原位电化学合成铁基电极材料及其超级电容器性能
2020-07-20梁晰童薛冬峰
梁晰童 薛冬峰*,
(1中国科学院长春应用化学研究所稀土资源利用国家重点实验室,长春 130022)
(2中国科学技术大学,合肥 230026)
0 Introduction
With the development of society,energy demand is increasing recently.Safe,clean and sustainable energy storage devices are necessary to satisfy the need for electrical energy storage[1-5].Electrochemical capacitors,also called supercapacitors,store energy in two closely spaced layers with opposing charges or electrodes with redox reactions near surface[6-8],which offer high power densities and ideal cycling ability.However,supercapacitors are still limited by low energy densities[9].A large variety of materials such as gra-phene[10-13],nanotube[14-16],MXene[17-20]and transition metal oxides/hydroxides have been explored in supercapacitors recently[21-24].
Iron oxides have a broad working window in negative range and high theoretical specific capacitances[25].Therefore,various iron oxides have received increasing interests in the field of supercapacitors.At present,the specific capacitance of most iron oxides is far from their theoretical value mainly due to relatively large particle size and poor conductivity.Porous Fe2O3nanoparticles on graphene delivered a specific capacitance of 357 F·g−1at 10 mV·s−1.Graphene improved the conductivity of the electrode and porous structure provided rich surface for redox reaction[26].A hydrothermal method was used to synthesize reduced graphene oxide(rGO)/Fe2O3aerogel.A specific capacitance over 700 F·g−1was achieved due to the synergetic effect of the higher conductivity of rGO,and the mesoporous structure of Fe2O3nanoparticles[27].Carbon fabric(CF),vertically aligned graphene nanosheets(VAGN)and FeOOH nanorods were incorporated into an electrode.ThisCF/VAGN/FeOOH electrodecan charge/discharge at a low potential window(0 to−1.1 V vs Hg/HgO),and exhibited a high specific capacitance of 909 F·g−1aided by the unique structure of VAGN[28].Fe3O4nanospheres were in situ decorated in graphene to form a nanocomposite,which exhibited a notably enhanced specific capacity of 268 F·g−1at 2 mV·s−1.The unique structure of the graphene/Fe3O4could provide more pathways for rapid transfer of electrons and transport or diffusion of electrolyte ions during fast charge/discharge processes[29].
Interface plays a key role in electrochemical reactions in supercapacitors.Interface consists of electrode materials and electrolyte in supercapacitors[30].In order to construct suitable interface,we can tune various electrolytes to construct different interface[31].Herein,we choose FeCl3·6H2O served as a precursor to synthesize electrode materials in different kinds of electrolytes.The electrode exhibited a specific capacitance of 1 113 F·g−1at a current density of 3 A·g−1in 2 mol·L−1KOH solution.The electrode exhibited specific capacitances of 927 and 755 F·g−1at a current density of 3 A·g−1in 1.5 mol·L−1NaOH and 1.5 mol·L−1LiOH electrolyte,respectively.
1 Experimental
1.1 Synthesis of Fe-based electrode
FeCl3·6H2O was purchased from Tianjin Huadong Reagent Factory and was used without further purification.FeCl3·6H2O salt was mixed with conductive carbon and polyvinylidene fluoride(PVDF)with a weight ratio of 6∶3∶1,then proper NMP was dropped into the mixture to form homogenous mixture.Then the mixture was incorporated onto nickel foams and put into the oven at 55℃for 7 hours.The nickel foam with paste was pressed at 15 MPa for 5 s.The geometrical area of working electrode was 1 cm×1 cm.The mass loading of FeCl3·6H2O in the electrode was 1.55 to 2.71 mg.The working electrode was put in electrolyte and carried out electrochemical reaction to form active Fe-based electrode.
1.2 Characterizations
The structural investigation of Fe-based electrodes was detected using a scanning electron microscope(SEM,Hitachi,S-4800 field emission scanning electron microscope,10 kV).All electrochemical measurements were carried out using electrochemical workstation(CHI 660e)in room temperature.
