PZT reacts with HNO3directly, and this promotes thermal decomposition of AN.
