Phytochemicals and cancer chemoprevention
2020-07-21AsimDaveFalguniParandeEunJungParkJohnPezzuto
Asim Dave, Falguni Parande, Eun-Jung Park, John M. Pezzuto,2
1Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Brooklyn, NY 11201, USA.
2College of Pharmacy and Health Sciences, Western New England University, Springfield, MA 01119-2684, USA.
★Contributed equally and should be viewed as first co-authors.
Abstract The unending morbidity and mortality that results from cancer, as well as adverse reactions due to chemotherapy and the enormous economic burden of treatment and hospitalization, advocates for the necessity of chemopreventive measures. Cancer chemoprevention refers to the use of agents capable of reversing, reducing, or slowing down the pathology of cancer at various stages. Fortunately, a few therapeutic drugs with relatively low toxicity (e.g., tamoxifen, finasteride), and a sparse number of vaccines (hepatitis B, HPV), are used to prevent specific cancers. In the general population, however, therapeutic options for cancer prevention are not common. Nonetheless, it is generally agreed that diet affects the genesis of cancer, and phytochemicals have the capacity of functioning as cancer chemoprevention agents. This is supported by epidemiological studies and clearly documented with animal models designed to mimic human carcinogenesis. Additionally, some public health strategies, such as recommendations for greater consumption of fruits and vegetables, reflect the merits of cancer chemoprevention. Here, we focus on some well-established natural product cancer chemopreventive agents, including resveratrol (grapes), epigallocatechin-3-gallate (green tea), sulforaphane (cruciferous vegetables), anthocyanins (grapes and berries), curcumin (turmeric), silibinin (milk thistle), and lycopene (tomatoes). As aptly demonstrated by genomic analysis and other methods, the mechanistic underpinning is variable and complex. In addition, responses may be mediated through indirect mechanisms, such as interaction with the microbiome. Furthermore, ancillary applications of chemopreventive agents are worthy of consideration, such as management of sequelae induced by chemotherapy. Recognizing the loss of millions of cancer patients every year, it is obvious that negating malignant metastatic conditions remains of paramount importance. In meeting this objective, cancer chemoprevention offers great promise.
Keywords: Chemoprevention, phytochemicals, resveratrol, curcumin, anthocyanins, silibinin, lycopene, epigallocatechin-3-gallate, anti-cancer activity
INTRODUCTION
Overall, cancer is the second leading cause of death in the US and a major public health issue throughout the world. Approximately 1,762,450 new cancer cases are estimated in the US in 2019[1], and 18.1 million new cases worldwide. In 2018, 9.6 million deaths were attributed to this disease[2]. Given these astounding demographics, and the associated pain, suffering and economic burden, the scientific and medical community continuously strive for better treatment options, improved palliative care, and effective preventative strategies. In this context, the role of diet in cancer has attracted considerable attention. This is especially compelling given that epidemiological studies have demonstrated regular consumption of phytochemicals from dietary sources like fruits, vegetables, herbs, and teas is associated with reduced risk of chronic diseases including cancer, cardiovascular disease and inflammatory disorders[3,4].
Cancer chemoprevention can be classified into primary, secondary, and tertiary measures. Populations with no overt cancer risk factors, or those ostensibly at high risk due to factors such as successful surgical resection or family history, can be grouped under primary measures. Patients with pre-malignant lesions bearing risk of progressing to an invasive cancer (e.g., ductal carcinoma in situ) can be grouped under secondary measures. In these cases, standard protocols of chemopreventive practice would be highly desirable, but scarcely come into play[5]. People in primary and secondary chemoprevention categories may be advised or decide on their own to increase dietary phytochemical consumption or to use over the counter products such as non-steroidal anti-inflammatory drugs. Finally, tertiary measures can be considered for patients with cancer relapses[6]. A unique example of a tertiary chemopreventive measure is the administration of tamoxifen (or structural relatives), or aromatase inhibitors, for patients diagnosed with breast cancer[7].
Around two and half thousand years ago, Hippocrates advised “let food be thy medicine and medicine be thy food”. This remains a powerful statement, as graphically illustrated in Figure 1. An inverse relationship between adequate fruit and vegetable consumption and cancer incidence has been established. In fact, it has been suggested that cancer incidence could be reduced by over 50% if people consume at least five servings of fruits and vegetables per day[9]. On the other hand, obesity is generally associated with poor health and chronic illness, and there are certain foods that can act as carcinogens and initiate tumor formation.
Secondary metabolites (phytochemicals) are typically generated in plants to afford protection against external threats such as UV, fungal infection, and the generation of free radicals. The compounds so produced show a remarkable array of structural diversity. Notably, ingestion of these phytochemicals provide human beings with protective eあects as well[10], perhaps by reducing oxidative stress (ROS) and inflammation[3,11]. However, the mechanisms by which phytochemicals function in a chemopreventive capacity are certainly intricate and multifaceted, as described to some extent in this review.
Interestingly, cancer and aging share several hallmarks in terms of the genetic pathways and biochemical processes. For example, DNA repair mechanisms are affected by ROS and this may result in the deregulation of signaling pathways such as p53 and nuclear factor-κB (NF-κB). In turn, such deregulation may accelerate aging and cancer development[12]. In principal, antioxidants sourced from phytochemicals may neutralize ROS and attenuate oxidative stress[13]. Further, as deregulation of signaling pathways is involved in progression of inflammatory diseases, modulation of these processes by phytochemicals may down-regulate proinflammatory factors[14]. Considering the general safety of dietary phytochemicals, especially compared with narrow therapeutic index chemotherapeutic agents, the potential merit of chemoprevention is obvious.

This review encompasses studies involving the dietary role of phytochemicals including curcumin (turmeric), epigallocatechin gallate (green tea), resveratrol (grapes), anthocyanidin (grapes and berries), sulforaphane (cruciferous vegetables), silibinin (herb milk thistle), and lycopene (tomatoes). Mechanisms of alleviating multiple pathological conditions, such as oxidative stress, epigenetic alteration, angiogenesis, chronic inflammation, and effects on stem cell transformation are taken into account. Finally, some thoughts are provided in regard to future directions.
RESVERATROL
Chemical properties of resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, MW: 228.25 g/mol) is a naturally occurring stilbene with two phenolic rings connected by an ethylene group[15]. It is a phytoalexin mainly synthesized as a protective mechanism in plants in response to environmental stress including fungal infection, UV radiation, and chemical exposure[16]. The dominant dietary source is grapes and grape products[17], but more recently, approximately 70 species of plants have been reported to produce resveratrol[18]. As such, in an average diet, relatively small amounts of resveratrol can be found in peanuts (Arachis hypogaea), blackberries (Morus spp.) and blueberries (Vaccinium spp.)[19,20]. Red wine is a major source of resveratrol in the Mediterranean diet, as grape (Vitis vinifera) is a rich source of resveratrol. Specifically, resveratrol is found in seeds, skin, woody parts, and petioles. Therefore, red wine generally has a higher content of resveratrol than white wine, since, during the production of the wine, parts of grapes in which resveratrol is present are macerated for a longer period of time[21]. During grape fermentation, the formation of alcohol facilitates the solubility of resveratrol which further leads to its extraction.
Polygonum cuspidatum is an extremely rich source of resveratrol and used as a therapeutic regimen for cardiovascular diseases in Chinese and Japanese traditional medicine practice[22]. Similarly, the rhizome of Veratrum formosanum, containing abundant resveratrol, has been applied to treat hypertension in East Asia[23].
Pharmacokinetic properties of resveratrol
Resveratrol is primarily metabolized by phase II enzymes in the liver. Through enterohepatic transport in bile, some of the compound returns to the small intestine[24]. Moreover, resveratrol can stimulate its own metabolism by increasing the action of phase II hepatic detoxifying enzymes[25]. Although trans-piceid, the naturally occurring glucoside, exhibits biological activities, glucuronide metabolites of resveratrol in humans seem to be less active.
The high rate of resveratrol metabolism produces conjugated sulfates and glucuronides which maintain some biological activity[26]. Although metabolites can differ in their nature and quantity between subjects due to inter-individual variability, there are major five types of metabolites in the human urine: two isomeric forms of resveratrol monoglucuronide, resveratrol monosulfate, monoglucuronide dihydroresveratrol and monosulfate dihydroresveratrol[27,28]. In human urine samples, cis metabolites have been found, mainly as cis-resveratrol-4′-sulfate, cis-resveratrol-4′-O-glucuronide and cis-resveratrol-3-Oglucuronide[29,30]. Even though the cis-isomer displayed comparable activities to the trans-isomer in some experimental settings[31,32], the trans-isomer is generally considered dominant and exerts greater activity. Other dietary components may influence metabolism. For example, quercetin has the potential to inhibit resveratrol glucuronidation and sulfation in the duodenal and liver, thus increasing bioavailability[33].
Biological activities of resveratrol
Spearheaded by conceptualization of the “French Paradox”, the potential health benefits of phenolic compounds present in wine and grapes have been extensively studied. As a brief background, in the northern region of France, the dietary intake of saturated fat is relatively high. However, relative to other parts of the world where a similar amount of high saturated fat is consumed, the mortality due to coronary heart disease is reduced. This phenomenon, termed the “French Paradox”, was attributed to the relatively high wine consumption of the French[34]. This possible benefit of wine was not ascribed to alcohol content, since alternative alcoholic beverages such as beer were not perceived to be eあective in this regard. In turn, this led to speculation regarding the eあectiveness of chemical constituents in wine other than alcohol. It is known that grapes, and consequently wine, contain scores of phytochemicals[35], but a conundrum exists since these components are also found in other sectors of the diet. Alas, when the cancer chemopreventive potential of resveratrol was first described[36], it was recognized this is a compound uniquely associated with the grape (and wine), and, in fact, grapes and wine are the dominant dietary source of this biologically active compound. Thus, resveratrol was perceived by some as a key to the “French Paradox” and many studies followed to explore broader biologic potential[37].
