Redefining infantile-onset multisystem phenotypes of coenzyme Q10-deficiency in the next-generation sequencing era
2020-07-30AndresBerardoCatarinaQuinzii
Andres Berardo, Catarina M.Quinzii
Department of Neurology, Columbia University Medical Center, New York, NY 10032, USA.
Abstract
Keywords: Coenzyme Q10, coenzyme Q10 deficiency, coenzyme Q biosynthesis, nephrotic syndrome, cardiopathy, encephalopathy
INTRODUCTION
Coenzyme Q10(ubiquinone; CoQ10, EC 206-147-9) is a lipid molecule widely but variably distributed among cellular organelles and tissues.Intracellular CoQ10concentration is highest in the lysosomes and Golgi vesicles, followed by microsomes and mitochondria[1,2].This essential molecule is required for multiple cellular functions and aspects of metabolism, including ATP synthesis via the mitochondrial respiratory chain; antioxidant defenses; regulation of the mitochondrial permeability transition pore; activation of uncoupling proteins; and metabolism of sulfides, proline, arginine, glycine, fatty acids, and pyrimidines[1,3,4].CoQ10contains a long polyisoprenyl tail of ten isoprene units, which positions the molecule in the mid-plane of membrane bilayer, as well as a fully substituted benzoquinone ring that undergoes reversible reduction and oxidation[3,5].The various functions of CoQ10depend on the capacity of the benzoate ring to assume three different redox states: (1) oxidized (ubiquinone); (2) semioxidized (semiubiquinone); and (3) reduced (ubiquinol)[1-4].Although the main ubiquinone antioxidant function is protection against lipid and protein peroxidation, ubiquinol also regenerates other powerful antioxidants, such as α-tocopherol and ascorbate, via electron donation, and recycles them back to their active reduced forms, thereby enhancing activities of other antioxidant defenses[1-4,6].
Among the non-mitochondrial enzymatic systems involved in the continuous regeneration of ubiquinol is selenoprotein thioredoxin reductase (TrxR1), an essential antioxidant enzyme known to reduce many compounds, as well as thioredoxin[6].TrxR1-mediated reduction of CoQ10is dependent on its selenocysteine, which may account for the relationship between levels of ubiquinone and selenium[7,8].
Similar to most other mitochondrial disorders, primary CoQ10deficiency is clinically heterogenous, presenting at different ages of onset, with variable, multiple organs involvement[9,10].In the past, diagnosis of this condition relied only on biochemical assays[10,11].Specifically, low levels of CoQ10in muscle, often, but not always, associated with deficiency of CoQ10-dependent respiratory chain enzymes (complexes I + III and II + III) activities[10]; however, identification of pathogenic gene variants, wider use of next-generation sequencing, and recognition of characteristic phenotypes have greatly facilitated diagnosis of this condition.For example, the two most frequent and earliest phenotypes associated with CoQ10deficiency, steroidresistant nephrotic syndrome (SRNS) and cerebellar ataxia, have been linked to specific molecular defects in CoQ10biosynthetic enzymes, and specific COQ genes have been added to targeted diagnostic panels [e.g.,COQ8A, previously known asADCK3, is included in ataxia gene panels because pathogenic variants in this gene cause autosomal recessive cerebellar ataxia 2 (ARCA2)][12,13].
In contrast, until very recently, diagnoses of the lethal, infantile or childhood-onset multisystemic forms were reached at late stage of disease or even postmortem, through linkage or homozygous analysis in the family, in conjunction with biochemical diagnosis, and thus fewer patients were reported, compared to the other two phenotypes.However, in the last few years, implementation of next generation sequencing (NGS)-based diagnostics such as whole exome sequencing (WES) and whole genome sequencing (WGS) has caused a dramatic shift in the diagnosis, from a biochemical approach towards a molecular one, of this phenotype too.The unbiased genetic screening approach enables early diagnosis in infants and children with complex multisystemic syndromes, unveiling novel phenotypes, and molecular defects[14]; however, it is important to note that some gene variants of uncertain significance have been reported, without the functional studies necessary to prove pathogenicity.
