Preparation of a versatile polyethylene glycol-bonded stationary phase based on silica monolith particles and its application in multi-modal separation in high performance liquid chromatography
2020-07-27LIANGQianZHOUYuhongZHANGZhilunHUANGMingxian
LIANG Qian, ZHOU Yuhong, ZHANG Zhilun, HUANG Mingxian
(College of Science, University of Shanghai for Science and Technology, Shanghai 200093, China)
Abstract: Silica monolith particles with sizes of 2-5 μm and pore sizes of 20-60 nm were obtained by grinding, flotation, pseudomorphic transformation, and hydrothermal treatment of the silica monolith prepared by the sol-gel method. The pseudomorphic transformation was performed with a dual micellar templating system consisting of Capstone FS-66, a partially fluorinated anion surfactant, and cetyltrimethylammonium bromide (CTAB), a commonly used cation surfactant. Hydrothermal treatment with a sodium carbonate solution was adopted to further expand the pore size. Scanning electron microscopy (SEM) images and N2 adsorption-desorption isotherm measurement results of the silica monolith particles before and after the treatments clearly demonstrated the changes in morphology caused by these treatments. Afterward, a long-chain polyethylene glycol (PEG) containing silane was bonded on the surface of the as-prepared particles, and the resulting products were characterized by elemental analysis and FT-IR spectroscopy analysis, and evaluated by high performance liquid chromatography (HPLC). Elemental analysis and thermogravimetric analysis (TGA) of the bonded stationary phase revealed that the bonding amount of PEG on the silica surface is about 8%. It has been shown that silica monolith particles can be treated and modified for the separation of proteins in size exclusion chromatography mode. It is also demonstrated that the bonded stationary phase can be used for the separation of ribonuclease A and lysozyme in hydrophobic interaction chromatography mode, and for the separation of highly polar compounds (picolinic acid, levodopa, melamine, and catechol) in hydrophilic interaction chromatography mode. The results indicate the versatility of the PEG-bonded stationary phase and its promising application to multi-modal separation in HPLC.
Key words: high performance liquid chromatography (HPLC); pseudomorphic transformation; protein separation; multi-modal stationary phase; silica monolith particles
Various separation modes in high performance liquid chromatography (HPLC) have been explored for a wide range of applications in chemical industry, biomedicine, food safety, and environmental protection [1-5]. In particular, different modes of HPLC are utilized for the separation and purification of proteins according to their size, charge, hydrophobicity, etc. [6]. At present, the most frequently used HPLC modes for protein separation and analysis are size exclusion chromatography (SEC), hydrophobic interaction chromatography (HIC), ion exchange chromatography (IEC), reversed-phase chromatography (RPC), and affinity chromatography (AC) [7-11]. In general, the separation mode is closely related to the stationary phase used in HPLC.
In SEC, proteins are separated based on their molecular weights and volumes [12]. Traditional SEC stationary phases for biological macromolecules are soft gels of polysaccharide, but they are vulnerable to high pressure [13], and the separation efficiency is low. Modern SEC packing materials include highly crosslinked hydrophilic gels, porous organic polymer microspheres, and porous silica microspheres [14-16]. HIC is a method for the separation of proteins by exploiting the hydrophobic interaction between the proteins and the stationary phase in a high-concentration salt buffer [17]. The advantages of HIC are mild analytical conditions and high recovery. Furthermore, the bioactivity of the target analytes is preserved [9]. The stationary phases used in HIC usually contain mild hydrophobic ligands such as butyl, phenyl, or polyethylene glycol (PEG) chains. Therefore, in HIC, a high salt concentration is required to ensure sufficient hydrophobic force for the adsorption of proteins; subsequently, the salt concentration is lowered to realize desorption for the separation of different proteins [18].
Hydrophilic interaction chromatography (HILIC) is suitable for the separation and analysis of highly hydrophilic and polar compounds. The stationary phases used in HILIC usually have strong hydrophilic ligands to allow for longer retention times and better separation of small hydrophilic compounds as compared to those achieved with reversed-phase chromatography stationary phases. Therefore, HILIC has recently attracted much attention [19,20].
