A review on exosome-based cancer therapy
2020-07-21AlexVonSchulzeFengyanDeng
Alex Von Schulze, Fengyan Deng
Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS 66160, USA.
Abstract Cancer is one of the leading causes of mortality and morbidity globally. Many types of cancer treatments have been developed, such as chemotherapy, surgery, radiotherapy, and immunotherapy. However, these therapies can also kill healthy cells and lead to severe side effects. Therefore, scientists are looking for new strategies to eliminate cancerous cells specifically. Exosomes, nanometer-sized lipid bilayer-enclosed vesicles secreted from various cell types, exist in nearly all body fluids, including blood, breast milk, saliva, urine, bile, pancreatic juice, cerebrospinal, and peritoneal fluids. They carry myriad donor cell-derived bioactive molecules such as proteins, lipids, and RNAs (including microRNA and lncRNA) and can deliver them to both nearby and distant recipient cells. Due to these characteristics, exosomes have attracted great interest in cancer treatment (especially serving as a biological carrier for some drugs, microRNA, lncRNA, inhibitors, and antibodies). In this paper, we will review the current knowledge of exosome therapeutic applications in cancer.
Keywords: Exosomes, cancer, cancer therapy, gene carrier, drug carrier
INTRODUCTION
Cancer remains the leading cause of death globally. There are over 200 types of cancer which claim more than 10 million lives annually[1]. Despite a lot of research focusing on this grave disease, cancer therapeutics still have the lowest clinical trial success rate of all major diseases. This is likely due to the fact that it is hard for our immune system to distinguish cancerous cells from healthy cells. For instance, current therapeutics like radiotherapy and chemotherapy not only kill the cancerous cells, but also healthy cells[1]. Therefore, developing new therapeutic strategies in order to precisely eliminate cancerous cells is an urgent need.Exosomes are nanosized extracellular vesicles which are secreted by various cells throughout the body[2-5]. They carry important bioactive molecules including proteins, lipids, DNA (e.g., mitochondrial DNA and genomic DNA), and RNAs [e.g., microRNAs (miRNAs), long non coding RNAs (lncRNAs), and messenger RNA (mRNA)], and can transfer them to the recipient cells - thus playing a crucial role in cellcell communication[2-6]. Exosomes are present in most bodily fluids including blood, breast milk, saliva, urine, bile, pancreatic juice, cerebrospinal, and peritoneal fluids[2-6]. Through the circulating flow, they can transfer from their original cells to distant tissues where they localize to target cells by binding their surface molecules to receptors on the surface of the target cells[2-6]. Due to the advantage of their unique biocompatibility and high stability, exosomes have attracted great interest in cancer treatment as an eあective anti-cancer drug delivery carrier or gene carrier. In this review, we provide an overview for studies of exosomes application in cancer therapies, as well as their advantages and challenges.
DUAL ROLES OF EXOSOMES IN TUMORIGENESIS
According to the exosome database ExoCarta (www.exocarta.org), 9,769 proteins, 3,408 mRNA, 2,838 miRNA, and 1,116 lipids have been identified in exosomes from different organisms and bodily fluids[7]. By transferring bioactive molecules from the donor cells to the recipient cells, exosomes play crucial roles in cell-cell communication[2-5]. Many studies have shown that cancer cells release a larger number of exosomes to exchange information with other cells both nearby and at distance[8-10]. Through delivering their bioactive cargoes (including proteins, miRNAs, and lncRNAs), cancer cell-derived exosomes contribute to the formation of the pre-metastatic microenvironment, tumor growth and progression, immune escape, angiogenesis, anti-apoptotic signaling, drug-resistance, and so on[8-10][Figure 1]. Meanwhile, exosomes from healthy cells, su ch as dendritic cells (DCs), B cells, and T cells, play an important role in inhibiting tumor growth[11-14][Figure 1]. To date, numerous miRNAs, lncRNAs, and proteins have been found to play important roles in cancer progression [Table 1]. Therefore, depending on their cell of origin and their bioactive cargo, exosomes can play dual roles in cancer regulation, either inhibiting or promoting growth.
EXOSOME-BASED CANCER THERAPIES
Since exosomes can unload bioactive cargo to cancer cells, they have attracted great interest in cancer treatment[66-72]. Currently, several different methods have been developed for cancer therapies: (1) using naturally derived exosomes from immune cells to suppress cancer cells[66]; (2) inhibiting the release of cancer-derived exosomes; (3) using exosomes as gene carriers[69]; and (4) using exosomes as anti-cancer drug carriers[68,71][Figure 2].