1.3 Electrochemical measurements
LiOH·H2O was purchased from Tianjin Huadong Reagent Factory.KOH and NaOH were purchased from Beijing Chemical Works.Then they were mixed with deionized water to form different concentrations of electrolyte for electrochemical reaction.Electrochemical measurements were carried out in a three-electrode cell.The counter electrode and reference electrode were Pt wire and saturated calomel electrode(SCE),respectively.The electrolytes were different concentrations of KOH,NaOH and LiOH aqueous solution.Cyclic voltammograms(CV)were carried out at scan rates of 2,5,10,25,and 50 mV·s−1,respectively.Galvanostatic charge and discharge(GCD)measurement was carried out at current densities of 3,5,10,15,and 30 A·g−1,respectively.The specific capacitance of Febased electrode was calculated from GCD curves ac-cording to equation[32]:
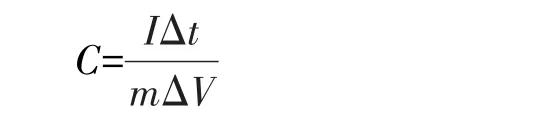
whereIis the current(A),mis the mass of active ions loaded in electrode(g),Δtis the discharge time(s)and ΔVis the potential window(V).
2 Results and discussion
2.1 Material synthesis and morphology characterization
In a typical synthesis process,FeCl3·6H2O salt was mixed with conductive carbon and PVDF to load on nickel foam,forming working electrode.The electrode was put in electrolyte,in which pristine salts were distributed in conductive matrix.CV measurement was used for the working electrode at 1 mV·s−1about 10 cycles in a three-electrode cell to obtain active Fe-based electrode.Salts dissolve into ions and ions interact with OH−instantly to form colloids,which was shown in Scheme 1.By tuning the kind of electrolytes,we can obtain various colloids with different compositions.The morphologies of the Fe-based electrode were investigated by SEM.Pristine salts,conductive carbon and PVDF were pasted on nickel foam(Fig.1a).The sample showed rough after experiencing cyclic voltammetry measurement.It seemed that active mate-rials had been formed in Fig.1b.After GCD measurement,there were so many particles ensuring enough surface areas for electrochemical reaction,which could lead to superior specific capacitances(Fig.1c and 1d).
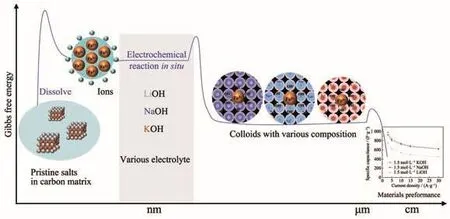