Silica remains the most widely used substrate for HPLC stationary phases because of its strong mechanical strength as well as stable morphology, pore structure, and specific surface area. Besides, the silica surface is rich in silanol groups that can be bonded and modified easily [21]. The particle size, pore distribution, and specific surface area of silica are important factors that affect the efficiency of HPLC separation [22]. Recently, Cheong’s group [23-28] published a series of reports on the preparation of stationary phases based on silica monolithic particles, which were prepared by the sol-gel method and then ground into microscale particles. The resulting irregular particles can help improve the permeability of the HPLC column while reducing the column pressure, thereby allowing for fast and efficient chromatographic separation.
The pore size and pore size distribution of the silica-based stationary phase play a crucial role in protein separation. In order to fine-tune the pore structure of silica materials, a pseudomorphic transformation technique can be adopted [29-32], which can aid the transformation of nonporous or porous silica shells into ordered mesoporous silica shells. In general, different pore expansion effects can be achieved by using cetyltrimethylammonium bromide (CTAB) in NaOH aqueous solution and adjusting the ratio of NaOH, SiO2, H2O, and CTAB [33,34]. In this process, silica in the surface layer was dissolved in the weak alkaline solution under hydrothermal conditions and re-assembled around the surfactant micelle template onto the original silica surface, while the shape and size of the original silica particles are preserved. Finally, the surfactant is removed by washing or calcination to realize the pore expansion effect, which can be further manipulated by varying the type and amount of surfactants.
In this study, irregular silica monolith particles with a size range of 2-5 μm were fabricated by grinding the silica monolith. Then, the silica particles were subjected to pseudomorphic transformation with a dual surfactant templating system, with Capstone FS-66 and CTAB as the anionic and cationic surfactants, respectively [35]. Subsequent hydrothermal treatment with a sodium carbonate solution was performed to further expand the pore size to the range of 20-60 nm. Finally, PEG silane was bonded onto the surface of the as-prepared silica, the resulting stationary phase was evaluated for different modes of separations in HPLC.
1 Experimental
1.1 Materials
PEG-20000, tetramethoxysilane (TMOS), acetonitrile (ACN), CTAB, ammonium sulfide, picolinic acid, melamine, levodopa, and catechol were purchased from Aladdin Reagents (China). Urea, uracil, acetic acid, hydrochloric acid, methanol, ethanol, isopropanol, trimethylamine (TEA), sodium dihydrogen dihydrate phosphate, egg albumin (Ova), thyroid globulin (Tg), anhydrous toluene, and trichloromethane were obtained from Sinopharm Chemical Reagent (China). Capstone FS-66, lysozyme (Lyz, from egg white), and ribonuclease A (Rib) were purchased from Sigma-Aldrich (USA). 2-[Methoxy (polyoxyethylene)6-9propyl] trimethoxysilane (PEG silane) was obtained from Gelest (USA). All the reagents were used as-received without further treatment, unless otherwise specified.
1.2 Synthesis of silica monolith particles
The silica monolith particles were prepared by modifying reported methods [25-30]. PEG (4.86 g) and urea (4.96 g) were dissolved in acetic acid solution (45 mL, 0.01 mol/L in water) in a Teflon vial. The mixture was magnetically stirred for 10 min in an ice-bath. Then, 15 mL of TMOS was added to the solution, and the mixture was stirred for 40 min. Next, the solution was transferred to an autoclave, followed by incubation in an oven at 40 ℃ for 48 h and then at 120 ℃ for 48 h. Thereafter, the monolithic solid cake was sequentially washed twice with water and ethanol each, and dried at 60 ℃ under vacuum for 15 h.
The dried bulk silica monolith was pulverized using a mortar and pestle, ground in a ball mill at a rotation speed of 150 r/min for 1 h, and finally calcined in a furnace at 550 ℃ for 5 h.
1.3 Flotation of silica monolith particles
The silica particles were suspended in a flotation solution (ethanol-water (95∶5, v/v)), which was continuously stirred in a beaker for 15 min and left to stand for 8 h. The supernatant was discarded, and the solid was dried overnight at 70 ℃.