To date, 10 genes encoding CoQ10biosynthetic proteins have been shown to cause primary CoQ10deficiency:PDSS1, PDSS2, COQ2, COQ4, COQ5, COQ6, COQ7, COQ8A, COQ8B, andCOQ9[Figure 1].The presentations include: infantile multisystem disease, with variable combinations of encephalopathy, cardiopathy, nephropathy (including SRNS), and cerebellar ataxia; SRNS; and cerebellar ataxia[9].In this review, we focus on the molecular defects in CoQ10biosynthetic genes that cause early-onset multisystemic disease (PDSS1, PDSS2, COQ2, COQ4, COQ5, COQ7,andCOQ9), propose genotype-phenotype correlation, and potential novel therapeutic strategies.

Figure 1.Schematic representation of CoQ10 biosynthesis
CLINICAL FEATURES AND MOLECULAR DEFECTS ASSOCIATED WITH EARLY ONSET MULTISYSTEMIC FORMS OF PRIMARY COQ10 DEFICIENCY
PDSS1 (MIM607429) and PDSS2 (MIM610564)
Mutations in the gene encoding subunit 1 of the decaprenyl diphosphate synthase [decaprenyl diphosphate synthase subunit 1 (PDDS1)], responsible for the synthesis of the decaprenyl tail of CoQ10, the first and ratelimiting step of CoQ10biosynthesis [Figure 1][5], are very rare[15-21].In 2007, Molletet al.[15]reported the first molecularly proven cases: two siblings with CoQ10deficiency manifesting with early-onset deafness, encephaloneuropathy, obesity, livedo reticularis, and valvulopathy, carrying a homozygous missensePDSS1pathogenic variant (c.924T>G, p.Asp308Glu).
Another patient, with compound heterozygous for two novel variants (p.Arg221Leufs★ and p.Ser370Arg) inPDSS1was reported in 2012.The infant presenteddevelopmental delay, nephrotic syndrome, and failure to thrive, and died at 16 months of age due to renal failure.Brain MRI showed leukoencephalopathy and brainstem lesions[16].
In 2000, Rötiget al.[17]described three siblings with similar symptoms, albeit varying degrees of severity, which included: severe SRNS, neurological impairment (ataxia, dystonia, and amyotrophy), retinitis pigmentosa, sensorineural deafness, and cardiomyopathy.Trans-prenyltransferase deficiency was identified, which was subsequently demonstrated to be due to a homozygousPDSS2variant.
In 2006, Lópezet al.[18]described an infant with severe Leigh syndrome, nephrotic syndrome, and CoQ10deficiency in muscle and fibroblasts due to compound heterozygous pathogenic variants inPDSS2(c.964C>T, p.Glu322★ and c.1145C>T, p.Ser382Leu).The patient was hypotonic at birth with rapid evolution of the encephalopathy.At 3 months of age, low dose CoQ10supplementation (50 mg) was initiated, and he developed intractable seizures, progressing to refractory focal status epilepticus, and death at 8 months.Quinzii and Loos reported another infant, withPDSS2pathogenic variants, who presented at age 2 months with severe global developmental delay and failure to thrive.Later evaluations showed bilateral optic atrophy, severe hypotonia, lactic acidosis, renal glomerular dysfunction, Leigh syndrome, and hypertrophy of the left ventricle.At 8 months oral therapy withl-carnitine (50 mg/kg/day), CoQ10(10 mg/kg/day), riboflavin (100 mg/day), and thiamine (50 mg/day) was started without clinical response.The proband developed generalized status epilepticus; his neurological status deteriorated and he died at 19 months.Postmortem sequencing identified two novel heterozygous missense mutations: c.590 C>A, p.Ala197Glu and c.932 T>C, p.Phe311Ser[19].
