miR-1与miR-499对心肌细胞增殖与凋亡调控作用及机制研究
2020-08-21李倩晓于勤那荣妹刘百亭
李倩晓 于勤 那荣妹 刘百亭

[摘要] 目的 研究miR-1與miR-499在心肌细胞增殖与凋亡中的调控作用及其机制。 方法 通过脂质体2000转染试剂将miR-1 mimics、miR-499 inhibitor转染至H9C2心肌细胞。设置空白对照组、H2O2组(未进行转染的H9C2心肌细胞)、干预组A(miR-1 mimics转染的H9C2心肌细胞)、干预组B(miR-499 inhibitor转染的H9C2心肌细胞)、干预组C(miR-1 mimics+miR-499 inhibitor转染的H9C2心肌细胞)。通过H2O2诱导建立H9C2心肌细胞氧化应激模型。采用CCK-8法检测细胞增殖情况,流式细胞仪检测细胞凋亡情况,Western Blot检测Bim、Mcl-1蛋白表达水平。 结果 与空白对照组比较,H2O2组细胞增殖显著降低而细胞凋亡率显著升高,差异有统计学意义(P<0.05)。与H2O2组相比,干预组A、干预组B细胞增殖进一步降低而细胞凋亡率进一步升高,这种改变在干预组C中更加显著,差异有统计学意义(P<0.05)。与空白对照组比较,H2O2组Bim蛋白表达水平明显升高,Mcl-1蛋白表达水平明显降低,差异有统计学意义(P<0.05)。与H2O2组相比,干预组A、干预组B的Bim蛋白表达水平进一步升高,Mcl-1蛋白表达水平进一步降低,这种改变在干预组C中更加显著,差异有统计学意义(P<0.05)。 结论 miR-1与miR-499在心肌细胞增殖与凋亡中发挥重要调控作用,miR-1可能通过上调Bim、下调Mcl-1蛋白表达促进心肌细胞凋亡,miR-499则通过下调Bim、上调Mcl-1蛋白表达抑制心肌细胞凋亡。
[关键词] miR-1;miR-499;调控;心肌细胞;凋亡
[中图分类号] R587.2 [文献标识码] A [文章编号] 1673-9701(2020)17-0037-04
A study of the regulatory effect of miR-1 and miR-499 on the proliferation and apoptosis of cardiomyocytes and the mechanism
LI Qianxiao1 YU Qin2 NA Rongmei2 LIU Baiting2
1.Department of Cardiology,Zhejiang Integrated Traditional and Western Medicine Hospital,Hangzhou 310000,China; 2.Department of Cardiology,Affiliated Zhongshan Hospital of Dalian University,Dalian 116001,China
[Abstract] Objective To study the regulatory effect of miR-1 and miR-499 on the proliferation and apoptosis of cardiomyocytes and the mechanism. Methods MiR-1 mimics and miR-499 inhibitor were transfected into H9C2 cardiomyocytes by liposome 2000 transfection reagent. Those groups were set up such as the blank control group, the H2O2 group(H9C2 cardiomyocytes not transfected),the intervention group A(H9C2 cardiomyocytes transfected by miR-1 mimics), the intervention group B(H9C2 cardiomyocytes transfected by miR-499 inhibitor), and the intervention group C(H9C2 cardiomyocytes transfected by miR-1 mimic+miR-499 inhibitor). The oxidative stress model of H9C2 cardiomyocytes was established by H2O2 induction. The cell proliferation was detected by the CCK-8 method, the cell apoptosis was detected by flow cytometer,and Bim and Mcl-1 protein expression levels were detected by Western Blot. Results Compared with that in the blank control group, the proliferation of cells in the H2O2 group was significantly reduced while the apoptosis rate of cells was significantly increased, with statistically significant differences(P<0.05). Compared with that in the H2O2 group,the proliferation of cells in the intervention group A and the intervention group B further decreased while the apoptosis rate of cells in the two groups further increased, and the correspondent changes were more significant in the intervention group C, with statistically significant differences(P<0.05). Compared with those in the blank control group,the Bim protein expression level in the H2O2 group significantly increased and the Mcl-1 protein expression level significantly decreased, with statistically significant differences(P<0.05). Compared with those in the H2O2 group, the Bim protein expression level in the intervention group A and the intervention group B further increased and the Mcl-1 protein expression level further decreased, and the correspondent changes were more significant in the intervention group C,with statistically significant differences(P<0.05). Conclusion MiR-1 and miR-499 play important regulatory roles in the proliferation and apoptosis of cardiomyocytes. MiR-1 may promote the apoptosis of cardiomyocytes by up-regulating Bim protein expression and down-regulating Mcl-1 protein expression, while miR-499 may inhibit the apoptosis of cardiomyocytes by down-regulating Bim protein expression and up-regulating Mcl-1 protein expression.
