羟基修饰MOFs的合成、表征和光催化机理
2020-07-20李石雄黄凤兰莫巧玲廖蓓玲
李石雄 黄凤兰 莫巧玲 廖蓓玲
(1梧州学院化学工程与资源再利用学院,梧州 543002)
(2河池学院化学与生物工程学院,河池 546300)
0 Introduction
Solving the environmental pollution and energy shortages will help develop the economy and promote technologicalinnovation.As a renewable energy source,the solar energy has attracted widespread attention.The photocatalytic oxidation technology is one of the directions[1-5].It can use solar energy to carry out a series of reactions,which has low energy consumption,simple equipment,mild reaction conditions,and no secondary pollution.It is an effective method to solve the environmental pollution and energy shortages.However,to achieve this technology,it is necessary to find and develop photocatalysts,which can be used in life.A large number of studies have been conducted on the inorganic semiconductor catalyst since its discovery[6-11].These studies had found that most of the pollutants can be effectively removed by photocatalytic techniques.But,the inorganic semiconductor photocatalysts have large forbidden band width,and small utilization efficiency for light,which limit their applications in life[12].Although the loading or doping precious metals on inorganic semiconductors can improve photocatalytic performance[13-15],but their structural are instability,difficulty in modification,and small specific surface area also affect their applications.It is extremely urgent to develop new,efficient and practical photocatalysts.
Metal-organic frameworks(MOFs)are coordination polymers with framework structure,they are formed by self-assembly of organic ligands with metals or metal ions[16].They have large specific surface area,abundant photocatalytic active sites,and easy regulation of structure[17-20].They have been deeply studied in the adsorption and separation of gas or pollutants[21-26],solid fluorescence[27-30],magnetism[31-33],drug loading[34],and heterogeneous catalysts[35-44].Since MOFs have a high specific surface area,which make them easy to adsorb and enrich organic or inorganic pollutant.In addition,many active sites in MOFs are prone to photocatalytic reactions.Therefore,MOFs show great potential application value in life.
In this paper,terephthalic acid,2,5-dihydroxyterephthalic acid,and Cr(NO3)3·9H2O are used as raw materials to synthesize MIL-101(Cr)and MIL-101(Cr)-2OH by hydrothermal synthesis.Their structures are characterized by Fourier transform infrared(FT-IR),X-ray powder diffraction(PXRD),and X-ray photoelectron spectroscopy(XPS).The conventional organic pollutant methylene blue is used as the research object to analyze the effect of hydroxyl groups on the photocatalytic properties of the constructed MOFs.The regulatory mechanisms are systematically studied by UV-Vis diffuse reflectance spectroscopy(UV-Vis DRS),ζpotential,and electrospray ionization mass spectrometry(ESI-MS).
1 Experimental
1.1 Instruments and reagents
The functional group of MOFs was characterized by Fourier transform infrared spectrometer(5DX FTIR,Nicolet Company,USA).The structure of MOFs was analyzed by X-ray diffractometer(PXRD,Rigaku′s D/max 2500,Rigaku Corporation,Japan)with CuKαradiation(λ=0.156 04 nm),the tube voltage was 40 kV,the tube current was 150 mA,a graphite monochromator was used,and 2θwas 5°to 50°.The elemental composition and valence state of MOFs were analyzed by X-ray photoelectron spectroscopy(XPS,ESCALAB250,Thermo Scientific,USA)with a base pressure of 1.333 22×10−7Pa.The light absorption behavior of MOFs was analyzed by UV-visible spectrophotometer with BaSO4as a reference(UV-2550PC,Shimadzu Corporation,Japan).The specific surface area of MOFs was analyzed by a fully automatic specific surface area and pore analyzer(Auto chem II2920,Micromeritics,USA).The surface charge of MOFs was analyzed by aζpotential analyzer(JS94H,Shanghai Zhongchen Digital Technology Equipment Co.,Ltd.,China).The photocatalytic degradation products were analyzed by electrospray ionization mass spectrometry(LCQ/AD,Thermo-Finnegan,USA).The pH value of solution was measured by a pH meter(PHS-25CW-CN,Shanghai Precision Instrument Co.,Ltd.,China).The photocatalytic reaction was carried out by using xenon lamp(5-100L,Shanghai Yanzheng Experimental Instrument Co.,Ltd.China).The hydrothermal synthesis of MOFs was carried out using an electric thermostatic blast drying oven(DHG-9145A,Shanghai Heheng Instrument Equipment Co.,Ltd.,China).