1.4 Pseudomorphic transformation
First, 16.33 g of CTAB and 0.66 g of NaOH were dissolved in a mixture of 420 mL of deionized water and 106 mL of ethanol. Then, 3.26 g of Capstone FS-66 dissolved in 5 mL of acetone was added to the above solution and stirred at 40 ℃ for 30 min. After the mixture was cooled, 10 g of silica monolith particles was added. The resulting mixture was stirred for 30 min, transferred to an autoclave, and reacted at 110 ℃ for 48 h. After cooling, the particles were centrifuged and washed with deionized water and ethanol, dried at 70 ℃ for 12 h, and calcined in a furnace at 580 ℃ for 5 h.
1.5 Treatment with sodium carbonate
The above mentioned treated silica particles were suspended in a solution of sodium carbonate (3%). The mixture was stirred at 90 ℃ for 2 h, transferred to an autoclave, and reacted at 120 ℃ for 9 h. The particles were centrifuged, washed with ethanol and deionized water, and dried at 70 ℃ for 12h.
1.6 Preparation of PEG-bonded stationary phase
The above mentioned treated silica monolith particles (6 g) were immersed in 50 mL of 6 mol/L HCl solution for 4 h with shaking. After the reaction, the particles were washed with deionized water until neutral pH and dried at 80 ℃ for 8 h. The desiccated particles were suspended in 150 mL of anhydrous toluene under nitrogen and continuously stirred for 2 h at 110 ℃. Then, 3 mL of PEG silane and 0.5 mL of TEA were added to the mixture, and refluxed for 12 h. Finally, the products were washed sequentially with toluene, ethanol, ethanol-deionized water (1∶1, v/v), and ethanol, and then dried at 60 ℃ for 12 h.
1.7 Characterization
Scanning electron microscopy (SEM) images of the particles were recorded using an XL-30ESEM-TMP microscope (Philips, Holland) with a built-in elemental analyzer. The surface properties of the silica were evaluated from N2adsorption-desorption isotherms (TriStar Ⅱ 3 020 surface area and pore size analyzer, Micromeritics, USA). FT-IR spectra were obtained on a Thermo Nicolet 380 apparatus (Thermo, USA). Thermogravimetric analysis (TGA) was performed on a TGA-50 system (Shimadzu, Japan). HPLC separation was performed on a P230Ⅱ instrument (Dalian Elite, China).
1.8 Packing HPLC columns
The PEG-bonded stationary phase (3 g) was suspended in 50 mL chloroform-isopropanol (1∶1, v/v) under ultrasound conditions and packed into stainless-steel columns (250 mm×4.6 mm i. d.) by the slurry packing technique (pressure: 35 MPa) with methanol as the displacing solvent.
2 Results and discussion
2.1 Properties of silica monolith particles
As demonstrated by Cheong’s group [25] and our group [36], silica monolith particles featured high permeability, porosity, and separation efficiency. In those studies, PEG and TMOS were used as the porogen and silica precursor, respectively, and monolithic silica was obtained under the catalysis of acetic acid and thermally decomposed urea. The monolithic silica was ground by a ball mill and then treated by flotation and pseudomorphic transformation into the desirable particles. The SEM images of the silica monolith particles before and after pseudomorphic transformation are displayed in Fig. 1. The particles were found to be irregular, with a size range of 2-5 μm, and a part of the Y-shaped silica skeleton from the silica monolith was retained. Moreover, the particle surface contained numerous mesoporous pores, and both the grain size of the silica nanoparticles on the silica particle surface and the pore size of the particles increased after the treatment.