[Key words] miR-1;miR-499;Regulation;Cardiomyocyte;Apoptosis
心肌细胞凋亡与心脏疾病密切相关,包括心肌缺血再灌注损伤、缺血性心脏病、心力衰竭、心肌病等[1]。近年来,关于微小RNA(microRNA,miRNA)调控心肌细胞凋亡的研究已成为热点,为心脏疾病的诊治提供了重要方向[2]。本研究将miR-1 mimics、miR-499 inhibitor转染至H2O2处理的心肌细胞,探讨miR-1与miR-499在心肌细胞增殖与凋亡过程中的调控作用及其机制,现报道如下。
1 材料与方法
1.1 材料来源
大鼠H9C2心肌细胞株,购自中科院上海细胞库;胎牛血清、DMEM培养基、0.05%胰酶/EDTA(美国Hycloneo公司);miR-1 mimics、miR-499 inhibitor、竞争性短核糖核苷酸阴性对照序列(scramble-NC)、miR-499、miR-1和U6引物序列(上海吉凯基因化学技术有限公司);H2O2、CCK-8、Annexin V-FITC/PI细胞凋亡检测试剂盒(中国碧云天试剂公司);β-actin、Bim、Mcl-1单抗(美国Epitmics公司);ECL化学发光试剂盒(美国millipore公司);脂质体2000转染试剂(美国Invitrogen公司);二喹啉甲酸(BCA)试剂盒(美国Thermo公司);miRNeasy Serum/plasma Kit、miScript SYBR Green PCR Kit、RNase-free ddH2O、逆转录试剂盒(德国QIAGEN公司)。RT-PCR检测仪(美国ABI公司),流式细胞仪(美国BD公司),酶标仪(美国Bio-Tek公司)。
1.2 细胞培养
配置含10%胎牛血清的DMEM培养基,加入H9C2心肌细胞,置于37℃、5%CO2培养箱中培养。每隔2~3 d细胞传代1次。在实验前24 h取对数生长期的H9C2心肌细胞换液备用。
1.3 细胞分组及转染
将培养好的H9C2心肌细胞分为空白对照组、阴性对照组、miR-1 mimics组、miR-499 inhibitor组。除空白对照组外,其余三组H9C2心肌细胞分别采用随机合成的miRNA片段、miR-1 mimics片段、miR-499 inhibitor片段通过脂质体2000转染试剂进行转染,浓度均为200 nm,处理时间均为6 h。转染结束后将细胞置于37℃、5%CO2培养箱中培养,48 h后收集细胞。
1.4 RT-PCR检测转染情况
采用miRNeasy Serum/plasma Kit试剂盒提取血清RNA,并检测RNA的纯度。采用逆转录试剂盒将miRNA逆转录为特定的cDNA,并置于-80℃冰箱保存备用。RT-PCR反应:应用制备好的cDNA作为模板,分别配置空白对照组、阴性对照组、miR-1 mimics组、miR-499 inhibitor組反应管,以U6作内参。采用ABI 7500软件分析熔解曲线,用2-△Ct计算miRNA基因相对表达量。
1.5 实验分组及H2O2处理
设置空白对照组、H2O2组(未进行转染的H9C2心肌细胞)、干预组A(miR-1 mimics转染的H9C2心肌细胞)、干预组B(miR-499 inhibitor转染的H9C2心肌细胞)、干预组C(miR-1 mimics+miR-499 inhibitor转染的H9C2心肌细胞)。除空白对照组外,其余四组H9C2心肌细胞均采用200 μmol/L的H2O2处理6 h。