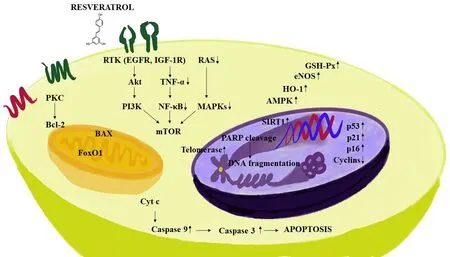
Figure 2. Some mechanisms of action mediated by resveratrol. Resveratrol exhibits an anti-inflammatory response by down-regulating pro-inflammatory factors. The compound decreases survival related proteins including phosphatidylinositol-3-kinase (PI3K) and Akt. Further, signaling cascades are impeded by down-regulating NF-κB, MAPKs and TNF-α, resulting in the inhibition of mTOR. In addition, resveratrol decreases the expression of anti-apoptotic marker BCL-2, and apoptotic pathways are controlled by up-regulating proapoptotic proteins which are responsible for the cell death, such as, BAX and caspase 3, with an increase in DNA fragmentation. The pleotropic mechanisms of resveratrol are also accentuated by induction in the signaling pathways such as AMPK, SIRT1, HO-1, p53, p21, p16, eNOS and GSH-Px. NF-κB: nuclear factor-κB; MAPK: mitogen-activated protein kinase; TNF-α: tumor necrosis factor-α
Because of its physical and chemical properties, resveratrol can either interact with receptors present on the cell surface or move passively through cell membranes. Therefore, at the cellular level, action may be initiated by either triggering signaling pathways when binding to the cell membrane receptors, or by facilitating intracellular mechanisms[38]. Accordingly, as discussed in previous reviews[39,40], resveratrol is capable of mediating a myriad of responses. For example, resveratrol is known to down-regulate pro-inflammatory factors [Figure 2], thereby exhibiting an anti-inflammatory response[41]. During the initial stage of an anti-inflammatory response, polymorphonuclear leukocytes play a key role in the process. Resveratrol abates the inflammatory responses initiated by calcium ionophore A23187, fMLP, or component fragment C5a[42]. Inducible nitric oxide synthase (iNOS) activates macrophages, and resveratrol has been shown to decrease the production of iNOS[43,44]. Resveratrol also impedes pro-inflammatory signaling which leads to inhibition of adenosine nucleotide secretion by activated platelets and reduces neutrophil functions through inhibition of P2 and PAP receptor signaling via mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK)[45]. Resveratrol induces extracellular signaling regulated kinases (ERK), p38 MAPK and JNK in the mouse epidermal cells resulting in the phosphorylation of serine 15 of p53, a tumor suppresser gene[46]. Further, resveratrol inhibits macrophage expression of EMMPRIN by inducing PPAR-γ[47]and inhibits NF-κB[48]. In addition, many other studies have confirmed that resveratrol inhibits activation by tumor necrosis factor-α (TNF-α)[49-51].
Janget al.[36]demonstrated an anti-inflammatory response in a rat model of inflammation by treatment with resveratrol, and further demonstrated reduction of prostaglandin synthesis by inhibition of cyclooxygenase-1 (COX-1). Later, it was found that resveratrol could selectively reduce COX activity by suppressing the COX-1 pathway; however, not through the COX-2 pathway. Szewczuket al.[52]verified this observation. However, in yet another study, it was found that resveratrol inhibits the synthesis of prostaglandin E2by suppression of COX-2, but not by altering COX-1[53]. One more study corroborates this result where resveratrol reduced colonic injury, neutrophil infiltration, and prostaglandin D2concentration by inhibiting COX-2 without affecting COX-1[54]. It was also shown that resveratrol attenuates COX-2 expression[53].
Chemopreventive mechanism of resveratrol
Many studies have been published describing the potential chemopreventive eあect of resveratrol. In brief, resveratrol induces apoptosis by interacting with the αVβ3 integrin receptor in the breast cancer cell line MCF-7[55]. The compound inhibits aryl hydrocarbon receptor activity thereby suppressing tumor growth and exhibiting anti-cancer properties[56]. Further, with MCF-7 cells, resveratrol inhibits NF-κB and BCL-2[57]. It antagonizes the aryl hydrocarbon receptor, which can relate to carcinogenic and immunosuppressive eあects in cells[58]. Some studies suggest that resveratrol shows antitumor eあects at the level of initiation, promotion, and progression with prostate cancer cells[59]. As noted above, resveratrol can inhibit COX-1 and COX-2, enzymes that are involved in tumor progression. With HL-60 cells, phenotypic markers indicative of reduced proliferation are induced. Also, in the initiation phase of carcinogenesis, resveratrol inhibits free radical generation[36]. In human lymphoblast cell lines, resveratrol induces apoptosis through p53 activation[60]. It also inhibits COX-2 activity[61]and ribonuclease reductase[62]. With osteosarcoma stem cells, resveratrol inhibits self-renewal, cell viability and tumorigenesis. Mechanically, resveratrol inhibited JAK2/STAT signaling and suppressed cytokine synthesis, which was consistent with the decline of CD133 cancer stem cell (CSC) markers[63]. Thus, when evaluated within vitromodels, resveratrol mediates a variety of eあects consistent with antitumor activity.
EPIGALLOCATECHIN-3-GALLATE
Chemical and pharmacological properties
Tea as a beverage is popularly consumed around the world, second only to water[64]. Consumption of green tea has been advocated for several health benefits, such as ameliorating cardiovascular risk and prevention of cancer. It has exhibited various biological properties proven favorable for hepatic function and improved metabolic profiles[65,66]. Pharmacologically, the polyphenols present in green tea demonstrate properties that can lead to anti-oxidative, anti-inflammatory, anti-atherosclerosis, anti-hypercholesterolemic and anticarcinogenic activities[67,68].
Active green tea polyphenols include catechins (members of the flavonoid family), like epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG). ECG and EGCG are found in the high concentrations (50 to 80% of the catechins)[64,69]. EGCG is the ester of epigallocatechin and gallic acid and known to be the most potent catechin in exhibiting anti-cancer and antioxidant eあects. The hydroxyl group on the B-ring plays an important role in antioxidant reactions and this is increased by the trihydroxyl group on the D-ring[70].
Inflammation is one dominant factor in the initiation of cancer. Increased oxidative stress promotes cell growth. Manyin vitrostudies and studies with animal models have conclusively demonstrated the potential of green tea to reduce tumorigenesis, although clinical trials are still not definitive. EGCG is hydrolyzed to EGC in the intestine by bacteria. Later, EGC and gallic acid undergo several conversions leading to metabolites such as 5-(3,5-dihydroxyphenyl)-4-hydroxyvaleric acid and 5-(3′,5′-dihydroxyphenyl)-γvalerolactone in glucuronide forms[71]. In a clinical study, the plasma levels were 0.17 μmol/L after having 2 cups of tea, while thein vitroconcentrations used in many studies have been in the range of 10-100 μmol/L[66]. Epidemiological studies have suggested green tea has a positive eあect on cancer prevention in certain types of cancer, namely breast, colon, and skin[72-74]. Although there is lesser or no positive clinical eあect seen with other types of gastrointestinal or oral cancers, there is bactericidal activity againstEscherichia coli,Streptococcus salivarius, andStreptococcus mutans[75-77].
Chemoprevention mechanism of EGCG
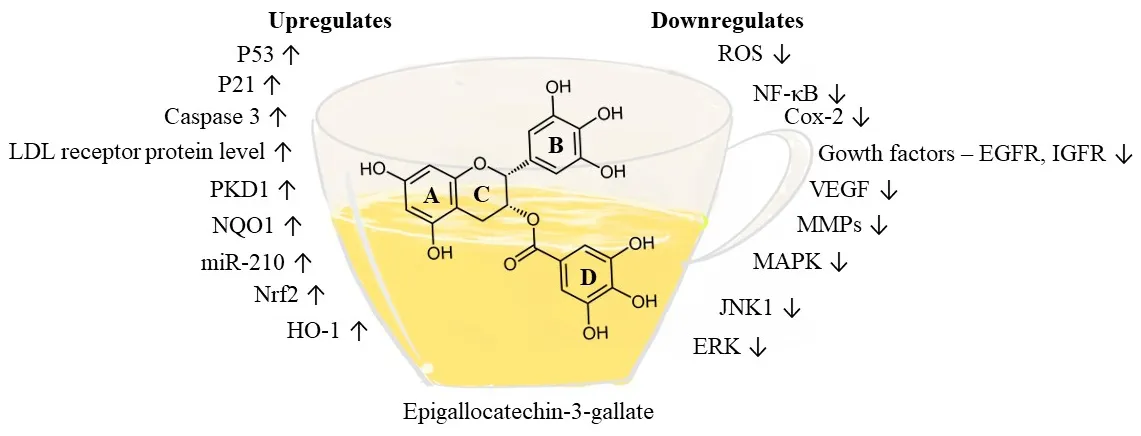
Figure 3. Pharmacological targets of epicatechin-3-gallate. EGCG affects various targets relevant to cancer prevention as shown in the figure. EGCG attenuates oxidative stress by down-regulating reactive oxygen species; inflammatory factors such as NF-κB and COX-2 are down-regulated. In addition, EGCG down-regulates several kinases, such as MAPK, JNK1, and ERK, and targets several growth factors, such as IGF1R, HGFR, and VEGF, that abate tumor growth and progression. Apoptosis factors such as p53, p21 and caspase 3 are up-regulated by EGCG, and the expression of NAD(P)H:quinone oxidoreductase 1 (NQO1), HO-1, and Nrf2 are increased to reduce oxidative stress. EGCG: epigallocatechin-3-gallate; NF-κB: nuclear factor-κB; COX: cyclooxygenase; MAPK: mitogen-activated protein kinase; ERK: extracellular signaling regulated kinases; VEGF: vascular endothelial growth factor
Since it has been established that cancer stem cells play a vital role in tumor progression, studies have been performed with lung CSCs such as A549 and H1299 illustrating the potential of EGCG to suppress tumor formation through the Wnt-β-catenin pathway[78]. Other studies performed with chemotherapeutic agent-resistant cancer stem cells such as A549/CDDP (cisplatin-resistant cells) and 5-flurouracil-resistant colorectal cancer cells have demonstrated the anti-cancer activity of EGCG[79,80]. Mechanistic evaluations with variousin vitromodels have led to the recognition of MAPKs as an important molecular target for EGCG [Figure 3]. These factors are associated with cell proliferation, diあerentiation, migration, senescence, and apoptosis[81]. ECGC inhibits signal regulated protein kinase and p38 phosphorylation. EGCG is also associated with inducing apoptosis, inhibition of transcription factors like NF-κB and activator protein (AP-1), and reduction of receptor tyrosine kinase activity[82].