Fig. 1 SEM images of silica monolith particles (a, b) before and (c, d) after treatment
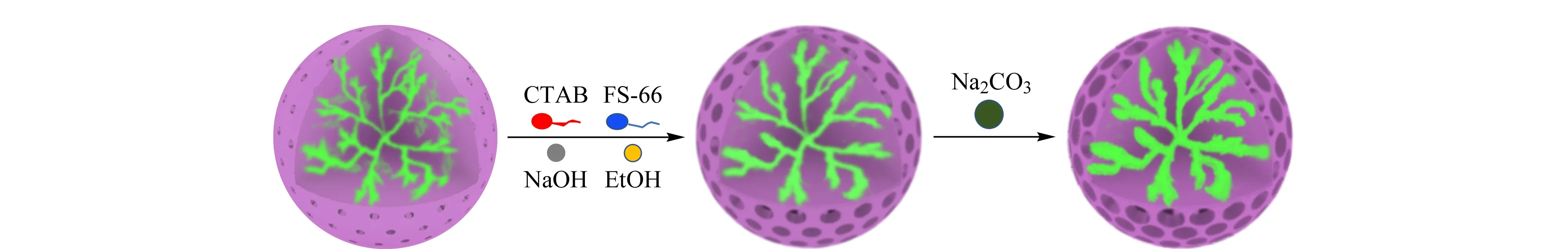
Fig. 2 Schematic of pore enlargement
2.2 Pore control of silica monolith particles
Fig. 2 shows a schematic representation of the pore enlargement process. In the pseudomorphic transformation step, it was hypothesized that the silica species on the pore surface dissolved in the weak alkaline solution and re-precipitated around the surfactant micelles to form an ordered mesoporous phase on the surface of and/or inside the silica. The silica particles were treated with a H2O-ethanol-NaOH-CTAB-Capstone FS-66 mixture at a molar ratio of 140∶35∶0.1∶0.27∶0.05 based on the information from our earlier study [35]. We have shown that Capstone FS-66, a partially fluorinated anion surfactant, worked along with CTAB as a dual micellar templating system and increased the pore size as compared to that obtained with the use of CTAB alone. The mesoporous channels are aligned in an orderly fashion in the silica shell, and are arranged perpendicularly to the surface. These features make the pores conducive for chromatographic processes, especially SEC, where fast transfer of solutes in and out of the pores is preferred.
After the pseudomorphic transformation and further hydrothermal treatment with a weak base, sodium carbonate, the pores of the silica particles became larger, as shown by the pore size distribution curves and N2adsorption-desorption isotherms in Fig. 3. The pore diameter of the original silica gel was 10-25 nm, which became 15-35 nm after the pseudomorphic transformation and further increased to 20-60 nm after the sodium carbonate treatment.
2.3 Characterization of PEG-bonded stationary phase
The silica particles after pore treatment were acidified to convert some of the Si-O-Si bonds on the surface to free silanol (Si-OH) groups. Then, PEG silane was bonded onto the particles with the surface silanol groups, as shown in Fig. 4.

Fig. 3 (a) Pore size distribution curves and (b) N2 adsorption-desorption isotherms of the silica particles (after Na2CO3 treatment)
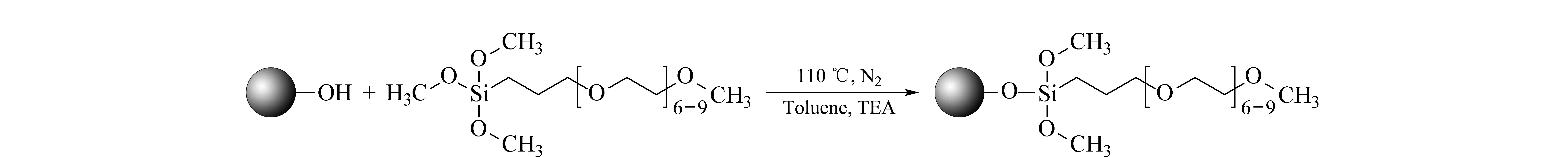
Fig. 4 Schematic procedure for the preparation of PEG-bonded stationary phase
As shown in Table 1, the carbon content of the PEG-bonded particles increased from 0% to 6.49%, indicating the successful bonding of PEG silane on the silica surface. The bonding amount of PEG on the silica surface was calculated to be around 8% based on the structure of the bonded PEG silane.
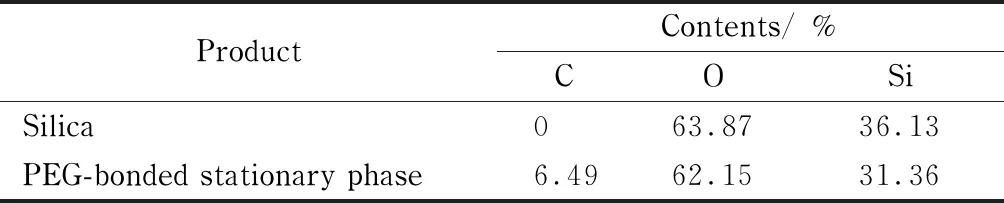
Table 1 Elemental analysis of the product in the preparation of PEG-bonded stationary phase
The IR spectra of the PEG-bonded stationary phase are shown in Fig. 5a. The peak at 3 440 cm-1is due to the absorption of residual silanol and water. The peak near 2 900 cm-1is due to C-H stretching vibrations arising from the CH2group of the bonded PEG molecules.