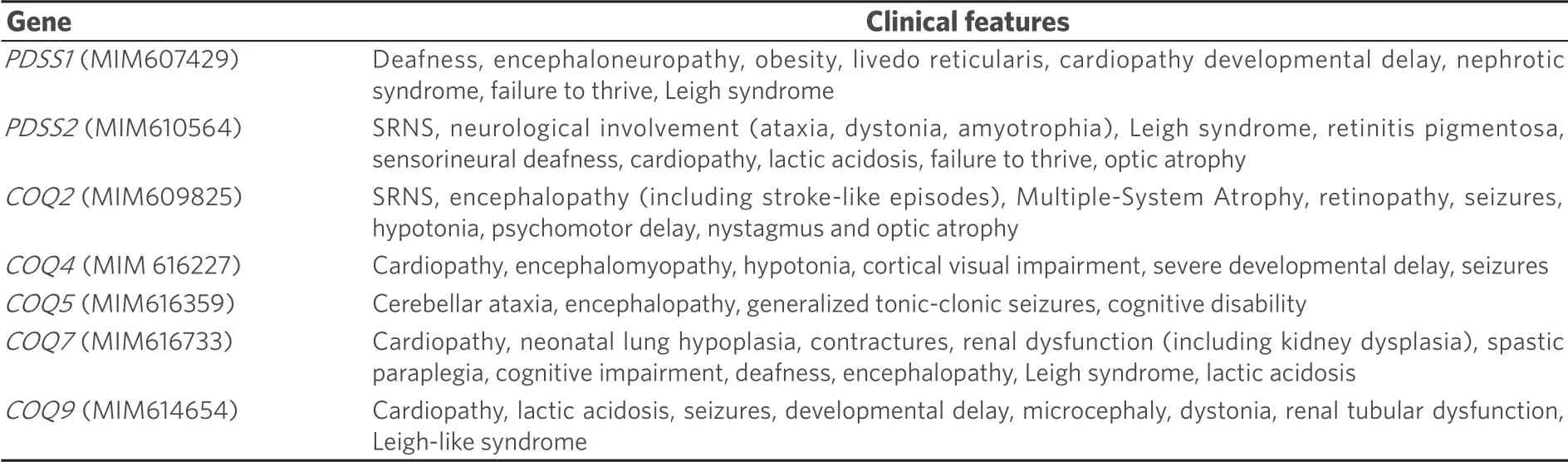
Table 1.Clinical features associated with specific defects in CoQ biosynthesis
More recently, two novel mutations inPDSS2were reported in a 7-month-old infant with nephrotic syndrome, along with encephalomyopathy, hypertrophic cardiomyopathy, deafness, retinitis pigmentosa, and elevated serum lactate level.Clinical exome sequencing revealed a heterozygous missense variant c.485A>G (p.His162Arg) and a heterozygous 2923-bp deletion (c.1042_1148-2816del), which causes a 107-base-long deletion of exon 8.The patient died at 8 months of age, despite CoQ10supplementation (20 mg/kg/day)[20].Pathogenic variants inPDSS2were also reported in two patients with isolated SRNS[21].
COQ2 (MIM609825)
COQ2encodes 4-para-hydroxybenzoate:polyprenyl transferase, the second enzyme in the biosynthetic pathway of CoQ10that condenses the benzoquinone ring with the decaprenyl side chain [Figure 1][22].
Mutations in theCOQ2gene have been associated with a wide spectrum of phenotypes [Table 1 and Figure 2], which ranges from a rapidly fatal, neonatal-onset, multisystemic disease[15,23-28], to a milder form characterized by SRNS in isolation or associated with encephalopathy[23,28,29].Mutations in COQ2 have also been reported in a patient with Multiple-System Atrophy with retinopathy[30].
The combination of neurological symptoms with SRNS are the hallmark of the neonatal multisystemic presentation of mutations inCOQ2.SRNS may be the first and predominating feature within the first year of life, followed by later onset of other manifestations such as refractory seizures, hypotonia, psychomotor delay, nystagmus, and optic atrophy[15,25,26].Nevertheless, a few exceptional cases have lacked renal involvement[26,27].
In 2006, Quinziiet al.[24]reported the first genetic cause of primary CoQ10deficiency, a homozygous c.890 A>G (p.Tyr297Cys) variant inCOQ2, in a 33-month-old boy[23].The clinical picture was dominated by nephro-encephalopathy with SRNS (proteinuria 4.3 g/day), psychomotor regression, optic atrophy, tremor, and acute-onset status epilepticus with focal electroencephalogram abnormalities predominantly in the left occipital region.Brain magnetic resonance imaging showed cerebellar atrophy, mild diffuse cerebral atrophy, and stroke-like lesions in the left cingulate cortex and subcortical area.His sister presented only with SRNS at 12 months but was treated before she developed significant neurological manifestations[23,24].