ERK signaling
ERK is one of the major signaling cascades of the MAPK signaling pathway that is targeted by EGCG. EGCG inhibits ERK activation in a concentration-dependent manner, suggesting its eあectiveness as an anticancer agent with severalin vitromodels. With MCF10A and MDA-MB-231 breast cancer cells, 5 μmol/L EGCG inhibited hepatocyte growth factor-induced activation of ERK and AKT[83]. In cervical tumor cell lines, like HeLa, Caski and SiHa, EGCG inhibited phosphorylated ERK1/2 by 83% and Akt by 50% at a concentration of 50 μmol/L[84].
Activation of nuclear factor-κB and activator protein signaling pathways
Nuclear factor-κB (NF-κB) plays a vital role in the regulation of several genes central for cellular responses like inflammation, growth, and cell death. It is sequestered in cytoplasm in an inactive form and activated on phosphorylation. AP-1, a transcription factor, is known to be involved in tumor promotion and progression of cancer[85]. EGCG exerts inhibitory effects on the binding of NF-κB to DNA and thereby reduces inflammation and cell proliferation within vitromodels. However, concentrations required to mediate these effects were between 10-100 μmol/L[86,87]. EGCG evidently reduces binding of AP-1 along with NF-κB to DNA; a process which promotes MMP-9 for tumor progression. This has been demonstrated with severalin vitrostudies using human breast cancer cells[88], gastric AGS cells[89], and bladder cancer cells[90], in a dose-dependent manner, with concentrations ranging from 10 to 50 μmol/L.
c-Jun N-terminal kinase 1/2
JNK is involved in cancer cell apoptosis, although recent studies have indicated that Janus signaling promotes cancer cell survival by acting synergistically with NF-κB, JAK/STAT, and other signaling molecules. The situation is complicated because JNK1 and JNK2 have opposite eあects in relation to cancer cell survival. JNK1 promotes apoptosis while JNK 2 promotes cancer cell survival. The expression of p53 is negatively regulated by JNK1 and positively regulated by JNK2[91]. EGCG attenuated reduced expression of JNK1 and oxidative damage and, at the same time, inhibited JNK2, thereby augmenting apoptotic signaling in cancer cells[87,92-94].
p38/MAPK signaling pathway.
p38/MAPK is a third major signaling cascade in MAPK, playing a major role in controlling the process of apoptosis. Activation occurs by several environmental factors such as stress and inflammatory cytokines. p38 activates several downstream kinases that induce apoptosis[95]. EGCG increases p38 levels and thereby inhibits growth in leukemic cells, HCC cells, and U373MG cells[94,96,97].
SULFORAPHANE
Chemical and pharmacological properties
Sulforaphane (1-isothiocyanato-4-methylsulfinylbutane) is an aliphatic hydrocarbon that is the major byproduct obtained during the hydrolysis of glucoraphanin[98]. It was isolated in 1947 from radish. Later, glucoraphanin was found to be present in larger quantities in cruciferous vegetables such as cauliflower, broccoli, Brussel sprouts, and cabbage[99]. It is generally found in broccoli and broccoli sprouts, yielding the highest concentrations found in any plant source[100]. Broccoli and other cruciferous vegetables are widely consumed throughout the world for various health benefits.
Hydrolysis of glucoraphanin occurs due to disruption of the plant cell and the subsequent activity of the intrinsic enzyme myrosinase. Sulforaphane is not heat stable, but greater stability is retained when exposed to light and acidic pH levels, rendering the compound useful under gastric pH conditions[99-101]. The presence of epithiospecifier protein (ESP) disrupts the process of glucoraphanin hydrolysis, reducing the bioavailability of sulforaphane and sulforaphane nitrile, with the nitrile form being less active in its binding to pharmacological targets[102]. Since ESP is temperature insensitive, heating the broccoli at 60 °C decreases the formation of sulforaphane nitrile[103]. Once absorbed, sulforaphane is conjugated with glutathione and metabolized by the meracaptopurine pathway; it is then excreted asN-acetylcysteine conjugates[104]. Pharmacokinetic studies have demonstrated that the peak plasma levels of sulforaphane are relatively high after oral administration of broccoli, for 1.6 to 6 h. with 95% elimination after 12 h[105]. In clinical studies, the plasma concentrations of sulforaphane following oral consumption of broccoli are in the range of 0.02-0.2 μmol/L[106]. With animal models, the consumption of broccoli has been found to exhibit protection against cancer[107].
Sulforaphane has also exhibited protective properties in the central nervous system by activating nuclear factor (erythroid derivative)-like 2 (Nrf2) and reducing oxidative stress and inflammation in nerve cells[108]. Further, the compound shows insulin-sensitizing and hepatoprotective eあects in rats fed a high fructose diet[109]. Patients with type 2 diabetes treated with broccoli sprout powder (5 to 10 g for 4 weeks) showed reduced serum glucose levels and improved insulin levels[110]. Patients with deregulated type 2 diabetes treated with 5 g of broccoli extract along with metformin (500 mg to 3000 mg) showed reduced HbA1c levels and there was a reduction in glucose production[111]. In healthy male individuals, cholesterol and LDL cholesterol levels were reduced; in women, HDL cholesterol levels increased significantly after consumption of 100 g of fresh broccoli sprouts for 1 week[112].
Sulforaphane reduced levels of iNOS with lipopolysaccharide (LPS) activated macrophages in a mouse model[113]. Anti-inflammatory properties were further demonstrated in another study conducted with mice treated with sulforaphane; cytokine production was reduced in a concentration-dependent manner by activating the Nrf2 pathway. T-cell proliferation was also significantly inhibited[114]. Sulforaphane demonstrates inhibition of phase I and phase II enzymes, induces cell-cycle arrest and inhibits angiogenesis. At 15 μmol/L, sulforaphane promotes apoptosis and cell-cycle arrest in prostate cancer cells (LNCaP and PC3) by decreasing histone deacetylase (HDAC) enzymes[115].
Carcinogen-treated Wistar rats at 10 weeks of age were treated with 150 μmol of sulforaphane by oral gavage, mammary glands were extracted, and sulforaphane concentration after 12 h was 22 μmol/L. In addition, there was an increase of NQO1 and HO-1 levels observed in rat mammary gland. Subsequently, healthy women were placed on a cruciferous-free diet and administered 200 μmol of sulforaphane. The amount of sulforaphane distributed in breast tissue was found to be 0.92 ± 0.72 μmol/L[116].
It is documented in various epidemiological studies that the consumption of isothiocyanates from cruciferous vegetables is inversely proportional to the incidence of lung cancer cases[117]. This inverse correlation was even stronger in a study conducted in female patients who do not smoke cigarettes[118]. There is also substantial evidence based on studies conducted with cisplatin-resistant cancer stem cells [such as human non-small cell lung cancer (NSCLC)] that up-regulation of miR-214 induced by sulforaphane may lead to anti-cancer activity[119].
Chemopreventive mechanisms of sulforaphane
KEAP-nrf2 signaling
Genetic deletion of nrf2 can lead to detrimental eあects on the survival of mice; they are more prone to brain injury and lung injury and other pathological conditions involving inflammation. To the contrary, the activation of KEAP1-nrf2leads to protective eあects in various animal models[120,121]. Nrf2 is important for the regulation of antioxidant genes such as enzymes that produce glutathione (GSH) and NADPH[122]. Sulforaphane, on entering cells, reacts with Kelch-like ECH associated protein, which functions as a sensor protein complex. Under basal conditions, KEAP1 binding to nrf1 leads to ubiquitination and proteasomal degradation. Sulforaphane protects nrf2 from degradation [Figure 4], allowing escape and the regulation of downstream target genes capable of mediating anti-inflammatory and antioxidant activities[123]. NQO1 and GST levels are significantly elevated in sulforaphane-treated wild-type mice (nrf2+/+) whereas nrf2-/-deficient mice exhibited no changes in NQO1[124].
Activity of cyclin dependent kinase and reduced cyclin D1
Cyclin D1 is a cell-cycle regulator and a transcriptional modulator for histone deacetylase 3. Overexpression of these factors has been linked to cancer progression. Therefore, reduction of cyclin D is considered as a potential strategy for chemoprevention[125]. In this context, sulforaphane-treated A549 cells showed concentration-dependent reduction of cyclin D1 as well as increased expression of thep21[126]. In DU-145 prostate cancer cells, sulforaphane reduced CDK4 activity and cyclin D1 levels when treated with 9 and 50 μmol/L, respectively. CDK4 activity was also affected by a concentration of less than 1 μmol/L, but not significantly[127]. In anin vivostudy, sulforaphane reduced tumor promotion and polyp formation in an ApcMin/+ mouse small intestine cancer model in a dose-dependent manner. However, biomarkers including cyclin D1 remained unaあected[128].