The TGA results for the PEG-bonded stationary phase are shown in Fig. 5b. The PEG-bonded silica particles show a weight loss of approximately 7% during the 200-700 ℃ heating period, indicating that the bonding amount of PEG on the silica surface is lower than the amount (8%) calculated from the elemental analysis. This phenomenon is probably due to the mild carbonization of the organic molecules on the silica surface.
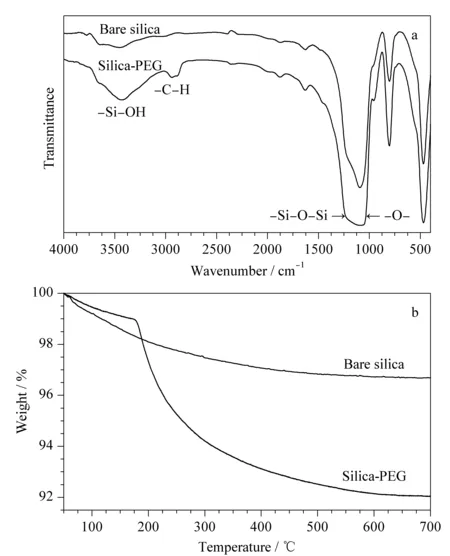
Fig. 5 (a) FT-IR spectra and (b) TGA curves of silica and PEG-bonded stationary phase
2.4 SEC performance test
PEG molecules are known to be inert to proteins, which makes the PEG-bonded particles suitable for use as a SEC stationary phase when the separation solely depends on the pore structure. The PEG-bonded stationary phase was evaluated for the separation of proteins having various molecular weights, as shown in Fig. 6. The stationary phase showed good separation performance for the selected proteins in SEC mode.