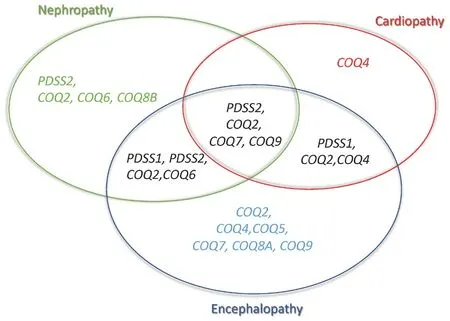
Figure 2.Phenotypes associated with CoQ10 biosynthesis defects
In 2007, Diomedi-Camasseiet al.[28]described two other patients with early-onset glomerular lesions.The first patient presented with SRNS at the age of 18 months due to collapsing glomerulopathy, with no extrarenal symptoms.He had compound heterozygousCOQ2variants c.590G>A (p.Arg197His) and c.683A>G (p.Asn228Ser).The second patient presented at 5 days of life with oliguria, with severe extracapillary proliferation on renal biopsy.He rapidly developed end-stage renal disease and died at the age of 6 months after a course complicated by progressive epileptic encephalopathy.He harbored a homozygous c.437G>A (p.Ser146Asn) variant.In 2018, Erogluet al.[29]reported four patients from two different families with SRNS and three with insulin dependent neonatal diabetes were described.Despite initial response to CoQ10supplementation in three, all patients developed neurological features, including intractable seizures that did not improve with oral CoQ10treatment.
In contrast to the original cases withCOQ2defects and encephalonephropathies, Desbatset al.[25]described a neonatal case with severe lactic acidosis, proteinuria, dicarboxylic aciduria, hepatic insufficiency, hypokinetic, and dilated left ventricle on echocardiography, although without clinical signs of cardiomyopathy, who died within the first 24 h of life.Scalaiset al.[27]described a patient without renal involvement, who presented at 3 weeks of age with myoclonic epilepsy and hypertrophic cardiomyopathy.Serial brain MRIs performed at 4 months showed bilateral and symmetrical increased signal intensities within the posterior putamen and temporal areas and in the rolandic and parasagittal cerebral regions as well as cerebral atrophy and increased CSF lactate.Jakobset al.[26]described dizygotic twins from consanguineous Turkish parents born prematurely who died at the ages of five and 6 months, respectively, after fluctuating disease courses with apneas, seizures, feeding problems, and generalized edema.Again, in these patients, there was no evidence of renal involvement.The patients carried a novel homozygous mutation inCOQ2(c.905C>T, p.Ala302Val).
COQ4 (MIM 616227)
COQ4is responsible for the stabilization of CoQ multienzyme biosynthetic supercomplex [Figure 1][31].Mutations inCOQ4have emerged lately as common causes of primary CoQ10deficiency manifesting with a variety of phenotypes [Table 1 and Figure 2], dominated by cardiopathy and/or encephalopmyopathy, without renal involvement[32-40].
The initial evidence ofCOQ4dysfunction as cause of encephalomyopathy was the report of Salviatiet al.[32], who, in 2012, reported a 3.9-Mb deletion of chromosome 9q34.13 encompassing COQ4 in a 3-year-old boy with mental retardation, encephalomyopathy, and dysmorphic features who responded to CoQ10supplementation (30 mg/kg per day of ubiquinone).
In 2015, the first five patients with point mutations inCOQ4were described.Four of them had prenatal or perinatal onset with early fatal outcome.Two unrelated individuals presented with severe hypotonia, bradycardia, respiratory insufficiency, and heart failure.Two sisters showed antenatal cerebellar hypoplasia, neonatal respiratory-distress syndrome, and epileptic encephalopathy.Only one patient had a gradually progressive condition characterized by spastic ataxic gait and seizures.Except for the solitary patient with the progressive condition, CoQ10supplementation was not administered due to fatal early onset.All these individuals carried homozygous or compound-heterozygous variants, clearly indicating that the disease is inherited as autosomal-recessive trait, indicating that haploinsufficiency might not be pathogenic because the parents, heterozygous for the nonsense variant, were unaffected[33].
Chunget al.[34]described five recessive missense mutations inCOQ4segregating with disease in four families.All patients presented with a severe multisystemic neonatal form including nervous system manifestations such as hypotonia, encephalopathy with EEG abnormalities, neonatal seizures, and cerebellar atrophy.Other manifestations included lactic acidosis, cardiomyopathy, and secondary breathing difficulties.Cerebellar hypoplasia was a common finding and nephropathy was not present.Only two patients received CoQ10supplementation, without response.
Sondheimeret al.[35]identified novel mutations inCOQ4in an infant presenting with early onset biventricular hypertrophic cardiomyopathy, hypotonia, hearing loss, seizures, and lactic acidosis associated with severe muscle CoQ10deficiency.