Scheme 1 Schematic fabrication of Fe-based electrodes with various compositions
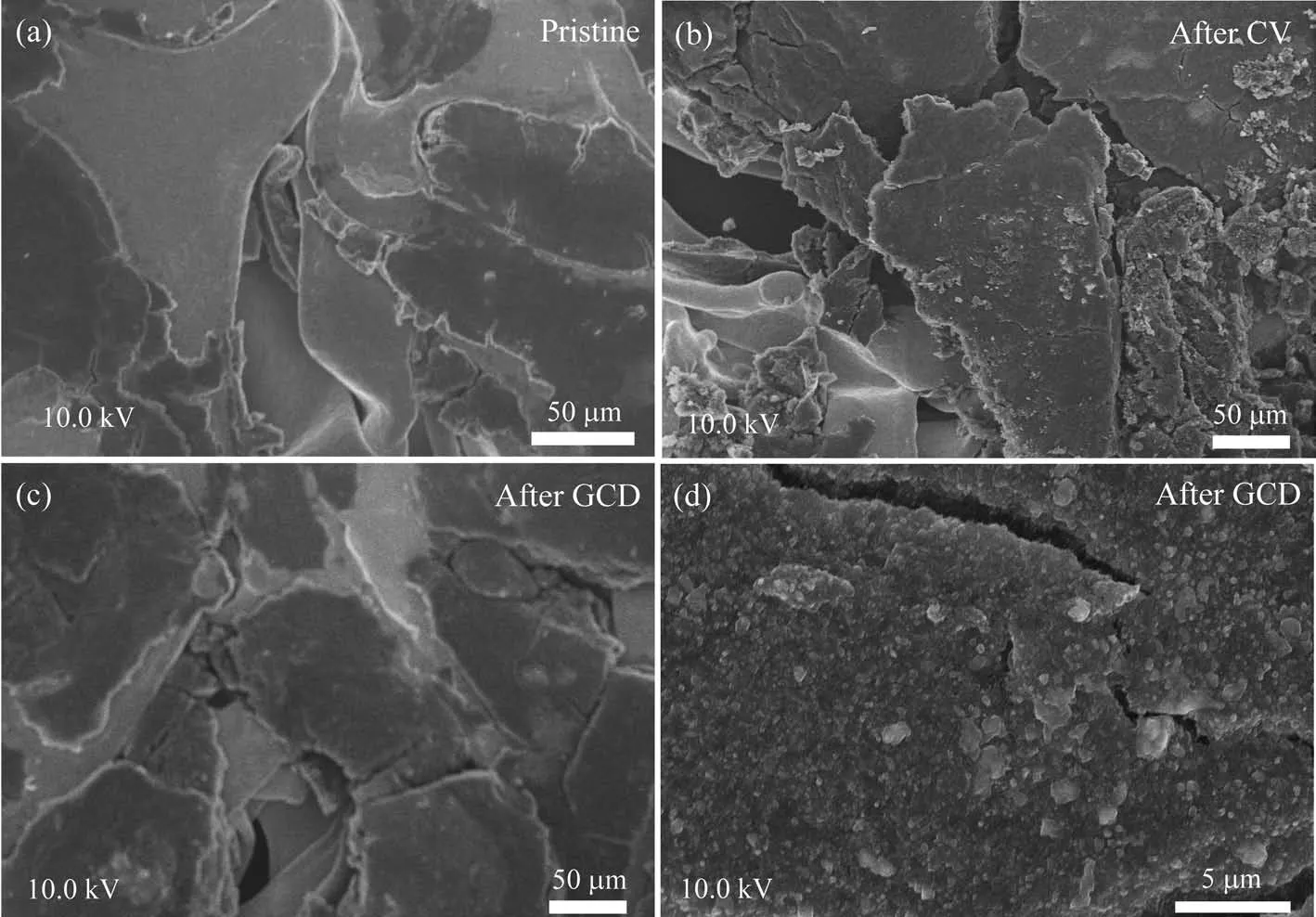
Fig.1 SEM images of FeCl3·6H2O electrodes:(a)Salts in carbon and PVDF matrix;(b)Electrode materials react with KOH electrolyte after CV cycles;(c,d)Electrode materials after GCD
2.2 Electrochemical characterizations
In order to compare the electrochemical characteristics of Fe-based electrode with different compositions,the electrochemical measurements were carried out using a three-electrode cell in different concentrations of various kinds of electrolytes.The detailed values were shown in Table S1.It can be found that the specific capacitance could be effectively tuned by changing the kind of electrolytes.Fig.2 showed electrochemical performance of Fe-based electrode materials in various aqueous electrolytes.In KOH electrolyte,the synthesized electrode exhibited a highest specific capacitance of 1 113 F·g−1at a current density of 3 A·g−1in 2 mol·L−1aqueous solution.The value was higher than data reported in relatively literatures(Table S2)[28-29,33-38].Moreover,the Fe-based electrode synthesized in KOH solution showed relatively high capacitances in 1.5 mol·L−1(Fig.2a).Fe-based electrode showed a capacitance of 927 F·g−1at a current density of 3 A·g−1in 1.5 mol·L−1NaOH(Fig.2b).In LiOH aqueous solution,Fe-based electrode showed the highest value of 766 F·g−1in 2 mol·L−1LiOH.Fig.2d showed CV curves of Fe-based electrodes in 1.5 mol·L−1electrolyte,whereEpawas anodic peak potential andEpcwas cathodic peak potential.The difference between anode peak potential and cathode peak potential indicates the reversible degree of the reaction.A pair of redox peak was obtained over a 1.2 V potential window at a scan rate of 2 mV·s−1.Fig.2e showed specific capacitances of Fe-based electrodes at various current densities in different electrolytes.It can be seen that Fe-based electrode synthesized in KOH electrolyte exhibited the highest values under the same concentration.Under the same concentration of 1.5 mol·L−1,the capacitance calculated from GCD curves was determined to be 982,927 and 755 F·g−1in KOH,NaOH and LiOH,respectively(Fig.2f).