Inhibition of HDAC activity in human prostate and colorectal cancer cells
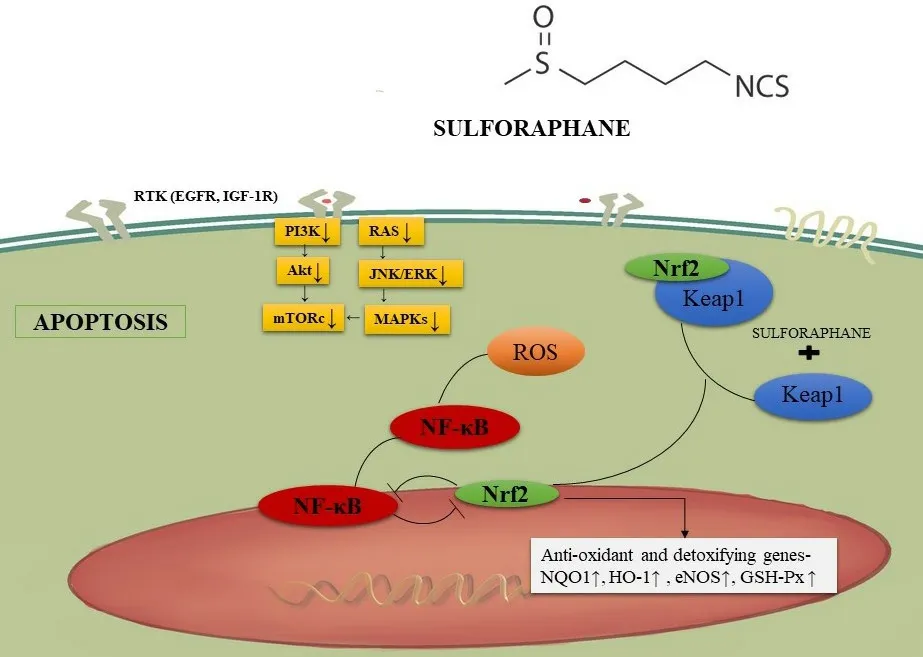
Figure 4. Sulforaphane and KEAP-nrf2 signaling. Sulforaphane enters cells and interacts with Kelch-like ECH associated protein. This prevents nrf2 degradation and leads to downstream anti-inflammatory and antioxidant effects. NF-κB: nuclear factor-κB
Increased HDAC expression has been associated with the deregulation of cell-cycle and apoptotic processes. HDAC inhibitors have shown potential in clinical studies for chemoprevention. Sulforaphane has demonstrated potential to reduce HDAC activity in prostate and colorectal cancer cells[129]. Sulforaphane (15 μmol/L) reduced HDAC activity by 30%, 40%, and 40% in LNCaP, BPH-1, and PC-3 cell lines, respectively. Expression ofp21associated with histone H4 was increased in all three cells lines leading to apoptosis[130]. In 4-6 week-old NOD/SCID mice inoculated with A549 lung cancer cells, administration of 9 μmol sulforaphane by oral gavage for 4 weeks attenuated the increase of tumor volume, significantly reduced HDAC activity, and increased acetylated histones 3 and 4[131]. HDAC inhibitory effects of sulforaphane were also demonstrated with a mouse model bearing a colon cancer xenograft[132]. The United States Food and Drug Administration approved the use of HDAC inhibitors for the treatment of cancer, so investigation of phytochemicals such as sulforaphane is reasonable[133]. In a phase II clinical trial involving patients with recurrent prostate cancer, administration of 200 μmol/day of sulforaphane rich extracts for 20 weeks did not produce a reduction in prostate specific antigen (PSA) levels by at least 50%. However, PSA doubling time was increased[134]. In a double-blinded study, there was a significant reduction in PSA levels in prostate cancer patients post-prostatectomy when given 60 mg sulforaphane orally with cancer therapy, followed by 2 months of sulforaphane with no other treatment[135]. The promising results observed with sulforaphane in these clinical trials suggest the use of an HDAC inhibitor in combination with a chemopreventive agent for the treatment of prostate cancer.
ANTHOCYANINS
Chemical properties of anthocyanins
Anthocyanins are water-soluble secondary polyphenolic metabolites produced by plants[136]. The substances are classified under the flavonoid group and provide blue, red, and purple pigmentation for plants. The basic chemical structure of an anthocyanin contains anthocyanidin without a sugar moiety. The anthocyanidins are comprised of an aromatic ring (A) which is bonded to a heterocyclic ring (C) that includes oxygen, and this is similarly attached to carbon-carbon bonds linking to a third aromatic ring (B)[137]. There is a vast number of anthocyanins present in nature, the most common being petunidin, pelargonidin, delphinidin, peonidin, malvidin, and cyanidin. The only difference between these compounds is the nature and the number of sugars attached to their structure, the number of hydroxylated groups in the molecule, the aromatic or aliphatic carboxylates attached to the sugar, and the position of these bonds[138]. Studies have indicated theortho-dihydroxyphenyl structure is the active moiety on the B-ring that suppresses tumor growth and metastasis[139,140].
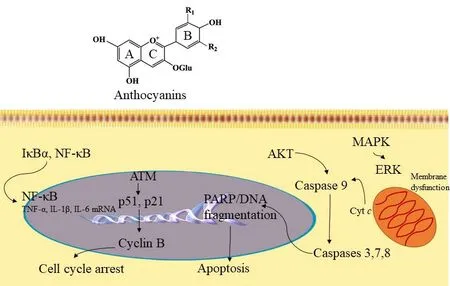
Figure 5. Anthocyanin mechanism of action. Based on structural-activity relationships (SAR), the ortho-dihydroxyphenyl functional group on the B-ring of anthocyanidins plays a key role in inhibiting the action of AP-1 and cell transformation. The compound inhibits MAPK/ERK and mitochondrial associated pathway caspases 3, 7, 8 to induce apoptosis. Further, anthocyanins have exhibited inhibition of cyclin-B by acting on p51 and p21 signaling pathways to induce cell cycle arrest. In addition, anthocyanins can act through the NFκB signaling pathway to mediate anti-inflammatory functions. NF-κB: nuclear factor-κB; MAPK: mitogen-activated protein kinase; ERK: extracellular signaling regulated kinases
Chemopreventive mechanism of anthocyanins
MAPK pathway and AP-1
With mouse epidermal cells (JB6+), tumor promoters such as epidermal growth factor (EGF), 12-O-tetradecanoylphorbol-13-acetate (TPA), and TNF-α can stimulate AP-1 activity and neoplastic transformation by inducing MAPK comprising ERK, p38 kinase, or JNK[141,142]. Cyanidin, petunidin, and delphinidin suppress TPA-stimulated AP-1 transcriptional activity and cell transformation with JB6+cells[139].
Evaluation of structure-activity relationships have shown that theortho-dihydroxyphenyl functional group on the B-ring of anthocyanidins plays a key role in inhibitory action since malvidin, pelargonidin, and peonidin, devoid of theortho-dihydroxyphenyl structure, fail to block AP-1 activity or cell transformation. Signal transduction analysis showed that delphinidin blocked ERK phosphorylation at early times and JNK phosphorylation at later times, but p38 was not blocked [Figure 5][139]. Furthermore, delphinidin inhibits the phosphorylation of c-Jun (a phosphorylation target of JNK and ERK), SAPK/ERK kinase (SEK, a JNK kinase), and MAPK/ERK kinase (MEK, an ERK kinase). Suppression of TPA-induced AP-1 activity and cell transformation by delphinidin involves inhibition of JNK and ERK signaling cascades. This eあect is increased in combination with superoxide dismutase (SOD) with any anthocyanidins bearing theorthodihydroxyphenyl arrangement on the B-ring. Multiplicative analysis demonstrated that inhibition between delphinidin and SOD is synergistic in nature[139]. Therefore, even though the signaling pathways aあected by SOD or delphinidin are diあerent, both are believed to play a crucial role in the cancer preventive activity of anthocyanidins.
Suppression of inflammation through NF-κB and COX-2
There are some antioxidants that block the expression of COX-2 by inhibiting the signaling mechanism that controls theCOX-2gene[143]. In one study, the molecular mechanism of anthocyanins was evaluated using the mouse macrophage cell line RAW264. Bilberry and purified delphinidin derived anthocyanin extracts suppress LPS-induced COX-2 expression at the transcription and protein levels. Signal pathway analysis showed that delphinidin blocks LPS-induced IκB degradation and inhibits NF-κB stimulation andCOX-2gene expression. Thus, it appears anthocyanins act through the NF-κB signaling pathway which is involved in the suppression ofCOX-2gene expression[144].
Apoptotic induction of cancer cells (by targeting ROS and JNK mediated caspase activation)
Petunidin, delphinidin, and cyanidin induced apoptosis with HL-60 cells, as demonstrated by DNA fragmentation and morphological changes, although peonidin, malvidin, and pelargonidin did not induce this response[145]. The number of hydroxyl groups at the B-ring is proportional to the potency of apoptosis induced by anthocyanidins; theortho-dihydroxyphenyl structure at the B-ring is vital for apoptosis induction[145].
Mechanistic analysis indicated the induction of apoptosis by delphinidin may involve an oxidation/JNK-mediated caspase pathway. Delphinidin increases intracellular ROS which may trigger JNK. With delphinidin treated cells, JNK phosphorylation, caspase-3 activation, and c-jun gene expression were observed[145]. Delphinidin-induced JNK phosphorylation, DNA fragmentation, and caspase-3 activation were eあectively blocked by antioxidants such asN-acetyl-L-cysteine[145]. Thus, delphinidin can potentiate the apoptotic death program in HL-60 cells through JNK signaling mediated through oxidative stress.
Chemopreventive mechanisms of anthocyanins
Anti-carcinogenic activities at the initial stage of tumorigenesis
Anthocyanins can prevent the occurrence of tumors by acting on antioxidant systems[146]. These compounds scavenge free radicals reducing damage to the genome of the normal cells by oxidative stress and later malignant transformation fostered by gene mutations[147,148]. Anthocyanins can induce the antioxidant effect by acting on the antioxidant response element through KEAP1-Nrf2. Furthermore, by regulating the expression of phase II antioxidases (quinone oxidoreductase, glutathione transferase, glutathione peroxidase, and glutathione reductase), anthocyanins can suppress the activity of caspase-3. The abnormal secretion and overexpression of inflammatory elements are important for tumorigenesis. It has been reported that anthocyanins can mediate anti-inflammatory functions by regulating the secretion and expression of inflammatory agents. This eあect occurs due to the suppression of transcription factors NF-κB through multiple pathways[149,150]. In anin vitrostudy, anthocyanin containing purple-fleshed potato extracts elevated apoptosis and suppressed proliferation in a p53-independent manner in colon CSCs[151].In vivo, purple-fleshed potato decreased the number of crypts containing cellswith nuclear β-catenin (which is an indicator of colon CSCs) by induction of apoptosis[151]. These results provide evidence that anthocyanins may reduce the initial stage of tumorigenesis.
Anti-carcinogenic activities in the cancer formation stage
Anthocyanins can block tumorigenesis and induce terminal diあerentiation of tumor cells. It was found that diあerentiation of the acute promyelocytic leukemia cell line HL-60 could be induced by cyanidin-3-O-βglucopyranoside (Cy-g) in a dose-dependent manner by the activation of PKC and PI3K. Upon treating HL-60 cells with Cy-g (200 μg/mL), diあerentiation characteristics were observed such as enhanced activity of esterase, increased adhesion, and reduced expression of the oncogene c-Myc. When cells were treated with PKC or PI3K inhibitors, the eあect of diあerentiation induced by Cy-g was substantially decreased[152]. Since the degree of differentiation correlates with the degree of tumor malignancy, it may be suggested anthocyanins can act at the cancer formation stage by stimulating diあerentiation.
Anti-carcinogenic activities in the cancer development stage
Anthocyanins can induce apoptosis of cancer cells through the external death receptor pathway and the internal mitochondrial pathway. It was determined that delphinidin could activate p38-FasL and the Bid pathway, which is a pro-apoptosis protein that induces apoptosis with HL-60 cells, in a dose- and timedependent manner[153]. In vascular smooth muscle cells, delphinidin and cyanidin strongly inhibited the expression of vascular endothelial growth factor (VEGF) (stimulated by platelet derived growth factor) by repressing the JNK and p38-MAPK pathways[154].