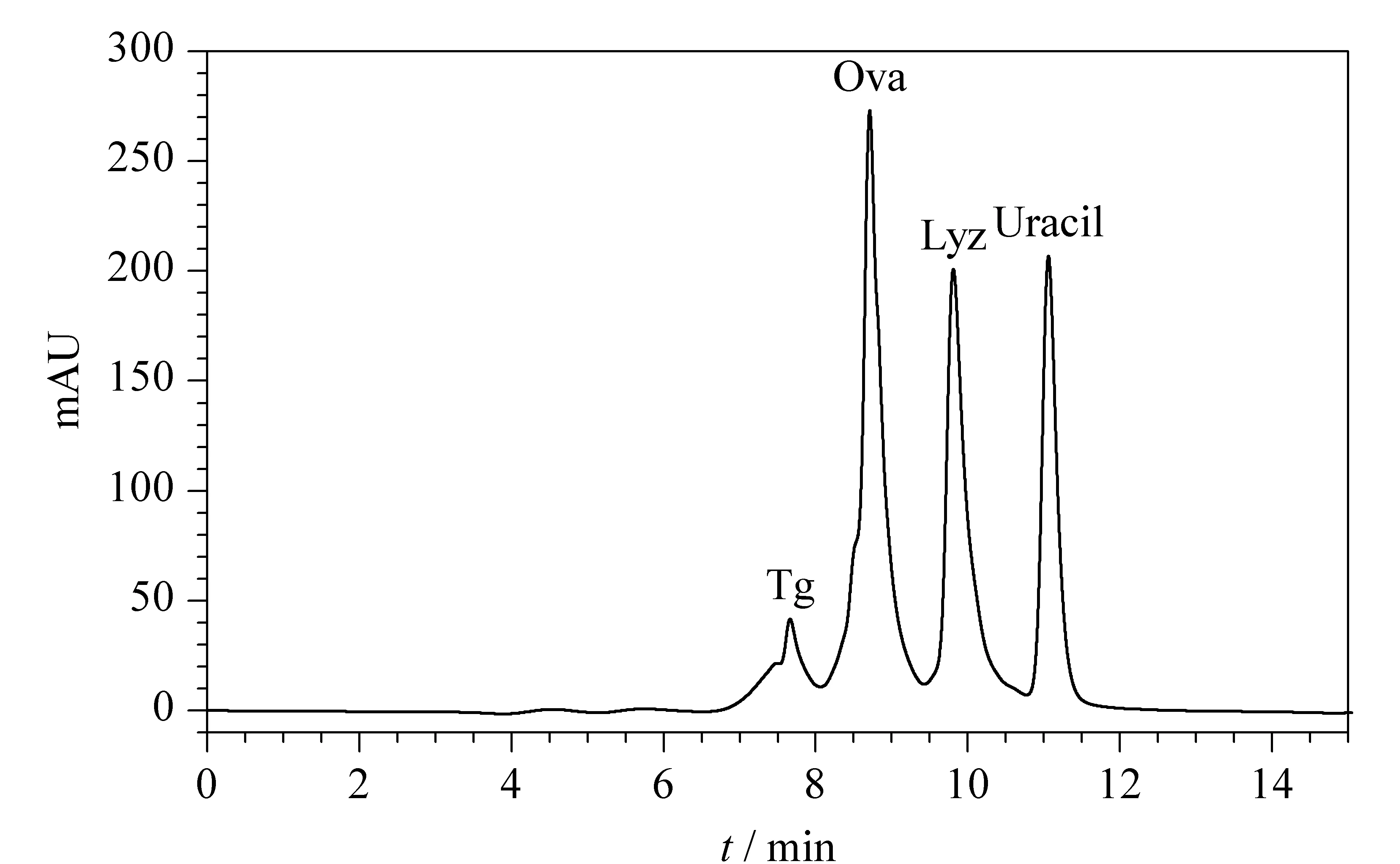
Fig. 6 Chromatogram of the standard proteins separated by the PEG-bonded stationary phase using SEC Mobile phase A: 0.3 mol/L NaCl and 100 mmol/L NaH2PO4, (pH 6.8); mobile phase B: H2O; isocratic elution; A-B (75∶25, v/v); flow rate: 0.3 mL/min; detector wavelength: 220 nm; column temperature: 25 ℃.
2.5 HIC performance test
It has been shown that PEG-bonded stationary phases can also be used for the separation of protein by HIC [37]. When using a mobile phase with a high salt concentration, proteins can be adsorbed onto the PEG ligands and then desorbed when the salt level is decreased; thus, the proteins can be sequentially eluted according to their surface hydrophobicity. Under such mild separation conditions, proteins can maintain their bioactivity during the HIC analysis. In order to avoid mutual adsorption and aggregation of proteins, the addition of a small amount of arginine is necessary [38]. Fig. 7 shows the separation of ribonuclease A and lysozyme in HIC mode using the PEG-bonded stationary phase.