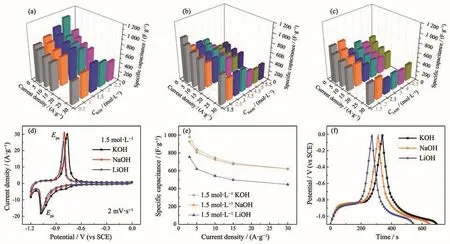
Fig.2 Electrochemical performance of FeCl3·6H2O based electrode materials in various aqueous electrolytes:(a~c)Specific capacitances of Fe-based electrodes;(d)Comparison of the CV curves at 2 mV·s−1;(e)Specific capacitances of Fe-based electrodes;(f)GCD curves at a current density of 3 A·g−1
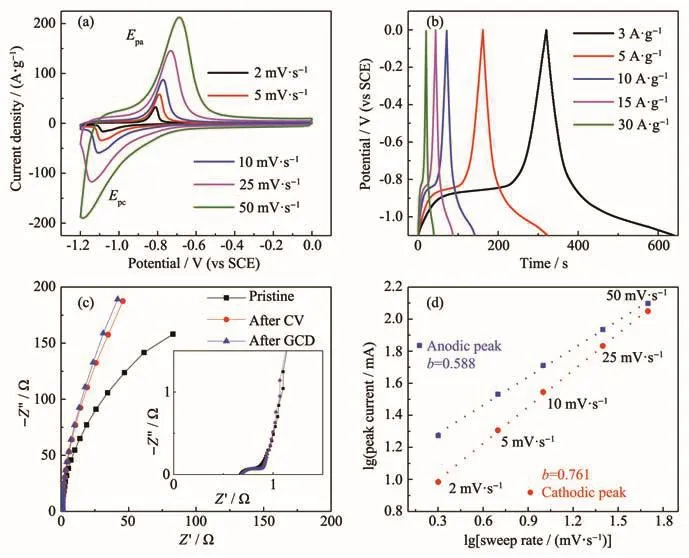
Fig.3 Electrochemical performance of FeCl3electrode in 2 mol·L−1KOH:(a)CV curves at scan rates from 2 to 50 mV·s−1;(b)GCD curves tested at various current densities from 3 to 30 A·g−1;(c)Nyquist plots of Fe-based electrode;Insert shows the high frequency region of the plots;(d)Relationship between peak current and sweep rate
To further evaluate the electrochemical behavior of the Fe-based electrode materials in KOH electrolyte,a series of electrochemical results were shown in Fig.3.CV curves at different scan rates from 2 to 50 mV·s−1were shown in Fig.3a.One pair of redox peaks at various scan rates was clearly observed.The linear slopes and presence of triangular with respect to the charge and discharge demonstrated that the capacitive characteristic of redox reaction in the electrodes(Fig.3b).The specific capacitances were calculated according to galvanostatic charge and discharge curves.The capacitances were 1 113,731,641,592 and 535 F·g−1at current densities of 3,5,10,15 and 30 A·g−1,respectively.Electrochemical impedance spectroscopy was collected with a frequency ranging from 0.01 to 100 000 Hz at 0 V.Nyquist plots were exhibited in Fig.3c.The insert was the local enlargement in high frequency region,which showed a semicircle.And the intercept on thex-axis was equivalent series resistance with a value of about 0.7 Ω,which was attributed to intrinsic resistance of ionic resistance of electrolyte,and contact resistance with current collector.Theb-value was calculated according to CV curves at different scan rates,which was between 0.5 and 1.Diffusion process played a key role in redox reaction.Cycling stability was shown in Fig.S1.Capacitance was maintained 87.4%after 50 cycles.
Electrochemical behavior of the Fe-based electrode materials in 1.5 mol·L−1NaOH electrolyte were shown in Fig.4.CV curves were measured at the scan rates from 2 to 50 mV·s−1.Galvanostatic charge and discharge measurements were conducted at current densities ranging from 3 to 30 A·g−1(Fig.4b).The charge and discharge curves were close to ideal capacitive behaviour.The specific capacitance was 927 F·g−1at a current density of 3 A·g−1.The value still maintained 622 F·g−1at 30 A·g−1.Equivalent series resistance was about 0.85 Ω(Fig.4c),which was higher than that in KOH aqueous solution.In anodic process,theb-value was about 0.596.In cathodic process,thebvalue was 0.752,which was higher than that in anodic process.It demonstrated that anodic process could be controlled by diffusion process.
Electrochemical behavior of the Fe-based electrode materials in 2 mol·L−1LiOH electrolyte were shown in Fig.5.A pair of redox peak at different scan rates was shown in CV curves(Fig.5a).Galvanostatic charge and discharge measurements were conducted at current densities ranging from 3 to 30 A·g−1(Fig.5b).The specific capacitances were 766,620 and 540 F·g−1at current densities of 3,5 and 10 A·g−1,respectively.Equivalent series resistance was about 1 Ω(Fig.5c),which was higher than that in KOH and NaOH aqueous solution.In anodic process,theb-value was about 0.600.In cathodic process,theb-value was 0.690,which was similar to that in anodic process.