Linget al.[36]showed three unrelated Chinese families presenting with theCOQ4c.370G>A (p.G124S) variant, manifesting as either encephalopathy with intractable seizures and developmental delay or cardiomyopathy with left ventricle hypertrophy.In the first case of this series, CoQ10supplementation (600 mg/day) was started at six years, which resulted in improvement in the patient’s alertness only.In the second patient, CoQ10supplementation was started at 250 mg per day; then, it was increased to 400 mg per day, 3 months after symptom onset with some improvement in the control of seizures and patient’s alertness.The patient had only one further episode of epilepsy at the age of three.The third patient was not treated.The same homozygous c.370G>A (p.G124S)COQ4variant was reported in another Chinese patient, who presented in the second month of life with Leigh syndrome, respiratory distress, lactic acidosis, dystonia, seizures, and failure to thrive, without renal involvement[37].
A recent paper reported 11 additional southern Chinese patients, the largest cohort ofCOQ4deficient patients to date.Five had classical neonatal-onset encephalo-cardiomyopathy, while the other six had infantile-onset characterized by different constellations of symptoms such as hypotonia, cortical visual impairment, severe developmental delay, and seizures.Although dystonia was observed in two out of the six patients with infantile-onset presentation, none displayed basal ganglia lesions.The patients carried the variant c.370G>A, (p.Gly124Ser), previously reported by Linget al.[36]and Luet al.[37], suggesting a founder effect in the southern Chinese population.Among the 10 patients who received CoQ10supplement and with continuous follow-up, only 3 showed stabilization of the cardiopathy or seizure control; all were homozygous for c.370G>A, p.(Gly124Ser).Some improvement was observed in one patient with the heterozygous missense variants c.370G>A and c.371G>T.Five patients harbored the splicing mutation c.402+1G>A, inducing a severe early onset phenotype that was not responsive to CoQ10supplementation[38].
A recent report expanded the spectrum phenotype ofCOQ4mutationsto include childhood-onset spinocerebellar ataxia with stroke-like episodes, associated with a homozygous variant in theCOQ4gene c.230C>T (p.Thr77Ile), reported in two siblings.After the diagnosis at ages 11 and 13 years, CoQ10supplementation (1000 mg/day) was initiated for both siblings.Although motor outcomes were stable for the first year of treatment, one of the patients developed a second stroke-like episode at age 14[39].
Finally, a homozygous mutation c.164G>T, p.Gly55Val inCOQ4was reported in two siblings with a combination of slowly progressive ataxia, spasticity, and seizures, constituting an autosomal recessive cerebellar ataxia (ARCA) syndrome.The more severely affected patient received high-dose CoQ10(2000 mg/day) and showed clinically significant improvement; he was originally wheelchair-bound, unable to walk with support or standing unaided.With treatment, he became able to ambulate with a walker and stand without support.After this response, the other patient was also treated, with some improvement as well[40].
COQ5 (MIM616359)
COQ5catalyzes the only C-methylation in the biosynthesis of CoQ10[Figure 1][41].Mutations inCOQ5have been reported in only three sisters of non-consanguineous Iraqi-Jewish descent.They had varying degrees of cerebellar ataxia, encephalopathy, generalized tonic-clonic seizures, and cognitive disability, with childhood onset and slow progression [Table 1 and Figure 2].Neither WES nor WGS was able to identify a potential pathogenic variant, whereas a SNP array study, performed on the parents and all siblings, identified a tandem duplication affecting the last four exons of the gene, confirmed by Sanger analysis[42].
COQ7 (MIM616733)
COQ7is required for one of the three hydroxylations of CoQ benzoquinone ring [Figure 1][43].In 2015, Freyeret al.[44]described a 9-year-old boy withCOQ7pathogenic variants with complex clinical multiple organ involvement.The child had a history of neonatal lung hypoplasia, joint contractures, early infantile hypertension, and left ventricular cardiac hypertrophy, likely secondary to his prenatal kidney dysplasia with renal dysfunction resulting in oligohydramniosis.Although renal dysfunction normalized during the first year of life, he progressively developed mental retardation, axono-demyelinating neuropathy, hypotonia, and hearing loss.The homozygous c.422T>A (p.Val141Glu) variant inCOQ7was identified through WES.Additional functional studies in the patient fibroblasts confirmed the pathogenicity of the variant.
A second report described a patient carrying the combination of a novel homozygous mutation (p.Leu111Pro) inCOQ7, with the mitochondrial DNA m.1555A>G mutation, commonly associated with deafness.The phenotype was characterized by a mild form of spastic paraparesia and cognitive impairment as well as hearing loss.No functional studies were performed to define the cause of the deafness.The authors hypothesized that the combination of CoQ10deficiency and the m.1555A>G mutation leads to synergistic inhibition of mitochondrial function, causing irreversible damages and/or cell death and finally the clinical manifestation of hearing loss[45].