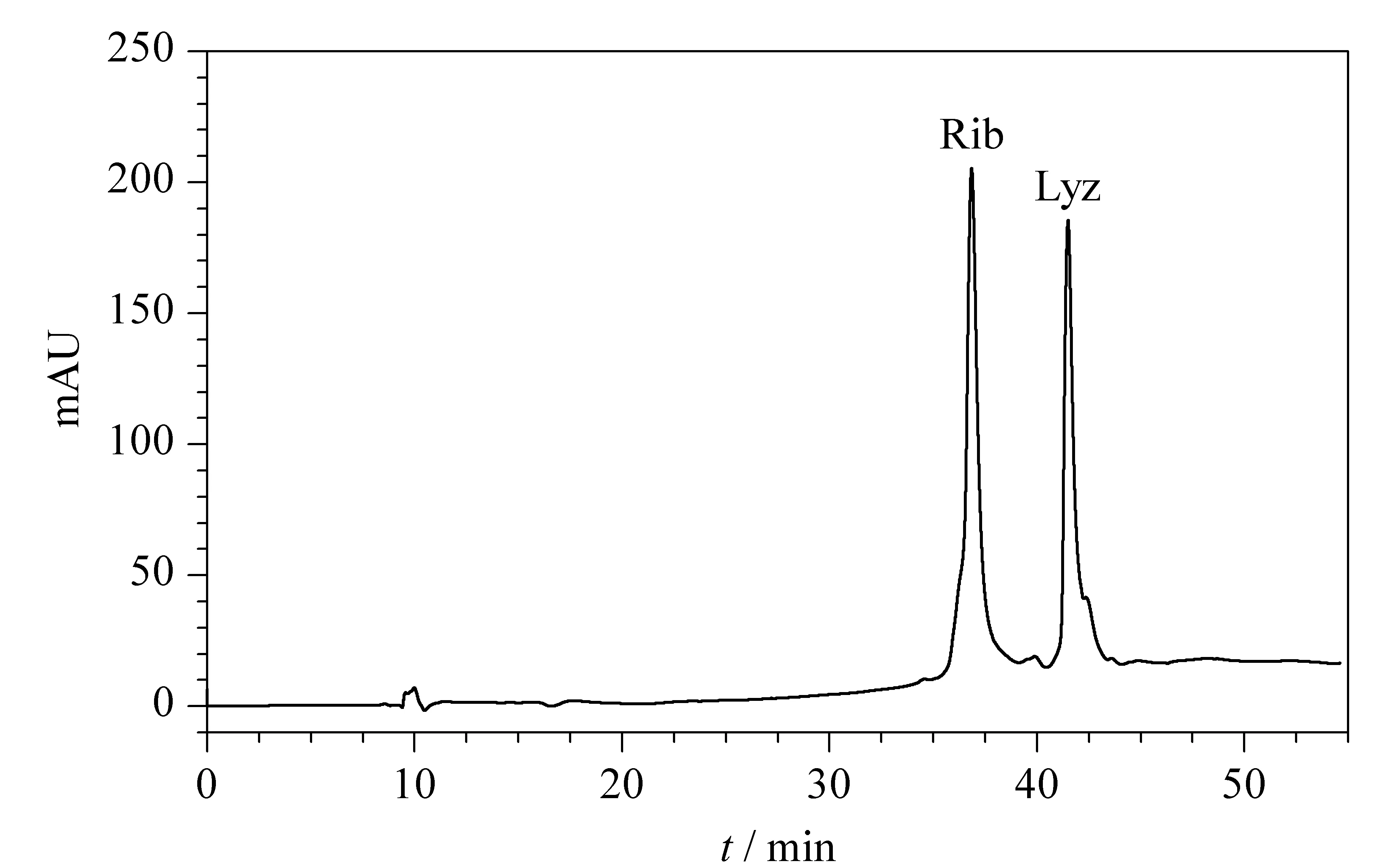
Fig. 7 Chromatogram of the standard proteins separated by the PEG-bonded stationary phase using HIC Mobile phase A: 0.1 mol/L L-arginine and 0.01 mol/L NaH2PO4(pH 7); mobile phase B: 0.01 mol/L NaH2PO4 and 3 mol/L (NH4)2SO4; gradient elution: 0-5 min: 10%A, 5-35 min: 10%A-100%A; flow rate: 0.3 mL/min; column temperature: 25 ℃; detection wavelength: 280 nm.
2.6 HILIC performance test
Recently, HILIC has been widely used for the separation of highly hydrophilic and polar compounds, where PEG molecules serve as ligands for the stationary phases [39]. As seen in Fig. 8, the four polar compounds (picolinic acid, levodopa, melamine, and catechol) are well separated on the PEG-bonded column. This result can be explained by the fact that the PEG molecules are hydrophilic, creating a highly polar water layer on the surface.