Naturally derived exosomes for cancer therapy
In cancer-immunity, DCs are involved in the first step of tumor cell growth inhibition by capturing neoantigens and triggering the tumor-specific cytotoxic lymphocyte response[73]. DC-derived exosomes (Dex) contain various bioactive cargoes responsible for antigen presentation, making them ideal for the treatment of cancer[74,75]. In 1998, Zitvogel et al.[76]found that tumor peptide-pulsed Dex were able to activate the antigen-specific cytotoxic T lymphocytes response in vivo and eradicate or suppress growth of established murine tumors in a T cell-dependent manner. Moreover, Munich et al.[77]found that Dex can directly kill tumor cells and activate naturel killer (NK) cells through TNF superfamily ligands. It has also been found that cancer cell-derived exosomes have an immunostimulatory eあect on anti-tumor DCs[66]. Thus, Dex represent an important strategy for cancer therapy.
lnterfering with cancer cell-derived exosomes for cancer therapy
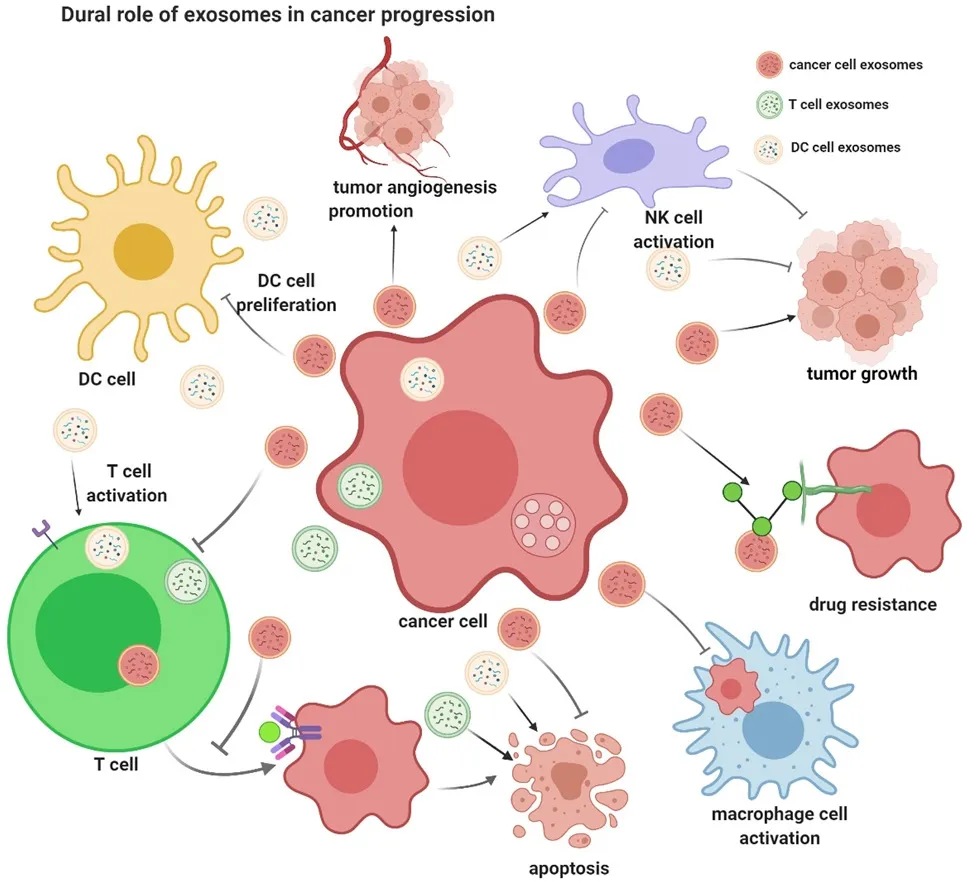
Figure 1. Dual role of exosomes in cancer progression. Cancer cell-derived exosomes contribute to the formation of the pre-metastatic microenvironment, tumor growth and progression, immune escape, angiogenesis, anti-apoptotic signaling, drug-resistance, and so on. While exosomes from healthy cells including dendritic cells, B cells, and T cells, play crucial role in inhibiting tumor growth (This figure was created with BioRender.com)
Cancer cell-derived exosomes are considered to accelerate cancer pathogenesis by contributing to the formation of the pre-metastatic microenvironment, tumor growth and progression, immune escape, angiogenesis, anti-apoptotic signaling, and drug-resistance[8-10]. Therefore, inhibition of the cancer cellderived exosomes synthesis, release, and uptake may serve as an eあective cancer therapy[78]. With the use of a mouse model, Bobrie et al.[78]found that blockade of Rab27a, a key mediator of exosome secretion, resulted in decreased primary tumor growth and lung dissemination of metastatic carcinoma (4T1) cells. It was found that miR-494 is enriched in melanoma-derived exosomes, and that exosomal transport of miR-494 promotes metastasis[79]. More specifically, inhibiting the function of Rab27a blocked exosomal transfer of miR-494 and resulted in inhibiting melanoma growth and metastasis[79]. Another possible way to mitigate tumor growth is to simple remove circulating exosomes via a hemofiltration system (such as the Aethlon ADAPTTMsystem). This alternative may also serve as another possible therapeutic approach for reversing immune dysfunction and improving the immune responses to tumor growth[80]. Finally, multiple studies have discovered that blocking exosomal uptake is another potentially eあective method for cancer therapy[81,82]. For example, the heparan sulfate (HS) proteoglycans has been discovered as internalizing receptors of cancer cell-derived exosomes[82]. HSPG deficiency, or the HS mimetic (heparin) treatment, significantly reduced exosome uptake and exosome-mediated stimulation of cancer cell migration[82]. In all three cases (blocking exosomal secretion, exosomal removal, and blocking exosomal uptake), disrupting cell-cell communication from cancer-cell derived exosomes appears to oあer an exciting new way to treat cancer.