Pharmacological properties
Hydroxylation of nonreactive carbons is a major function of phase I cytochrome P450s and the monooxygenase system[155]. Phase I hydroxylation of anthocyanins is noteworthy since hydroxyl groups structurally distinguish this group of compounds. Even though flavonoids are reported to have low bioavailability due to extensive metabolism, their metabolites may be present for a longer duration and result in significant bioactivity. This can be the case with anthocyanins, since metabolites have been found to retain basic structural characteristics, thereby preserving bioactivity[156,157]. The majority of the flavonoids present in the urine and circulation as glycated, glucuronidated, sulfated, and methylated conjugates[158,159]. Glucuronide conjugation is regarded as an important conjugation reaction in the metabolism of flavonoid[158-162]. UDP-glucuronosyltransferases, which catalyzes the glucuronidation reaction, are observed in high concentrations in the intestine, kidney, and liver[163,164]. However, in humans, following dietary consumption, evidence suggests that the initial site for flavonoid glucuronidation is the intestine[159,165,166]. Methylation is observed as the second major conjugation reaction of flavonoids[162].O-Methylation is the most common such reaction. Catechol-O-methyltransferase catalyzesO-methylation utilizing the cofactorS-adenosyl methionine. The liver is a major organ responsible for methylation with the highest catechol-Omethyltransferase activity[167]. The primary site of methylation is determined by the pattern of a flavonoid ring structure. Some studies have shown low oral doses of quercetin in animals and humans undergo extensive methylation[168]. Glycation or sulfation are common conjugation reactions that predominate when low dose phenolic drugs are administered. Sulfotransferases are a small group of cytosolic enzymes that are widely distributed in the body. They use phosphoadenosine-5’-phosphosulfate as a cofactor and their recognized substrates include polyphenols (i.e., flavonoids), hydroxylamines, 4-nitrophenol, iodothyronines, and phenols[168].
Studies suggest that anthocyanins have very low bioavailability (< 1% in plasma), although some amount has been found in the colonic tissues of patients, indicating the possibility of a local site of action[169,170]. It is still unclear whether anthocyanins are eあective against cancer in human beings and whether they can function as metabolites or as parent molecules.
CURCUMIN
Chemical and pharmacological properties
Curcumin [(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a yellow-colored phytochemical obtained fromCurcuma longa(turmeric), a member of the ginger family (Zingiberaceae). It is commonly cultivated in the southwest Asia region and is popularly used as a spice for various cuisines[171,172]. Curcumin is comprised of twoo-methoxy phenolic rings which are connected by seven carbon chains consisting of an α, β-unsaturated β-diketone component, and generally accounts for 60%-70% of curcuminoid extracts[173,174]. The o-methoxyphenol rings are the primary domains for the antioxidant activity of curcumin and the β-diketone component forms chelation complexes with metals rendering them useful for the treatment of heavy metal poisoning[175].
In a pre-clinical study, 500 mg/kg curcumin administered orally to rats gave a maximum plasma concentration of approximately 0.06 μg/mL, reaching the peak by approximately 41 min; the half-life was 28 min, giving an oral bioavailability of about 1%[176]. As suggested by this poor bioavailability, the tolerance for curcumin is very high for humans. In a dose escalation study, minimal adverse events were observed which were unrelated to the dose after oral administration of 10,000 or 12,000 mg in healthy subjects. Still, only traces were detected in serum[177]. At 10-12 g, the highest dose given to human subjects, the plasma concentration of curcumin and its metabolites were in the range of 0.075-10 μg/mL[178]. Curcumin is metabolized by the process of glucuronidation in the intestine and by the liver yielding metabolites such as tetra-hydrocurcumin, and hexahydrocurcumin[179].
Nevertheless, there are a vast number of studies conducted with curcumin to investigate anti-inflammatory, antioxidant, anti-carcinogenic, antiviral, and anti-infection activities. Owing to its antibacterial, antioxidant, anti-inflammatory, and antiseptic properties, curcumin has been proven useful in treating several skin diseases, such as psoriasis, infection, acne, skin inflammation, and skin cancer[180]. Curcumin controlled the in vitro growth of Staphylococcus aureus and Pseudomonas aeruginosa in an in vivo murine wound model, and topical administration accelerated healing[181]. In a psoriasis keratin (K) 14-VEGF transgenic mouse model, after oral curcumin administration, symptoms like ear redness, weight, thickness, and lymph node weight were reduced significantly[182].
There have been several conclusive in vitro, in vivo, and clinical studies conducted on the use and benefits of curcumin in treating inflammatory diseases. Curcumin exhibited anti-inflammatory eあects in adipose tissue of C57BL/6 mice fed a high fat diet by down-regulating inflammatory transcription factors like NF-κB and toll-like receptor-4[183]. The clinical effectiveness of curcumin in treating inflammation was studied in patients diagnosed with osteoarthritis, and significant reductions in myeloperoxidase, collagen degradation activity, and C-reactive protein levels were observed; also, additional symptoms like pain and effusion were reduced[184]. In 45 patients with active rheumatoid arthritis, curcumin (500 mg) was used in combination with diclofenac sodium (50 mg) and there was a significant reduction in their disease activity score compared with the group provided only with diclofenac sodium[185]. In yet another clinical study, patients with acute rhinitis were administered 500 mg of curcumin orally. Symptoms like sneezing, itching, and obstruction rhinorrhea were reduced significantly in patients treated with curcumin compared to the placebo treatment, and inflammatory markers such as IL-4, TNF-α, IL-8, PEG2were significantly decreased[186].
Curcumin as a chemopreventive and anticarcinogenic agent
Curcumin has been broadly studied for anti-cancer characteristics [Figure 6]. A pilot study conducted with patients diagnosed with benign prostatic hyperplasia (BPH) and administered 500 mg of curcumin twice a day along with the standard therapeutic regimen showed improved symptoms and improved quality of life relative to the group administered the standard treatment only[187]. In another study, 85 patients with prostate cancer were administered curcumin and soy isoflavones along with the standard treatment, and elevated PSA levels were significantly decreased in patients treated with curcumin and soy isoflavones compared with the group given the standard treatment[188].
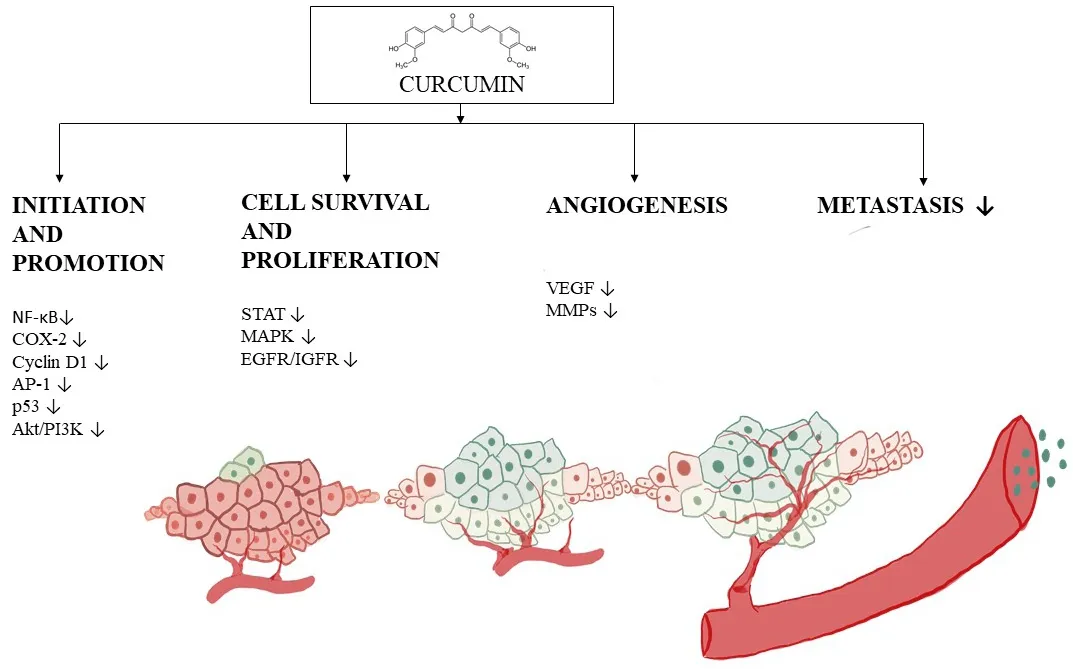
Figure 6. Progression of angiogenesis and the inhibitory mechanism of curcumin. Curcumin is capable of functioning through a myriad of mechanisms, but this figure illustrates down-regulation of factors involved in tumor initiation such as NF-κB, COX-2, cyclin D1, p53 and Akt/PI3k, and proliferation factors such as STAT, MAPK EGFR. The progression phase of tumorigenesis is also inhibited, for example, by alteration of VEGF expression, leading to reduced angiogenesis and metastasis. NF-κB: nuclear factor-κB; COX: cyclooxygenase; PI3K: phosphatidylinositol-3-kinase; MAPK: mitogen-activated protein kinase
In other forms of cancer, like breast and cervical, curcumin has proved eあective. In patients with advanced metastatic breast cancer, curcumin reduced biomarkers like CEA with a maximum tolerated dose of 8000 mg along with chemotherapy[189]. In addition, topical application of curcumin along with other herbal preparations for 30 consecutive days resulted in significant clearance of HPV. However, vaginal curcumin capsules did not show any statistical difference[190]. Oral administration of curcumin (8000 mg) is well tolerated in patients diagnosed with advanced pancreatic cancer[191]. And, in a Phase II clinical trial, despite its limited absorption, curcumin exerted biological activity in a few patients, such as down-regulation of transcription factor NF-κB and reduced COX-2 (8,000 mg/day dosage)[192].
Mechanism of anti-carcinogenesis
VEGF
Since VEGF is a crucial regulator for angiogenesis, the use of potentially active phytochemicals including curcumin as an anti-angiogenic compound has been explored[193]. With the H22 HCC cell line, curcumin inhibited the proliferation of cells in vitro and reduced VEGF expression and PI3K/AKT signaling in vivo[194]. Curcumin reduced high mobility group box 1 and VEGF-D expression in gastric cancer AGC and SGC-7901 cell lines, and induced cell apoptosis through caspase-3 activation, in a dose-dependent manner[195].