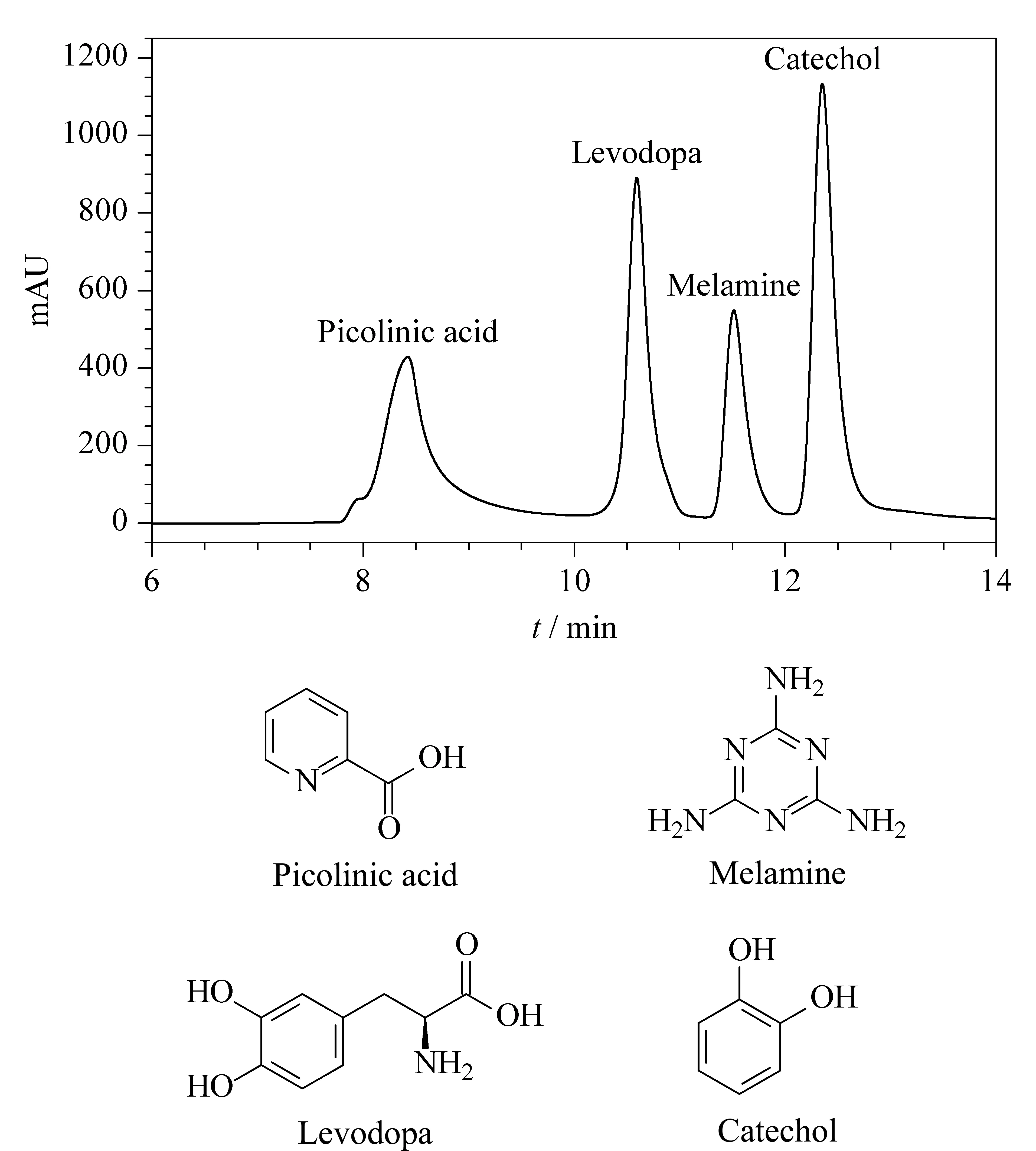
Fig. 8 Chromatogram of the four polar compounds PEG-bonded stationary phase (HILIC) and their structural formulas Mobile phase A: acetonitrile; mobile phase B: H2O; gradient elution: A-B (60∶40, v/v); column temperature: 25 ℃; flow rate: 0.3 mL/min; detector wavelength: 220 nm.
3 Conclusion
In this study, the use of a versatile PEG-bonded stationary phase based on silica monolith particles for various separation modes in HPLC has been demonstrated. The preparation method for this stationary phase is simple and cost-effective. Moreover, the scope of phases based on silica monolith particles is extended from reversed-phases in the previous publications to a polar phase in this work. In particular, the pore size enlargement by pseudomorphic transformation and hydrothermal treatment is found to be effective for making the silica monolith particles suitable base materials for the separation of proteins in SEC and HIC. In the meantime, the PEG-bonded stationary phase with silica monolith particles also has promising application to the HILIC mode. The separation efficiency of the as-prepared stationary phase can be increased by reducing the particle size and optimizing the column packing conditions. The HILIC performance for small or large molecules can be improved by using silica monolith particles with different pore sizes. The permeability of these silica monolith particles is worth exploring in further detail, and this will be the topic of our future work.