All reagents are purchased from Energy Chemical(Shanghai,China).They include P25,terephthalic acid(analytical grade),2,5-dihydroxyterephthalic acid(analytical grade),2-aminoterephthalic acid(analytical grade),Cr(NO3)3·9H2O(analytical grade),ZrCl4(analytical grade),anhydrous methanol and ethanol(analytical grade),formamide(analytical grade),acetone(analyti-cal grade),deionized water.
1.2 Synthesis of MIL-101(Cr)
The MIL-101(Cr)was synthesized by the method of reference[45].A mixture of terephthalic acid(0.166 1 g,1 mmol),Cr(NO3)3·9H2O(0.400 0 g,1 mmol)and sodium hydroxide(0.080 0 g,2 mmol)in a polytetrafluoroethylene reactor tank containing 15 mL of water.The mixture was stirred at room temperature for 15 min,then followed by the addition of 1 mL of hydrofluoric acid.The liner was then placed in a stainless steel sleeve and reacted at 180℃for 8 h.It was naturally cooled to room temperature after the reaction was completed.The reactor liner was opened,filtered,and collected the green solid.The green solid was ultrasonically washed three times with water,absolute ethanol,acetone,and anhydrous methanol,respectively.The green solid was dried in an oven at 80℃for 12 h.The yield of MIL-101(Cr)was about 55.1%(based on Cr).
1.3 Synthesis of MIL-101(Cr)-2OH
The synthesis method of MIL-101(Cr)-2OH was similar to the MIL-101(Cr).The 2,5-dihydroxyterephthalic acid(0.198 1 g,1 mmol),Cr(NO3)3·9H2O(0.400 0 g,1 mmol)and sodium hydroxide(0.080 0 g,2 mmol)were sequentially added to 15 mL of dimethylformamide.The mixture was placed in a 23 mL Teflon liner and stirred at room temperature for 15 min.The liner was then placed in a stainless steel sleeve and reacted at 180℃for 8 h.It was naturally cooled to room temperature after the reaction was completed.The reactor liner was opened,filtered,and collected the gray solid.The gray solid was ultrasonically washed three times with water,absolute ethanol,acetone,and anhydrous methanol,respectively.The gray solid was dried in an oven at 80℃for 12 h.The yield of MIL-101(Cr)-2OH was about 20.6%(based on Cr).
1.4 Synthesis of UIO-66-NH2
The reference photocatalyst UIO-66-NH2was synthesized by the method of the reference[46].ZrCl4(3.495 g,15 mmol)and 2-aminoterephthalic acid(2.715 g,15 mmol)were sequentially dissolved in 115 mL of DMF and dissolved by ultrasonication at room temperature for 5 min.The mixture were sealed in a stainless steel container after the mixed solution was uniformly dispersed,and heated in an oven at 120℃for 24 h.It was naturally cooled to room temperature after the reaction was completed.The liner was opened,the solvent was removed,and a pale yellow UIO-66-NH2was collected.The UIO-66-NH2was washed three times with 10 mL of DMF,methanol,acetone,and ethanol,respectively.The UIO-66-NH2was dried in a vacuum oven at 80℃for 12 h.The yield of UIO-66-NH2was about 85%(based on Zr).
1.5 Photocatalysis experiment
The photocatalytic properties of MIL-101(Cr)and MIL-101(Cr)-2OH were studied by using UIO-66-NH2and P25 as reference photocatalysts and methylene blue(MB)as organic pollutants.The photocatalytic reaction conditions were as follows:the dosage of photocatalysts was 0.010 0 g;the initial concentration of MB wasC0=5 mg·L−1;the pH value of the solution was 3~9;the amount of the solution was 50 mL;the photocatalytic reaction was carried out using an 800 W xenon lamp(A UV filter was added to the source to ensure removal the light of below 420 nm before the start of the photocatalytic reaction.At this time,the light irradiation intensity measured by the photometer was about 15 W·m−2).After the photocatalytic reaction was carried out for a certain period of time,the absorbance of MB in the solution was measured at 664 nm using an ultraviolet-visible spectrophotometer.The corresponding concentration of MB was calculated by the standard curvey=0.160 6x+0.000 7(R2=0.999 9).The photocatalytic degradation performance was evaluated by the change of MB concentration before and after the reaction.The degradation rate is calculated as follows:
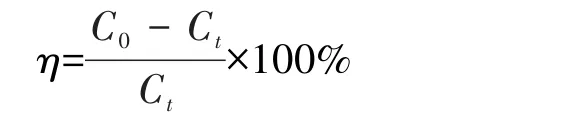
whereηis the degradation rate(%),andC0andCtare the concentration of MB before degradation and at a certain time of degradation,respectively(mg·L−1).