Table 1. List of exosomal bioactive molecules involved in various cancer progression

Figure 2. Exosome-based cancer therapies. Currently, four different methods have been developed for cancer therapies: (1) natural exosomes from some immune cells to suppress cancer cells; (2) inhibition of cancer cells-derived exosomes; (3) exosomes as gene carriers; and (4) exosomes as anti-cancer drug carriers (This figure was created with BioRender.com)
Exosomes as gene carriers for cancer therapy
Although, there is great potential for exosomes in cancer therapy, the use of natural exosomes is hard and rarely achieves the expected therapeutic result. Fortunately, engineered exosomes carrying specific proteins, RNAs, or drugs have been found to possess great potential for eあective cancer treatment.
Exosomes as miRNA carrier for cancer therapy
miRNAs are endogenous, small, non-coding RNAs that can regulate gene expression by binding to target mRNAs. Therefore, miRNAs could be a powerful tool for cancer therapy. However, miRNAs are easily degradedin vivoand delivery of miRNAs to their specific target cells/tissue/organ is a major challenge. As exosomes are stable small vesicles that can carry functional bioactive molecules long distances with a high degree of target specificity[2-6], they have been suggested as a potential carrier for miRNAs in cancer therapy. Over the last few years, many scientists have focused on exosomal-based delivery of miRNAs and miRNA inhibitors for cancer therapy[83-88]. For instance, in 2013, Katakowskiet al.[83]reported that exosomes enriched with the anti-glioma miRNA (miRNA-146b) can suppress glioma growthin vitroand can also significantly reduce glioma xenograft growth in rats. Similarly, miR-101-enriched exosomes can suppress osteosarcoma cell invasion/migrationin vitroand suppress metastasisin vivo[84]. Wanget al.[85]also reported that exosomes loaded with miR-335-5p can decrease cancer growth and invasion bothin vitroandin vivo. Interestingly, O’Brienet al.[86]used engineered mesenchymal stem cells to secrete exosomes enriched with miR-379 forin vivotherapy of breast cancer. Importantly, they found that systemic administration of miR-379 enriched exosomes can significantly reduce tumor activity. Another miRNA, miR-145-5p, was also found to inhibit pancreatic ductal adenocarcinoma cell proliferation and invasion, as well as increased apoptosis. Moreover, exosomes transfected with miR-145-5p were able to inhibit pancreatic ductal adenocarcinoma cell proliferation and invasion through TGF-β/Smad3 pathways[87]. On the other hand, inhibitors of exosomal miRNAs, which play key roles in cancer progression through exosome, have become another eあective method for cancer therapy. For example, exosomal miR-25-3p is a metastasispromoting miRNA of colorectal cancer. Exosomes enriched with miR-25-3p dramatically promoted vascular permeability and colorectal cancer metastasis in mice liver and lung. Nevertheless, these eあects can be rescued by blockage of exosomal miR-25-3p by a miR-25-3p inhibitor[88].