Role of curcumin in cell-cycle arrest
Curcumin inhibits the expression of cyclin E and cyclin D1 that enable cell-cycle arrest at the G1/S phase in LNCaP and PC-3 cell lines[196]. In another in vitro study, with human osteosarcoma cells, cell-cycle arrest by curcumin at the G1/S phase was related to a reduction of cyclin D1[197]. Human mantle cell lymphoma is characterized by overexpression of cyclin D1, which up-regulates NF-κB and several genes such as, bcl-2, COX-2 and IL-6. However, on treatment with curcumin, cyclin D1 expression was down-regulated, along with AKT activation[198]. Curcumin also improves the efficacy of chemotherapeutic agents such as cisplatin by targeting cancer stem cells [such as A549 and H2170 (NSCLC cell lines)], down-regulating cyclin D1 and increasing the expression of p21[199]. There is also evidence of involvement of curcumin in suppressing glioma growth and inducing apoptosis by AKT and signal regulated kinase pathway in U87-MG cells[200]. In T-cell acute leukemia malignant cells, curcumin causes de-phosphorylation of active AKT, FOXO, and releases cytochrome c, which activates caspase-3. This leads to inhibition of cell proliferation[201].
SILIBININ
Chemical properties of silibinin
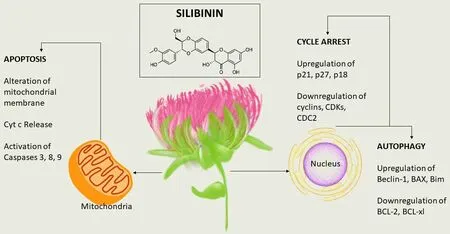
Silibinin is a key constituent of silymarin, which is a polyphenolic flavonoid obtained from milk thistle seeds[202]. It is constituted as two isomers, silybin A and silybin B, in the ratio of 1:1, functions as a weak acid in aqueous form, and is especially stable in the presence of acids and unstable in a basic environment[203]. Silibinin is a major component of the silymarin complex, constituting 50%-60%, depending on the formulation[204]. Many studies have demonstrated that silibinin has strong antioxidant properties, scavenging both reactive oxygen species and free radicals, which can lead to the enhancement of cellular antioxidant defense mechanisms[205-209]. Notably, silibinin can affect cancer development by various modes of action, including modulation of oxidative stress, proliferation, inflammation, metastasis, and angiogenesis[210-212]. Some studies have indicated a beneficial eあect on toxicity due to short- and longterm exposure to radiation treatment and chemotherapy[213].
Pharmacokinetics of silibinin
Silymarin is primarily conjugated and excreted into bile and urine and seems to undergo insignificant phase I metabolism; inadequate data exist concerning the role for phase II metabolism and transporters[214,215]. Silymarin (where silibinin is an active constitute) pharmacokinetic analysis was done with healthy volunteers. There was rapid metabolism, forming conjugates such as glucuronides, that were detected in plasma[214]. Also, it was found that conjugated silibinin metabolites are eliminated slowly as compared to free silibinin[214]. Factors such as inefficient intestinal absorption, low water solubility, elevated metabolism, and rapid excretion, significantly decrease the serum concentration of silibinin, thus reducing its ability to reach target organs and consequently therapeutic efficiency is reduced[216-218]. There have been many eあorts to produce formulations to increase the bioavailability of silibinin[219,220]. For example, silibinin that is complexed with phosphatidylcholine is known as “silipide”. In pharmacokinetic studies conducted with healthy subjects, it was shown that silibinin derived from silipide has greater absorption in plasma and liver as compared with conventional silibinin[221,222]. Silipide, when tested in cancer patients, demonstrated high plasma bioavailability[223-225]. A comparative study revealed high bioavailability of silibinin in colon tissue but relatively poor levels in prostate tissue[223-225]. This suggests organ specificity may be anticipated as a result of bioavailability following oral administration.
Chemoprevention mechanism of silibinin
Several studies suggest that silibinin may be eあective against lung cancer. Silibinin reduced the production of matrix metalloproteinase-2 and urokinase-plasminogen activator when metastatic A549 lung cancer cells were treated with different concentrations, up to 100 μmol/L[226]. Silibinin has been shown to reduce the development of human NSCLC, such as large cell carcinoma cells (H1299 and H460) and bronchioalveolar carcinoma cells (H322)[227]. Silibinin treatment of cultured cells (10-75 μmol/L) has been shown to target cell-cycle progression leading to a G1 arrest and altered protein levels of cyclins (D1, D3, and E), CDKIs (p18/INK4C, p21/Cip1, and p27/Kip1) and cyclin-dependent kinases (CDKs)[227]. Inhibitory eあects of silibinin (75 μmol/L) were shown in both a human large-cell lung cancer cell line and human lung adenocarcinoma A549 cells[228]. A combination of silibinin and indole-3-carbinol showed greater antiproliferative eあects than the single compounds alone. A549 cells, when treated with 100 μmol/L of indole 3-carbinol and 75 μmol/L of silibinin, or 200 μmol/L of indole-3-carbinol plus 75 μmol/L of silibinin, for 24 h, showed a reduction in proliferation by 40% and 62%, respectively. With H460 cells, the responses were 31% and 69%, respectively. At the molecular level, the combination of indole-3-carbinol and silibinin inhibited Akt and ERK activation and induced apoptosis[228]. Silibinin has also shown eあects against cancer stem cells (CSC) in lung cancer[229]. A model of acquired erlotinib resistance was established by growing NSCLC cells with a TKI-sensitizing EGFR exon 19 deletion in the constant presence of high doses of Erlotinib (Erlotinib-refractory PC-9/Erl-R cells). Treatment with silibinin (50-100 μg/mL) decreased the amount of lung cancer spheres in this model. These results suggest the possibility of using silibinin in combination with EGFR tyrosine kinase inhibitor Erlotinib to target CSC in EGFR-mutant NSCLC patients[229].
The anti-prostate cancer properties of silibinin have been investigated in various studies. The substance has shown inhibition of androgen- and serum-stimulated PSA protein levels in LNCaP cells, and this is associated with cell growth inhibition through G1 arrest in cell cycle progression[230]. Treatment with 25 and 75 μg/mL of silibinin for 24 h showed 45% and 59% decreases in PSA secretion in the medium, respectively. Silibinin has further shown up-regulation of insulin-like growth factor binding protein 3 expression and suppression of androgen-independent prostate cancer (PC-3) cell proliferation[231]. With PC-3 cells in a medium supplemented with 10% FBS, treatment with silibinin (2 and 20 μmol/L) reduced cell growth by 17.3% and 54%, respectively[231].
Silibinin has been shown to suppress the adhesion and migration of human prostate adenocarcinoma (PC-3) cells[232], and the motility, migration, and invasion of ARCaPM prostate cancer cells[233]. However, the response was weak with ARCaPM cells. When applied at a higher concentration (200 μmol/L), growth inhibition was only 18.5% in ARCaPM cells, but in the case of LNCaP, PC-3 and DU145 cells, the growth inhibition was found to be 48.7%, 60.0%, and 73.8%, respectively, after 48 hours of treatment. Moreover, silibinin induced morphological reversal to the epithelial phenotype from epithelial-mesenchymal transition (EMT), down-regulated MMP-2 and vimentin, and up-regulated cytokeratin-18. Also, silibinin inhibited NF-κB p50 translocation by up-regulating I kappa B alpha (IκBα) protein, and down-regulating the expression of two key EMT regulators, SLUG, and ZEB1 transcription factors[234].
Several studies have demonstrated the activity of silibinin against colon cancer. Silibinin mediated apoptosis in cultured human colorectal carcinoma LoVo cells, which was associated with high levels of cleaved PARP and cleaved caspases (3 and 9). When LoVo cells were treated with silibinin (50-200 μmol/L) for 24 hours, growth was reduced by 30%-49%. The substance also induced strong cell cycle arrest at the G1 phase, and a minor although significant G2/M phase arrest, at the highest concentration tested. Moreover, silibinin reduced the levels of cyclins (A, B1, D1 and D3), CDK1, CDK2, CDK4, and CDK6, and elevated the level of CDKIs (p21 and p27) and phosphorylation of Rb protein[235]. Anti-angiogenic eあects, such as a decrease in inducible COX-1 and COX-2, NOS and NOS3, and VEGF and HIF-1α, were exhibited by silibinin[236]. When administered along with TNF-related apoptosis-inducing agent (TRAIL), silibinin caused cell death in primary tumor cells (SW480), as well as TRAIL-resistant metastatic cells (SW620). Finally, silibinin induced up-regulation of death receptor 4 (DR4) and DR5, down-regulated the anti-apoptotic proteins XIAP and Mcl-1, and synergistically activated the mitochondrial apoptotic pathway[237][Figure 7].
LYCOPENE
Chemical properties lycopene
Lycopene is a lipophilic hydrocarbon carotenoid with red color due to a chromatophore with 11 conjugated double bonds[238]. The antioxidant properties of lycopene derive from this conjugated polyene structure[239]. Unlike α- and β-carotenes, lycopene is not provitamin A due to the absence of a β-ionone ring and its acyclic structure[239]. Having been studied for many decades, lycopene is found in tomatoes, watermelon, pink grapefruit, papaya, and other fruits[240]. Unlike other widely distributed carotenoids, however, lycopene is mainly found in tomatoes and tomato products[241]. Unique structural and chemical features may contribute to distinct biological properties[239].
Pharmacological properties of lycopene
Lycopene has been shown to function through a variety of mechanisms [Figure 8], several of which are described below.
Antioxidant activity
Oxidative stress caused by the ROS is linked with aging, carcinogenesis, and cardiovascular diseases. Lycopene can function as an antioxidant by various mechanisms, with the best documented mechanism being through quenching singlet oxygen (1O2) due to the extended system of conjugated double bonds[242].
In cell culture, V79 Chinese hamster lung fibroblasts were treated with peroxynitrite to cause DNA strand breakage and nitration of proteins. This was shown to be inhibited by lycopene in the concentration range of 0.31 to 10 μmol/L[243]. In another preclinical study, lycopene at concentrations of 0.25 to 10 μmol/L, reduced the oxidative DNA damage caused by the redox-cycling of catechol estrogens in Chinese hamster lung fibroblasts and plasmid DNA[244]. It was determined that 81% of the subcellular localization of lycopene in prostate cancer cells treated with lycopene was at the nucleus[245]. Consistent with this, it is observed that the DNA protective effects exhibited by lycopene correlate with the localization of lycopene in the nucleus[246].