2 Results and discussion
2.1 Structural characterization of MIL-101(Cr)and MIL-101(Cr)-2OH

Fig.1 Synthesis and color of MIL-101(Cr),and MIL-101(Cr)-2OH
The MIL-101(Cr)and MIL-101(Cr)-2OH were successfully synthesized by hydrothermal method(Fig.1)when the terephthalic acid,2,5-dihydroxyterephthalic acid and Cr(NO3)3·9H2O were used as raw materials.It can be clearly seen from Fig.1 that the white powder of terephthalic acid(Fig.1a)reacted with the dark purple solid of Cr(NO3)3·9H2O(Fig.1b)react to form green powder MIL-101(Cr)(Fig.1d).However,the yellow powder of 2,5-dihydroxyterephthalic acid(Fig.1c)reacted with the dark purple solid of Cr(NO3)3·9H2O to form gray MIL-101(Cr)-2OH(Fig.1e).It can be seen that modifying the organic ligand can regulate the color change of MOFs.The structure of MIL-101(Cr)and MIL-101(Cr)-2OH are characterized by FT-IR,PXRD,XPS,and N2adsorption desorption isotherm.
2.1.1 IR
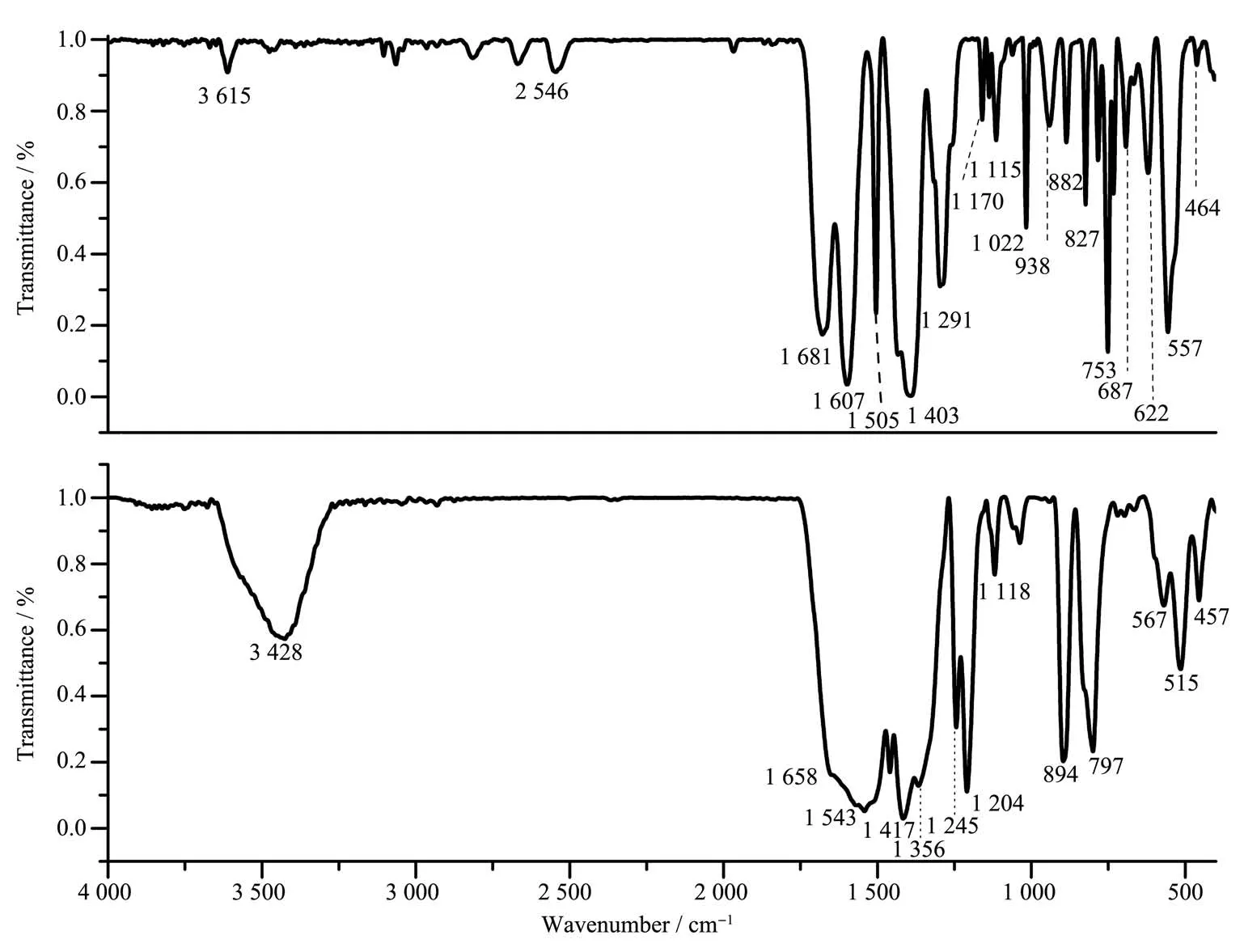
Fig.2 (a)IR of MIL-101(Cr)and(b)MIL-101(Cr)-2OH
The FT-IR characterization can be used to analyze and determine the functional groups contained in the material.The IR spectrum of MIL-101(Cr)(Fig.2a)shows that it has a strong absorption peak at 3 615 cm−1,which can be attributed to the tensile frequency vibration of intermolecular hydrogen bonds.In general,theν(C=O)characteristic absorption peak in the organic carboxylic acid was at 1 691 cm−1.However,in MIL-101(Cr),thisν(C=O)peak had shifted to low frequency of 1 681 cm−1.This phenomenon indicates that the O atom in terephthalic acid has been coordinated to Cr(Ⅳ).