In 2019, Kwonget al.[46]reported a patient with a severe phenotype characterized by encephalomyonephrocar-diopathy, persistent lactic acidosis, and basal ganglia lesions, who died at 12 months.The patient had intrauterine growth restriction, cardiomegaly, and tricuspid regurgitation since antenatal period.WES identified two compound heterozygous variants in theCOQ7gene: a deletion insertion resulting in frameshift c.599_600delinsTAATGCATC, p.(Lys200Ilefs★56) and a missense substitution c.319C>T, p.(Arg107Trp).The proband started CoQ10supplementation at 2 months of life; the initial dose is unknown, but it was increased to 20 mg/kg/day at 12 months of life.Nevertheless, the patient cardiorespiratory manifestations deteriorated and the patient died of sepsis.Skin fibroblast studies supported pathogenicity by revealing decreased combined complex II + III activity and reduction in CoQ10level.
COQ9 (MIM614654)
COQ9is required for the stability and function ofCOQ7[Figure 1][47,48].Mutations inCOQ9have been reported in few patients, presenting with the similar lethal neonatal phenotypes characterized by encephalomyopathy and kidney involvement, including tubulopathy [Table 1 and Figure 2].
In 2009, Duncanet al.[49]described the first variant inCOQ9(c.730C>T, p.Arg244★), in a patient from an apparently non-consanguineous Pakistani family, who presented with neonatal lactic acidosis, intractable seizures, global developmental delay, microcephaly, dystonia, left ventricular hypertrophy, and renal tubular dysfunction[50].
Danhauseret al.[51]described another infant carrying a homozygous splice-site variant c.521+1del, p.(Ser127_Arg202del) inCOQ9, manifesting with neonatal encephalopathy with hypotonia, poor breathing, and severe lactic acidosis with symmetrical hyperechoic signal alterations in the basal ganglia, suggestive of neonatal Leigh-like syndrome.The patient subsequently developed seizures and recurrent episodes of apnea and bradycardia and died at 18 days of life.
In 2018, Smithet al.[52]reported four siblings, who presented prenatally with an unknown and an ultimately lethal condition characterized by intrauterine growth retardation, oligohydramnios, variable dilated cardiomyopathy, anemia, abnormal appearing kidneys, and autopsy brain findings suggestive of Leigh disease.The patients had the variants c.521+2T>C and c.711+3G>C inCOQ9,which cause in-frame deletions (p.Ser127_Arg202del and p.Ala203_Asp237del).
In 2019, a novel frameshift c.384delG (Gly129Valfs★17) homozygous mutation was reported in a 9-month-old girl, born from consanguineous parents of Pakistani origin, presenting with growth retardation, microcephaly, and seizures.She was born at 38 weeks gestation, weighed 2000 g, after an uncomplicated pregnancy, and was hospitalized for 3 days due to respiratory distress.At age 4 months, she had sustained clonic seizures.Physical examination showed microcephaly, truncal hypotonia, and dysmorphic features.Abdominal ultrasonography revealed cystic kidneys.Non-compaction of the left ventricle was detected in echocardiography.Cranial MRI showed hypoplasia of the cerebellar vermis and brain stem, corpus callosum agenesis, and cortical atrophy.CoQ10supplementation (5 mg/kg/day) was started when she was 10 months old.Despite increasing the dose to 50 mg/kg/day after the molecular diagnosis, no neurological improvement was observed[53].
DIAGNOSIS OF EARLY ONSET MULTISYSTEMIC PHENOTYPE OF PRIMARY COQ10 DEFICIENCY
Early onset primary CoQ10deficiency is clinically heterogeneous, and genotype-phenotype correlation is based on a limited number of cases[9,10].Four phenotypic groups can be defined: (1) SRNS, isolated or with neurological involvement, associated with defects inPDSS2, COQ2, COQ6,orCOQ8B(the latter with later age-at-onset); (2) encephalomyopathy, hypertrophic/dilated cardiomyopathy, lactic acidosis, and tubulopathy with defects inPDSS2,COQ2, COQ7,orCOQ9; (3) neonatal cardio-encephalopathies withCOQ2, COQ4, orPDSS1;and (4) pure neurological syndromes, including isolated or combined Leigh syndrome, ARCA, and refractory epilepsy, in association with defects inCOQ2, COQ4, COQ5, COQ7,orCOQ9[Table 1 and Figure 2][9,10].