1.6 CCK-8法检测细胞增殖情况
取对数生长期的H9C2心肌细胞,接种到96孔板中(1×104个细胞/孔),置于37℃、5%CO2培养箱中,培养24 h,待细胞贴壁后,将H2O2处理后的各组细胞加入贴壁均匀的96孔板中,用CCK-8进行处理后,继续置于37℃、5%CO2培养箱中。孵育2 h后,测定各组细胞光密度(optical density,OD)值。
1.7 流式细胞仪检测细胞凋亡情况
取H2O2处理后的各组细胞,经PBS洗涤后,消化,离心,收集细胞。在收集到的细胞沉淀中加入200 μL binding buffer并吹打成单细胞悬液,再分别加入浓度为250 μg/mL的Annexin V-FITC和PI试剂各5 μL,避光室温下反应5~15 min,在1 h内采用流式细胞仪检测细胞凋亡率。
1.8 Western Blot检测Bim、Mcl-1蛋白表达水平
取H2O2处理后的各组细胞,加入裂解液处理30 min,收集蛋白并用BCA法测定蛋白浓度,煮沸10 min使蛋白变性,上样,按20 μg/泳道蛋白量经12%SDS-PAGE电泳后转至PVDF膜,以5%脱脂奶粉封闭60 min,分别加入β-actin、Bim、Mcl-1特异性抗体(均按照1:1000稀释),4℃孵育过夜。次日用PBS-T液洗膜后加入辣根过氧化物酶标记的羊抗兔二抗,室温孵育2 h后,电化学发光(Electro-chemi-luminescence,ECL)显影,采用图像分析软件测定目的条带灰度值,用甘油醛-3-磷酸脱氢酶(Glyceraldehyde-3-phosphate dehydrogenase,GAPDH)条带灰度值作为对照进行校正,分别计算出各样本相对表达量。计算方法:目的蛋白相对表达量=目的蛋白条带灰度值/GAPDH条带灰度值。
1.9 统计学方法
采用SPSS 23.0统计学软件处理数据,计量资料以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,两两比较采用LSD-t检验,P<0.05为差异有统计学意义。
2 结果
2.1 转染结果比较
与空白对照组和阴性对照组比较,miR-1 mimics组miR-1表达明显升高,miR-499 inhibitor组miR-499表达明显降低,差异有统计学意义(P<0.05)。空白对照组与阴性对照组间miR-1与miR-499表达均无显著差异(P>0.05)。见表1。
注:与空白对照组和阴性对照组比较,*P<0.05;与空白对照组比较,▲P>0.05
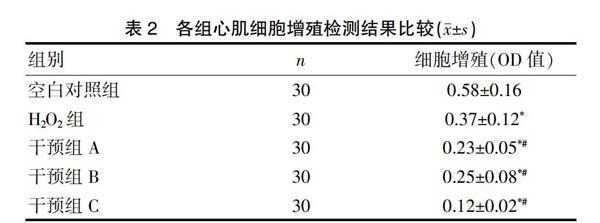
2.2 各组心肌细胞增殖检测结果比较
与空白对照组比较,H2O2组细胞增殖显著降低,差异有统计学意义(P<0.05)。与H2O2组相比,干预组A、干预组B细胞增殖进一步降低,这种改变在干预组C中更加显著,差异有统计学意义(P<0.05)。见表2。
注:与空白对照组比较,*P<0.05;与H2O2组比较,#P<0.05
2.3 各组心肌细胞凋亡检测结果比较
与空白对照组比较,H2O2组细胞凋亡率显著升高,差异有统计学意义(P<0.05)。与H2O2组相比,干预组A、干预组B细胞凋亡率进一步升高,这种改变在干预组C中更加显著,差异有统计学意义(P<0.05)。见表3。
注:与空白对照组比较,*P<0.05;与H2O2组比较,#P<0.05
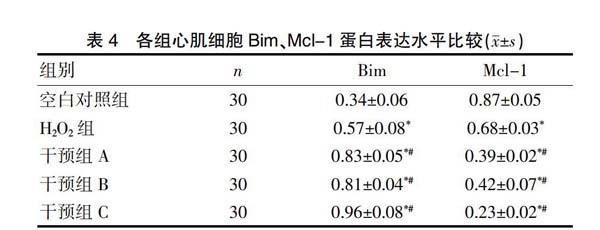
2.4 各组心肌细胞Bim、Mcl-1蛋白表达水平比较