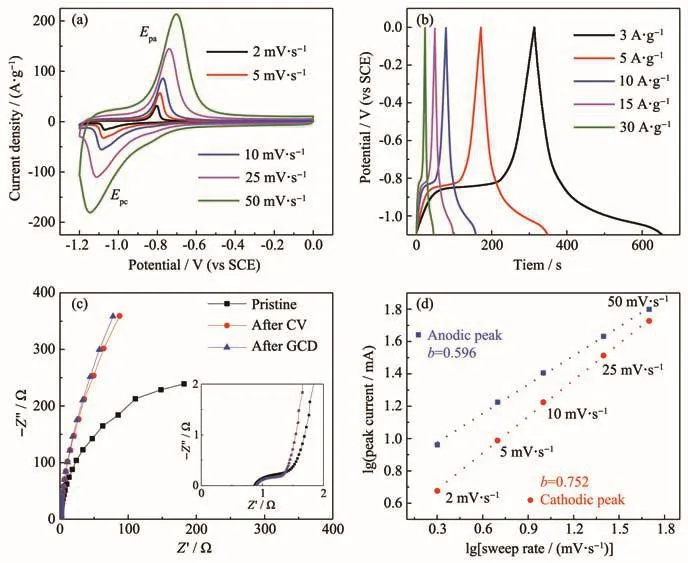
Fig.4 Electrochemical performance of FeCl3electrode in 1.5 mol·L−1NaOH:(a)CV curves at scan rates from 2 to 50 mV·s−1;(b)GCD curves tested at various current densities from 3 to 30 A·g−1;(c)Nyquist plots of Fe-based electrode;Insert shows the high frequency region of the plots;(d)Relationship between peak current and sweep rate
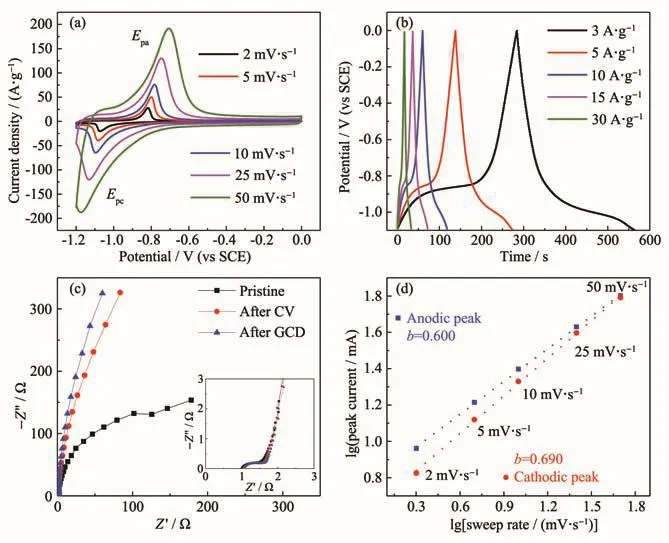
Fig.5 Electrochemical performance of FeCl3electrode in 2 mol·L−1LiOH:(a)CV curves at scan rates from 2 to 50 mV·s−1;(b)GCD curves tested at various current densities from 3 to 30 A·g−1;(c)Nyquist plots of Fe-based electrode;Insert shows the high frequency region of the plots;(d)Relationship between peak current and sweep rate
3 Conclusions
In conclusion,electrochemical interface could be tuned by various electrolytes with kinds of cations to achieve favourable specific capacitances.Fe3+colloidal systems showed the highest specific capacitance of 1 113 F·g−1at 3 A·g−1in 2 mol·L−1KOH aqueous electrolyte.The results demonstrated that tuning the kind of electrolytes may tune compositions of Fe-based electrodes to improve electrochemical performance.The active materials were synthesized in a three-electrode cell by in situ reaction,which exhibited superior reactive ability in the measurement conditions.Furthermore,by extending the colloid model,a novel method of in situ composition adjustment to cross-scale control of material properties is provided.This novel method may be applied in synthesizing other electrode materials with high specific capacitance.
Supporting information is available at http://www.wjhxxb.cn