Apoptosis
In one study, lycopene reduced mitochondrial transmembrane potential, decreased mitochondrial function, increased annexin V binding, and released mitochondrial cytochrome c[247]. In another study, apoptosis was induced with LNCaP and PC3 cells with lycopene concentrations as low as 10 nmol/L[248].
With human colon carcinoma (HuCC) cells, lycopene treatment at 2.0 or 4.0 μmol/L induced apoptosis. However, the compound was not eあective when incubated with a physiologically relevant concentration 1.0 μmol/L. The same group showed apoptosis in Raji cells, a prototype Burkitt lymphoma cell line, when exposed to 2.0 μmol/L lycopene. There were no anti-apoptotic eあects observed in human erythroleukemia (K562) or B chronic lymphocytic leukemia (EHEB) cells treated with lycopene at a concentration of 4 μmol/L[249]. These data indicate that lycopene shows greatest efficacy for inducing apoptosis in human prostate cancer cells.
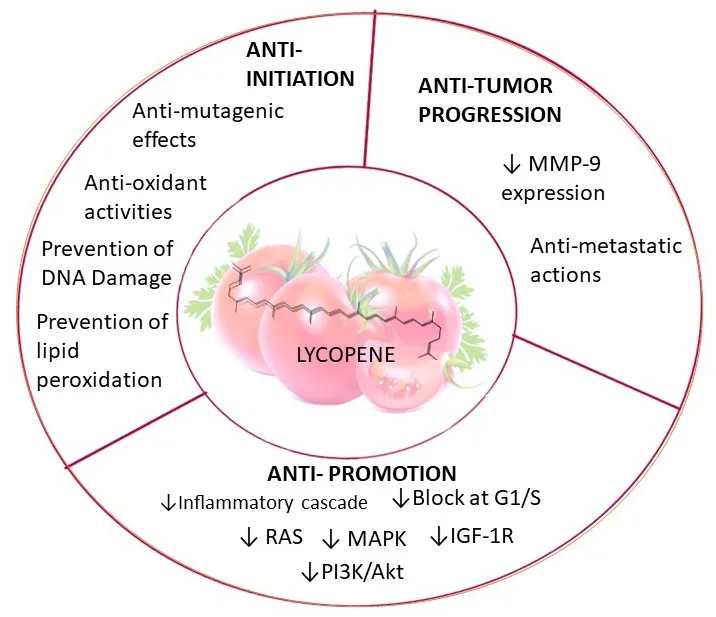
Figure 8. Pharmacological functions of lycoepene. Lycopene induces factors leading to anti-initiation and anti-progression by downregulating MMP-9 expression. The compound mediates anti-promotion effects by suppressing RAS, MAPK, IGZF-1R, PI3K/AKT, and inflammatory cascades. MAPK: mitogen-activated protein kinase; PI3K: phosphatidylinositol-3-kinase
Cell-cycle arrest
Studies have suggested that lycopene can induce cell-cycle arrest at the G1 phase. It was found that Hep3B human hepatoma cells were inhibited 20%-50% by lycopene at physiologically relevant concentrations as low as 0.2 μmol/L. Lycopene induced G0/G1 arrest[246]. Similarly, in a study with human prostate cancer cell lines PC3 and LNCaP, lycopene promoted mitotic arrest at the G0/G1 phase, facilitated by a decreased amount of cyclin dependent kinase 4 and cyclin D1 and E[248].
Pharmacokinetics and bioavailability
The pharmacokinetics of lycopene provides insight regarding relevant mechanisms by which intervention impacts disease. The ability to interpret epidemiologic findings, design clinical intervention trials, and plan physiologically relevant animal and in vitro mechanistic investigations hinges on overall bioavailability. Critical questions impacting interventions include absorption, distribution, tissue concentrations and metabolic breakdown.
One important parameter is plasma half-life. With patients given 20 mg of lycopene from tomato soup or a synthetic lycopene tablet for 8 sequential days, the systemic availability of lycopene in the form of a tablet was comparable to that of processed tomatoes (tomato paste available as a soup) and better than tomato juice. The plasma lycopene half-life was found to be 5.6 and 6.4 d for the tablets and the soup, respectively. Furthermore, the half-lives of 5-cis- vs. all-trans-lycopene were evaluated and found to be 7.4 and 5.4 d, respectively. These observations suggest differences in the pharmacokinetic parameter which may lead to distinctive tissue and plasma lycopene isomeric patterns[250]. The diあerence in the half-lives observed in the geometrical isomers of lycopene may be due to factors such as the higher bioavailability of cis vs. trans[251,252], greater thermodynamic stability of cis isomers at elevated temperature[253], and endogenous isomerization, which may or may not be enzymatic[254].
Cleavage of β-carotene gives homologous carbonyl products[255,256]; the pathway was confirmed by the identification of BCO2 in zebra fish, ferrets, mice and humans[257,258]. BCO2, an enzyme derived from mitochondria[259], is mainly expressed in testis and liver, with a comparatively lower amount found in lung, heart, brain, colon, stomach, intestine, prostate, spleen, and kidney[257,258]. In a study conducted with humans with lycopene, plasma of those who consumed tomato juice was found to contain apo-6-, apo-8’-, apo-10’-, apo-12’-, and apo-14’-lycopenal[260]. Moreover, it was reported that non-provitamin A carotenoids, including zeaxanthin and lutein, are preferentially cleaved relative to provitamin A carotenoids, which indicates a major role of BCO2 in the metabolism of non-provitamin A carotenoid[258,261]. In another investigation with humans given lycopene, a series of apo-lycopenals were identified in plasma, including apo-10’-lycopenal[260]. However, it is not known whether these metabolites are enzymatically cleaved or products originating due to chemical oxidative cleavage[260].
In a single blind, randomized, placebo-controlled study, lycopene supplements of 15 mg two times a day for 3 weeks (Lyc-O-Mato™, LycoRed, Beer-Sheva, Israel) were given to 15 of 26 men who were scheduled for radical prostatectomy due to organ confined malignancy. Over 3 weeks, in those taking lycopene supplements, a 22% increase in tissue and plasma levels and a statistically significant reduction in PSA was observed. Tumor volume was reduced. In addition, cellular proliferation biomarkers were decreased whereas cellular differentiation markers including connexin 43 and apoptosis were increased with intervention[262]. In another study, it was reported that combined intake of tomato sauce, tomatoes, tomato juice, and pizza decreased the risk of prostate cancer[263]. These findings suggest that tomato-based foods may be beneficial for prostate cancer.
CONCLUSIONS AND FUTURE DIRECTIONS
We currently review some well-studied phytochemicals with cancer chemopreventive potential. Of course, many other cancer chemopreventive agents are known, either naturally occurring or synthetic, such as genistein[264], 6-gingerol[265], diallyl disulfide[266], lupeol[267], honokiol[268], plumbagin[269], ellagic acid[270], and quercetin[271], but our arbitrary selection mainly focuses on agents found in the diet with strong evidence of bioactivity. Numerous studies have been completed or are ongoing with these compounds; copious amounts of data have been generated. As one example, more than 20,000 articles were disclosed when searching “resveratrol” as a topic on Web of Science (accessed on August 25, 2020), and there is no doubt a search involving other chemopreventive agents would reveal a multitude of publications as well. Given this huge corpus of data, and taking into account the current state of science, there is now an opportunity to further expand the field of chemoprevention. We currently suggest three such paradigm shifts, referred to as omics, indirectness, and sequelae.
Omics
The traditional dogma for the discovery of drug candidates is based on “one drug-one target”, where the goal is to find highly selective agents with one molecular target (or biomarker). The concept is to minimize adverse eあects caused by oあ-target responses. However, this approach is disingenuous. First of all, an agent functioning in such a manner would be highly susceptible to failure due to the possibility of developing drug resistance. Moreover, signaling pathways are exceedingly linked via interconnected networks (cf. Figure 9). A small change in one target can result in multiple changes in other pathways, akin to the socalled Butterfly Effect (“Does the Flap of a Butterfly’s Wings in Brazil Set Off a Tornado in Texas?”)[272]; Also, the genesis of cancer is comprised of multiple factors, not a single specific element. This is further complicated by a host of endogenous (e.g., aging, genetic susceptibility, DNA repair machinery, hormones, growth factors, inflammation) and exogenous (e.g., radiation, chemical carcinogens, viruses, smoking, lack of exercise, nutrient imbalance) risk factors, and typically long latency periods[273]. Further, cause-effect relationships are often nebulous.

Figure 9. Bubble map with target proteins of resveratrol using STRING software. Human target proteins of resveratrol that were selected from ChEMBL are visualized as a network of predicted associations. The network nodes represent proteins while the edges represent the protein-protein associations. The colored lines represent different types of evidence - Light blue line: curated database; purple line: experimentally determined; green line: neighborhood; red line: gene fusion; blue line: gene co-occurrence; yellow line: text mining; black line: co-expression. The colored nodes demonstrate the pathways. The KEGG pathway table demonstrates the top ten pathways enriched by resveratrol based on a gene list of human target proteins. Neuroactive ligand-receptor interaction appears at the top of the list with the lowest false discovery rate (2.36 × 1042)
In the context of chemoprevention, it is likely that dietary phytochemicals currently play an important role in cancer reduction or delay, but that role is difficult to quantify in a direct manner. Daily consumption varies, as does the phytochemical content of the foods consumed. For a more predictable response, especially for individuals at high risk for developing malignancies, a cocktail of chemopreventive agents would be preferred[8]. Development of such a cocktail needs to take into account the pleiotropic activities of typical chemopreventive agents, as demonstrated by those described in this review and elsewhere. On one hand, pleiotropic responses are considered a distinct advantage. On the other hand, the creation of a proper preparation becomes a daunting task as a result of complexity. However, unique tools are now available that can be put to use. For example, with agents know to function in a chemopreventive capacity, we can take into account novel pathways uncovered utilizing primary -omics data (e.g., genomics, proteomics, metabolomics) or data mining with publicly accessible biological data repositories (e.g., ChEMBL, PubChem).
Consider resveratrol as an example. Based on literature reports describing individual actions of this compound, we input human target proteins listed in ChEMBL (accessed on August 25 2020)[274]on STRING (accessed on August 25 2020)[275]. As shown in Figure 9, the gene list with network edges is visualized with enriched pathways (e.g., neuroactive ligand-receptor interaction, calcium signaling pathway, nitrogen metabolism, serotonergic synapse, cAMP signaling pathway). Clearly, this is a more realistic view of the action of resveratrol, relative to thinking of it as simply inhibiting or stimulating factor x, y or z. Now, if we consider a second chemopreventive agent, and the network of factors modulated by that agent, and overlay the two individual networks, it is easy to perceive the complexity of the actual response leading to a chemopreventive response.