与空白对照组比较,H2O2组Bim蛋白表达水平明显升高,而Mcl-1蛋白表达水平明显降低,差异有统计学意义(P<0.05)。与H2O2组相比,干预组A、干预组B的Bim蛋白表达水平进一步升高,而Mcl-1蛋白表达水平进一步降低,这种改变在干预组C中更加显著,差异有统计学意义(P<0.05)。见表4。
注:与空白对照组比较,*P<0.05;与H2O2组比较,#P<0.05
3 讨论
心肌细胞凋亡由氧化应激、缺血、缺氧、负荷过重、再灌注损伤等诱导[3]。心肌细胞凋亡机能异常是多种心脏疾病发生的重要机制[4]。细胞凋亡经典通路主要包括线粒体凋亡通路和死亡受体凋亡通路[5]。B细胞淋巴瘤-2(B-cell lymphoma-2,Bcl-2)基因家族是一类重要的凋亡调控蛋白,在线粒体凋亡通路中具有重要调节作用[6-8]。Bcl-2基因家族分为抗凋亡亚家族、促凋亡亚家族和仅含BH3结构域的BH3-only亚家族[9]。Bcl-2相互作用细胞凋亡调节因子(Bcl-2 interacting mediator of cell death,Bim)是仅含BH3结构域的BH3-only亚家族成员之一,与促凋亡亚家族成员高亲和力结合,发挥促凋亡作用[10]。髓细胞白血病基因-1(myeloid cell leukaemia-1,Mcl-1)是抗凋亡亚家族成员之一,可调控细胞凋亡、分化和细胞周期[11]。
miRNA是一类广泛存在于真核生物中内源性表达的小分子非編码单链RNA,几乎参与调控人类生长发育的各个阶段[12,13]。miRNA在转录水平通过与靶基因mRNA特定序列互补结合,阻止靶基因mRNA翻译或诱导其剪切,从而在基因调控中发挥重要作用,参与各种生物学过程和疾病的发生发展[14,15]。miRNA在心脏疾病的病理过程中具有重要调节作用[16]。研究发现,miRNA可作为心脏疾病早期筛查的生物标志物[17]。由于miRNA可同时调控细胞某一信号通路上与疾病相关的多个基因表达,因此对miRNA进行干预有望成为更理想的治疗方法[18]。miR-499是与心肌细胞分化密切相关的miRNA,在心肌细胞中特异表达[19,20]。miR-1在调节心肌细胞增殖、心脏节律性、心脏电传导等过程中具有重要作用[21]。本研究检测了miR-1 mimics、miR-499 inhibitor转染后心肌细胞增殖与凋亡情况,结果表明,上调miR-1表达或抑制miR-499表达后,心肌细胞增殖降低,而心肌细胞凋亡率升高。当两者同时进行干预后,心肌细胞增殖进一步降低,而心肌细胞凋亡率则进一步升高。通过检测促凋亡蛋白Bim与抗凋亡蛋白Mcl-1,发现转染miR-1 mimics、miR-499 inhibitor心肌细胞,Bim蛋白表达水平明显升高,Mcl-1蛋白表达水平明显降低,而联合干预组Bim蛋白表达水平升高幅度及Mcl-1蛋白表达水平降低幅度更为显著,提示miR-1可能是通过上调Bim、下调Mcl-1蛋白发挥促进心肌细胞凋亡作用,而miR-499则通过下调Bim、上调Mcl-1蛋白发挥抑制心肌细胞凋亡作用。
总而言之,miRNA的发现为心脏疾病的诊断与治疗提供了一个新的研究方向,但目前相关机制研究仍处于起步阶段,尚有待今后进一步深入探索。
[参考文献]
[1] Xia P,Liu Y,Cheng Z. Signaling pathways in cardiac myocyte apoptosis[J]. Biomed Res Int,2016,2016(19):1-22.