Exosomes as protein carrier for cancer therapy
Recently, many scientists have begun to develop an exosomal-based cancer vaccine[89-91]. For example, TNF-alpha-related-apoptosis-inducing-ligand (TRAIL), a cytokine, functions as a ligand that induces cell apoptosis[92,93]. Rivoltiniet al.[94]reported that TRAIL-armed exosomes could promote apoptosis in cancer cells and control tumor progressionin vivo. Furthermore, IL-18 enriched exosomes enhance Th1 cytokine release and proliferation of peripheral blood mononuclear cells, suggesting that IL-18 enriched exosomes harbor more capability to induce specific anti-tumor immunity as they trigger a bigger immune response[95]. Yanget al.[96]also found that IL-2 enriched exosomes induce the antigen-specific Th1-polarized immune response and cytotoxic T lymphocyte response more efficiently, leading a significant inhibition of tumor growth in mice. Exosomes can also be used as carriers of protein antagonists. For example, signal regulatory protein α (SIRPα) can interact with CD47, a “don’t eat me” signal that limits the ability of macrophages to engulf tumor cells. Exosomes carrying SIRPα antagonists could significantly increase tumor phagocytosis, as has been observed in tumor-bearing mice[97].
Exosomes as chemotherapeutic drug carriers for cancer therapy
Anti-tumor chemotherapeutic drugs can effectively kill fast-growing tumor cells. However, these drugs can also kill the normal, healthy cells that are fast-growing, causing serious side effects. Besides, for some hydrophobic drugs, it is a challenge for them to target tumor cells with any kind of specificity. Therefore, an eあective carrier for these drugs is badly needed. Due to their naturally derived origin and their stable lipid bilayer structure, exosomes have the great potential to serve as an effective carrier for chemotherapeutic agents. As early as 2012, Tanget al.[98]reported that tumor cell-derived microparticles can be used as chemotherapeutic drug carriers. They found that chemotherapeutic drugs loaded onto microparticles had a potent anti-tumor eあect bothin vitroandin vivo[98]. In 2015, Kimet al.[99]developed a exosome-based formulation of paclitaxel (PTX), a commonly used chemotherapeutic agent, to overcome multiple drug resistance (MDR) in cancer cells. Three methods including incubation at room temperature, electroporation, and mild sonication were used to incorporate PTX into exosomes; among which, the mild sonication method resulted in the highest loading efficiency and sustained drug release[99]. PTX-loaded exosomes (exoPTX) increased cytotoxicity more than 50 times in drug resistant MDCKMDR1(Pgp +) cells[99]. Furthermore, through a similarin vivomice model, Kimet al.[99]found that exoPTX can efficiently target cancer cells and produce strong antineoplastic eあects in mice with lung metastases. Similarly, Saariet al.[100]also found that delivery of PTX via cancer cell-derived exosomes enhances the cytotoxicity of PTX in autologous prostate cancer cells. Furthermore, by modifying the exosome surface proteins, exosomes can deliver chemotherapeutic drugs to cancer cells with a high degree of specificity. For example, Tianet al.[101]engineered Lamp2b-iRGD peptide (αv integrin-specific) expressing mouse immature DCs (imDCs), isolated their exosomes and used them to deliver doxorubicin (Dox). Using this approach, they found that iRGD-exosomes can efficiently target and deliver Dox to αv integrin-positive breast cancer cellsin vitro, and specifically to tumor tissues, resulting in inhibition of tumor growthin vivo[101].
CONCLUSION AND FUTURE DIRECTIONS
Due to their role in cancer progression and biological features, exosomes possess promising potential for cancer therapy. To date, numerous exosomes-based cancer therapies have been studied and developed including applying naturally derived immune cell exosomes to suppress cancer cells, inhibiting cancer cells-derived exosomal activity, and using exosomes as gene/drug carriers. However, there are considerable challenges to be solved. First, the difference among exosomes from different sources is still not clear. Second, the exosome number to get a therapeutic effect may be significantly different among different cancers. Third, scalability and heterogeneity of tumor may influence therapeutic outcome. Moreover, different functions of exosomes derived from different sources are not fully studied. Furthermore, knowledge on how exosomes can be modified so that they possess a high degree of specificity to particular cancer cells remains unclear. Finally, the storage and the stability of exosomes remains ill-defined. With these challenges, there is a vital need to systematically characterize exosomes derived from diあerent cells/tissue to choose the most efficient cells for specific cancer therapy. There is a further need to identify the specific cancer cell surface markers for designing exosomes as drug carriers with high specificity to cancer cells. Moreover, because of the complexity and the heterogeneity of the tumor, exosome-based cancer may need to combine with other approaches. Finally, clinical trials of exosome-based cancer therapy are urgently needed to determine the efficacy of the application. While there are still challenges ahead, it is clear that exosomes oあer novel and important applications for the treatment of cancer.
DECLARATIONS
Acknowledgments
All figures were created with BioRender.com.
Authors’ contributions
Conceived the general idea of the review, made up the structure and searched the literature: Deng F Drafted the manuscript: Schulze AV, Deng F
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