In general, clinical features alone are insufficient to definitively diagnose CoQ10deficiency or to distinguish between primary and secondary CoQ10deficiencies, or even from other mitochondrial conditions.Therefore, evaluation of patients with suspected CoQ10deficiency relies on genetic or biochemical studies.If the clinical picture and/or family history raise the possibility of a metabolic/genetic condition, WES, including sequencing of mitochondrial DNA, if available, should be considered the first step.However, only 35% of Mendelian diseases are solved by WES[54]because the majority of undiagnosed cases are subject to limitations in variant-calling and prioritization, as well as inability to detect intronic and regulatory pathogenic variants.WGS enables complete coverage of the genome; however, interpretation is often hindered by difficulty in prioritization of the vast numbers of variants detected and our incomplete understanding of the non-coding sequences.Consequently, the diagnostic yield with WGS is only modestly increased to just over 40%[55-57].In parallel with NGS, laboratory analyses should include routine tests such as blood lactate and urine organic acids, although normal values do not exclude CoQ10deficiency.
If genetic analysis shows pathogenic homozygous or compound heterozygous variants in any of the previously reported genes involved in CoQ10synthesis with a compatible clinical picture, definitive diagnosis of primary CoQ10can be established without further analyses.In presence of variants of uncertain significance, functional and/or complementary studies are needed.Blood mononuclear cells represent a readily accessible sample, which is often suitable as an alternative to muscle for the measurement of CoQ10, by high performance liquid chromatography or mass spectrometry[11,58].In contrast, plasma levels of CoQ10are influenced by the amount of plasma lipoproteins (carriers of CoQ10in circulation), dietary intake, or supplementation, therefore cannot be used for diagnostic purpose.In addition, COQ10levels can be measured in other tissues, such as lymphoblastoid cell lines or primary fibroblasts, although normal values in these tissues do not exclude the diagnosis of CoQ10deficiency, as some patients with genetically confirmed CoQ10biosynthetic defects have had normal CoQ10levels in fibroblasts.As mentioned above, reduced activity of complexes I + III and II + III (and I + III) is highly suggestive of CoQ10deficiency[10].
TREATMENT OF EARLY ONSET MULTISYSTEMIC PHENOTYPE OF PRIMARY COQ10 DEFICIENCY
Current treatments
Humans
Varying doses of CoQ10have been used for the treatment of primary CoQ10deficiencies, ranging from 5 to 50 mg/kg/day for both adults and children[10,17].We cannot compare the effects of different dosages because formulations and durations of treatment also varied[10].We recommend high doses of CoQ10supplementation (> 30 mg/kg), because inadequate dosage and duration of intake have often constrained uptake of exogenous CoQ10
[59-61], with few mild reported side effects[10].
Early intervention with CoQ10supplementation in high doses has been shown to improve renal function[62].However, in neonatal cases with neurological involvement, response of CoQ10supplementation is poor, probably due to the irreversible brain damage at the time of the diagnosis, as well as the poor bioavailability of CoQ10, which does not cross the blood-brain barrier[29,46,53].New solubilized and stabilized formulations that are able to preserve CoQ10in its reduced form (CoQH2or ubiquinol) have been developed and increase bioavailability after oral dosing compared to standard ubiquinone[63].Experience in patients with primary CoQ10deficiency is limited and there are no clear indications about the dose-equivalence of ubiquinone and ubiquinol.Short-tail Q10analogs, such as idebenone (IDB), are more bioavailable than CoQ10but are not effective in patients with primary CoQ10deficiency[64].
In vitro and in vivo studies
In vitrostudies in human fibroblasts show that short-tail Q10analogs, such as CoQ2and IDB, are not effective in primary CoQ10deficiency because they do not correct the respiratory chain defects[65].
Studies inPdss2mutant mice, a mouse model of CoQ-deficient NS, show that CoQ10supplementation prevents renal failure through rescue of sulfides metabolism and oxidative stress.In contrast, IDB treatment was ineffective and comparable to placebo[66,67].
In a mouse model of CoQ10deficiency and encephalomyopathy due toCoq9dysfunction, the water-soluble formulation of ubiquinol was shown to be more effective than ubiquinone in rescuing brain abnormalities[68].