There is little doubt that the intrinsic response of a mammal exposed to a chemopreventive agent correlates to some extent with the theoretical response shown in Figure 9. As an example, an area of great interest for our group is the potential eあect of grapes on health[276], and the corresponding mechanisms. In a recent study (unpublished) in which mice were provided diets with or without whole grape supplementation, RNA expression data were examined. As shown in Figure 10, remarkable alterations were observed that would likely relate to some of the beneficial properties. There is little doubt this modulation of genetic expression results from the action of phytochemicals contained within the grape. Grapes have received great notoriety for being a primary dietary source of resveratrol but, in actual fact, whole grapes contain over 1,600 phytochemicals[34]. Thus, it is perhaps not surprising that such a profound eあect on genetic expression was observed. This provides a good illustration of the complexity of a real-life response and accentuates the naivety of one drug-one target philosophy.
lndirectness
As noted above, the low bioavailability of phytochemicals may lead to discrepancies in effective doses observed with in vitro models but required for in vivo responses. For example, the oral bioavailability of resveratrol is reported as under 1% due to rapid metabolism in the intestine and liver[277]. As exemplified in Figure 11, this has led to the exploration of technology designed to enhance bioavailability. Here, a map was created based on bibliographic data (co-occurrence) of “resveratrol” from Web of Science, and words such as “drug-delivery” and “encapsulation”, and highlighting using VOSviewer[278].
Of course, metabolism of chemopreventive agents may have a major influence on efficacy. Several major metabolites derived from the chemopreventive agents described herein are shown in Figure 12. In some cases, these metabolites may be less active than the parent molecule. In other cases, such as with resveratrol 3’-sulfate[279], the activity may be similar or even greater.

Figure 10. Hierarchical clustering heatmap. A study was conducted with female C57BL/6J mice. Starting at age 10 weeks, animals were provided the following diets ad libitum: standard synthetic diet (STD); STD supplemented with 5% whole grape powder (STD5GP); a high-fat (Western) diet (HFD); HFD supplemented with 5% whole grape powder (HFD5GP). Cluster analysis was produced using the log10(FPKM)+1 value that shows the influence of grape on the expression levels of genes. Red color indicates up-regulated gene expression; blue color indicates down-regulated gene expression. The interrelationships between groups is presented as hierarchical clustering. The order of the rows and columns is based on resemblance correlations that can be interpreted based on the hierarchical clustering shown in the heatmap. The data indicate dietary supplementation with whole grape powder influences the expression levels of genes. The heatmap was produced by analyzing a total of 11,047 genes
In addition to well-known phase 1 and phase 2 mammalian enzymes that participate in drug metabolism, clearly, the overall metabolic profile of chemopreventive agents is influenced by the gut microbiome. Even further, however, health benefits mediated by phytochemicals may result from indirect eあects such as influencing products produced by the gut microbiome itself. For example, recent studies have shown enrichment of short-chain fatty acids (SCFAs)-producing bacteria (e.g., Bacteroidetes and Parabacteroides) in mice treated with resveratrol[280]. SCFAs, microbial-derived metabolites, are reported to affect CNS functions in the microbiota-brain-axis[281].Conversely, dietary chemopreventive agents may influence the actual composition, and therefore the metabolic capacity, of the gut microbiome. For example, in studies we have conducted with mice in which the diet was supplemented with whole grapes (unpublished), the overall organization of the gut microbiome was clearly altered [Figure 13]. This is yet another factor that needs to be considered in defining the overall action of chemopreventive agents.
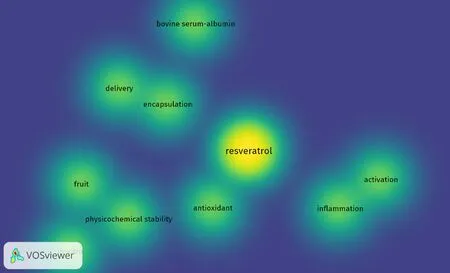
Figure 11. Density visualization of “resveratrol” and co-occurring words by VOSviewer. Co-occurrence words with resveratrol are displayed based on text data from Web of Science. The color illustrated in the figure changes from blue to yellow based on the frequency with which as a word is linked to resveratrol
Sequelae
Over the past decades, there have been significant advancements in understanding cancers and in improving early detection, treatment, and prevention. As a result, the survival rate of cancer patients has increased. And, as the number of cancer survivors increases, the quality of life for these individuals must receive greater attention, given that adverse eあects (sequelae) are frequently encountered during or after chemotherapy. One common sequela is cancer-related cognitive impairment (CRCI), or chemotherapyinduced cognitive impairments, colloquially called “chemobrain” or “chemofog”, a decline in cognitive function following cancer treatment. Central neurotoxicity can remain as long-term sequelae following the termination of treatment. For example, it has been reported that breast cancer and childhood cancer survivors may experience cognitive dysfunction[282]. Notably, some chemopreventive agents are at least touted to improve this condition[283,284].
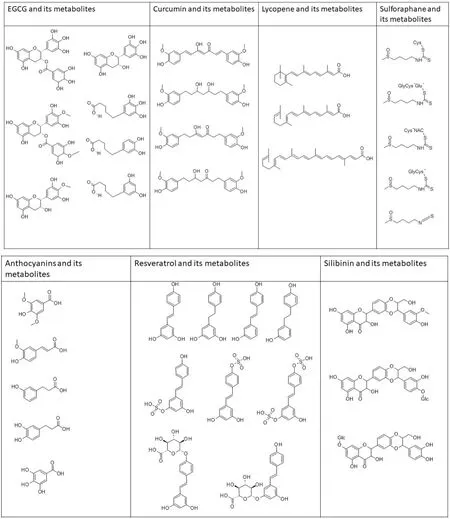
Figure 12. Chemical structures of phytochemicals and their metabolites. EGCG and metabolites - EGC, 4’-MeEGC; 4’,4’-diMeEGCG, 5-(3’, 4’-dihydroxyphenyl-γ-valerolactone (M6); 5-(3’, 4’, 5’- triihydroxyphenyl-γ-valerolactone (M4); 5-(3’, 5’- dihydroxyphenylγ-valerolactone (M6’). Curcumin and metabolites - tetrahydrocurcumin, hexahydrocurcumin, hexahydrocurcuminol. Lycopene and metabolites - apo-10’a-lycopenoic acid, acylclo-retinoic acid, all-trans-retinoic acid. Sulforaphane and metabolites - sulforapahane-GSH, sulforapahane cysteinylglycine. Anthocyanin and metabolites - syringic acid, ferulic acid 3,4-dihydroxyphenylpropionic acid, m-hydroxyphenylpropionic acid, gallic acid. Resveratrol and metabolites - trans-resveratrol (RSV), trans-resveratrol-3-O-sulfate, transresveratrol-4’-O-sulfate, trans-resveratrol-3,4’-O-disulfate, trans-resveratrol-3-O-glucuronide, trans-resveratrol-4’-O-glucuronide, dihydroresveratrol (DHR), 3,4’-O-dihydroxy-trans-stilbene, lunularin. Silibinin and metabolites - 20-O-β-D-Glu, 7-O-β-D-Glu, O-demethylated metabolite

Figure 13. The figure shows a Venn diagram where each circle represents one group of microorganisms. The values in overlapping parts represent common Operational Taxonomic Units (OTUs) while others are OTUs specific to each group. Female HSD: ICR (CD-1®) mice at the age of 4 weeks were divided into four groups and given the following diets ad libitum for 20 weeks: S1.0D, standard synthetic diet, S2.0D; standard diet supplemented with 5% whole grape powder; S4.0D, high-fat (Western) diet;S5.0D, high-fat diet supplemented with 5% whole grape powder. The gut microbiota was analyzed using fecal samples. The data illustrate dietary grape supplementation influenced the composition of the gut microbiome. The overall organization of the gut microbiome is altered by grape and a higher number of microbiome groups can be observed in the grape fed diet groups
Given the mode of action of chemopreventive agents, it seems logical that acute or chronic side-effects resulting from some forms of cancer chemotherapy could be ameliorated by concomitant administration. However, since chemotherapy is often designed to be overtly toxic, and therapeutic indices may be low, an eあective chemopreventive agent could actually have an adverse eあect on outcome by negating anti-cancer drug action. An appropriate balance is necessary, but some promising results have been described. For example, recent studies have demonstrated the neuroprotective potential of phytochemicals by reducing chemotherapy-induced CRCI in animal models. In addition, orally administered curcumin, at a dose of 100 mg/kg 1 h prior to cisplatin treatment, attenuated cisplatin-induced autophagy in the murine hippocampus accompanied by the activation of AMPK-JNK signaling[283]. Further, piceid, a resveratrol derivative, given orally at a dose of 50 mg/kg/d for 4 weeks, protected against doxorubicin-induced cognitive impairment concomitant with the up-regulation of Nrf2, inhibition of the NF-κB pathway, and reduction of apoptosis in the rat hippocampus[284]. It seems the ameliorative potential of chemoprevention is worthy of serious consideration.
In summary, over the past 50 years or so, many phytochemicals have been discovered and characterized that can delay, prevent, or reverse the process of carcinogenesis, and thereby modulate the incidence of the end stage cancer. Given the consequences of malignant metastatic disease, the promise of this therapeutic approach is evident yet remains largely untapped. As we continue to strive for primary intervention strategies that can be oあered to all of those in need, additional potential benefits have become apparent, some of which are described herein and depicted in Figure 14. Not only does chemoprevention offer significant promise for disease prevention, but given the enormous economic burden imposed by contemporary biologic and drug therapies[285], the approach would be cost-eあective.
Figure 14. Possible paradigm augmentation in cancer chemoprevention. As we realize shifts in the perspective of handling the dilemma of cancer, changing roles for chemopreventive agents can be envisioned. Emphasis is placed on sustaining “holistic health” and wellbeing
DECLARATIONS
Acknowledgments
The authors are grateful to the California Table Grape Commission for providing support for this work.
Authors’ contributions
Participated in the conception and preparation of this review: Dave A, Parande F, Park EJ, Pezzuto JM
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported in part by the California Table Grape Commission.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