[2] Chen-Scarabelli C,Saravolatz L,Murad Y,et al. A critical review of the use of carvedilol in ischemic heart disease[J]. Am J Cardiovasc Drugs,2012,12(6):391-401.
[3] Vila-Petroff M,Salas MA,Said M,et al. CaMKⅡ inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury[J]. Cardiovasc Res,2007,73(4):689-698.
[4] Bergmann MW,Rechner C,Freund C,et al. Statins inhibit reoxygenation induced cardiomyocyte apoptosis:Role for glycogen synthase kinase 3 and transcription factor β catenin[J]. J Molec Cell Cardiol,2004,37(5):681-690.
[5] Martinez BA,Petersen DA,Gaeta AL,et al. Dysregulation of the mitochondrial unfolded protein response induces non-apoptotic dopaminergic neurodegeneration in C.elegans models of Parkinson's disease[J]. J Neurosci,2017,37(46):11085-11100.
[6] Zhu Y,Tchkonia T,Fuhrmann-Stroissnigg H,et al. Identification of a novel senolytic agent,navitoclax,targeting the Bcl-2 family of anti-apoptotic factors[J]. Aging Cell,2016,15(3):428-435.
[7] Grancara S,Ohkubo S,Artico M,et al. Milestones and recent discoveries on cell death mediated by mitochondria and their inter actions with biologically active amines[J]. Amino Acids,2016,48(10):2313-2326.
[8] Zheng JH,Viacava FA,Kriwacki RW,et al. Discoveries and controversies in BCL-2 protein-mediated apoptosis[J].Febs Journal,2016,283(14):2690-2700.
[9] Aira LE,Villa E,Colosetti P,et al. The oncogenic tyrosine kinase Lyn impairs the pro-apoptotic function of Bim[J]. Oncogene,2018,37(16):2122-2136.
[10] Shukla S,Saxena S,Singh BK,et al. BH3-only protein BIM:an emerging target in chemotherapy[J]. European Journal of Cell Biology,2017,96(8):728-738.
[11] Petros AM,Swann SL,Song D,et al. Fragment-based discovery of potent inhibitors of the anti-apoptotic Mcl-1 protein[J]. Bioorg Med Chem Lett,2014,24(6):1484-1488.
[12] Navickas R,Gal D,Laucevi?ius A,et al. Identifying circulating microRNAs as biomarkers of cardiovascular disease:A systematic review[J]. Cardiovasc Res,2016, 111(4):322-337.
[13] Ye Y,Perez-polo JR,Qian J,et al. The role of microRNA in modulating myocardial ischemia-reperfusion injury[J]. Physiol Genomics,2011,43(10):534-542.
[14] Gurha P. Noncoding RNAs in cardiovascular diseases[J]. Curr Opin Cardiol,2019,34(3):241-245.
[15] Seeley JJ,Baker RG,Mohamed G,et al. Induction of innate immune memory via microRNA targeting of chromatin remodelling factors[J]. Nature,2018,559(7712):114-119.
[16] Li R,Geng HH,Xiao J,et al. MiR-7a/b attenuates post-myocardial infarction remodeling and protects H9C2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1[J]. Sci Rep,2016,6(1):1-11.
[17] Mayr B,Muller EE,Schafer C,et al. Exercise responsive micro ribonucleic acids identify patients with coronary artery disease[J]. Eur J Prev Cardiol,2019,26(4):348-355.
[18] Vegter EL,Ovchinnikova ES,van Veldhuisen DJ,et al. Low circulating microRNA levels in heart failure patients are associated with atherosclerotic disease and cardiovascular-related rehospitalizations[J]. Clin Res Cardiol,2017, 106(8):598-609.
[19] Sluijter JP,van Mil A,van Vliet P,et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells[J]. Arterioscler Thromb Vasc Biol,2010,30(4):859-868.
[20] Bell ML,Buvoli M,Leinwand LA. Uncoupling of expression of an intronic microRNA and its myosin host gene by exon skipping[J]. Mol Cell Biol,2010,30(8):1937-1945.
[21] Barwari T,Joshi A,Mayr M. MicroRNAs in cardiovascular disease[J]. J Am Coll Cardiol,2016,68(23):2577-2584.
(收稿日期:2020-03-11)