Investigational treatments
Administration of metabolic intermediates able to “bypass” the enzymatic block and to enable endogenous synthesis of CoQ10has been attempted in experimentalin vitroandin vivomodels of primary CoQ deficiency, as an alternative to CoQ10supplementation[69], whose therapeutic effects are hampered by its poor bioavailability.
In vitro studies
Treatment with 2,4-dihydroxybenzoic acid (DHB, β-resorcylic acid, β-RA) was shown to be effective in human fibroblasts carryingCOQ7pathogenic variants[44,45]and in COQ2-deficient cell lines, increasing the levels of CoQ10as well as increasing the viability of mutant cells growth in galactose medium[70].
Luna-Sánchezet al.[71]also investigated the effect of DHB in mouse embryonic fibroblasts from two different mouse models of COQ9 dysfunction (Coq9R239X/R239XandCoq9Q95X/Q95X) showing similar results to those obtained in COQ2 and COQ7 mutant cells, with different response to treatment based on the severity of the biochemical defect and the residual levels ofCOQ7.
Treatment with vanillic acid (VA) recovered CoQ10biosynthesis, ATP production, and reduced levels of reactive oxygen species in a human cell line lacking functionalCOQ6[72],a FAD-dependent monooxygenase responsible for the addition of the hydroxyl group in position C5 of the quinone ring[73].Mutations inCOQ6cause SRNS associated with sensorineural deafness and a variable degree of encephalopathy[74].
In vivo studies
The first studies to showin vivoefficacy of hydroxylated CoQ precursor compounds 3,4-dihydroxybenzoic acid, DHB, and VA to rescue endogenous CoQ biosynthesis were performed in yeast models ofCOQ6andCOQ7deficiencies[75,76].
More recent studies showedthatDHB ameliorated survival and phenotype inCoq7knock-out mice[77], while vanillic acid ameliorated proteinuria and prevented focal segmental glomerulosclerosis in podocyte-specificCoq6knockout mice (Coq6podKO), prolonging their survival[78].
DHB was found to rescue not only the clinical phenotype but also morphological and histopathological signs of encephalopathy in theCoq9R239Xmouse.The therapeutic effect of DHB was not attributed to the increase of CoQ10levels, but rather to the reduction of DMQ10, an intermediated metabolite that may be toxic for mitochondrial function when accumulated in the organelle.Thus, the authors proposed that DHB should be preferentially considered for the treatment of human CoQ10deficiency with accumulation of DMQ10, as mutations in COQ4, COQ7, andCOQ9[79].
Although all these experimental data suggest that biosynthesis intermediates might be a promising alternative, further studies are needed to assess therapeutic response, safety, and bioavailability and to understand their mechanism of action before their translation to the clinical practice.
CONCLUSION
Multisystemic forms of primary CoQ10deficiency are usually devastating conditions manifesting in prenatal, neonatal, or infantile period of life.Clinical symptoms include variable combinations of encephalomyocardionephropathy syndromes.Although the diagnosis of these primary CoQ10deficiency syndromes is usually not straightforward, renal involvement, particularly SRNS, can be a clinical clue.In the severe multisystemic forms, WES is often the first step in the diagnostic workup.Nevertheless, detection of novel genetic variants of uncertain significance should be followed by biochemical assays and/or functional studies in patient cells to prove pathogenicity.Eventually, comprehensive characterization of the clinical spectrum of these syndromes and associated molecular defects will establish pathogenicity of variants identified by WES and obviate further studies that are available only in specialized research laboratories.
Although in the suspect of primary coenzyme Q10deficiency high doses of coenzyme Q10supplementation are recommended, early-onset neurological features are often not responsive to supplementation.CoQ10biosynthetic analogs might be suitable alternatives to CoQ10supplementation, but additional analyses are required before these compounds can be translated to the clinical setting.
DECLARATIONS
Authors’ contributions
Wrote the manuscript, designed the study and performed data analysis and interpretation: Berardo A, Quinzii CM
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by NIH P01 HD080642-01 (CMQ).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
杂志排行
Journal of Translational Genetics and Genomics的其它文章
- Intellectual disability, the long way from genes to biological mechanisms
- Spectrum of MECP2 mutations in Indian females with Rett Syndrome - a large cohort study
- The North American mitochondrial disease registry
- Mitochondrial translation defects and human disease
- Role of transfer RNA modification and aminoacylation in the etiology of congenital intellectual disability
- Cryogenic electron paramagnetic resonance spectroscopy of flash-frozen tissue for characterization of mitochondrial disease