When the-OH was introduced into MIL-101(Cr),the infrared characteristic peak of the-OH was also observed.In the IR spectrum of MIL-101(Cr)-2OH(Fig.2b),the IR absorption peak at 3 428 and 1 356 cm−1can be attributed to the characteristic absorption peak ofν(-OH);The absorption peaks at 1 658 and 1 204 cm−1can be attributed to the characteristic absorption peak ofν(C=O).These IR characteristic peaks indicated that 2,5-dihydroxyterephthalic acid had coordinated with Cr(Ⅳ)to form complex.
2.1.2 XRD
The above FT-IR characterization has shown that the organic ligand has coordinated to Cr(Ⅳ).However,the structure need to further analytical determination.The XRD can accurate analysis the structure of the material.In the XRD patterns of MIL-101(Cr)and MIL-101(Cr)-2OH(Fig.3),the main diffraction peaks are relatively strong and sharp.This phenomenon indicates that both MIL-101(Cr)and MIL-101(Cr)-2OH have a good crystal structure.It can be clearly seen from Fig.3 that the MIL-101(Cr)and MIL-101(Cr)-2OH had strong diffraction peaks at 5.83°,6.46°,7.08°,8.26°,8.96°,11.56°,12.27°,13.52°,19.94°,21.35°and 22.22°.The positions of these diffraction peaks are the same as those of the theoretical MIL-101(Cr)diffraction peak[45].This indicates that MIL-101(Cr)and MIL-101(Cr)-2OH have been successfully synthesized.
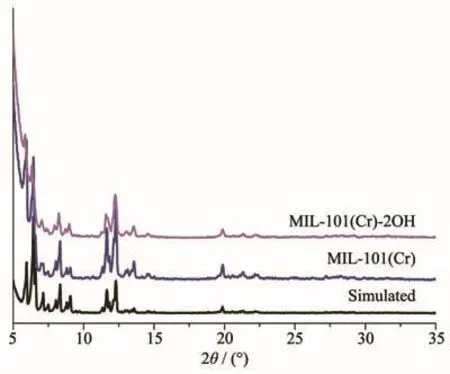
Fig.3 PXRD patterns of MIL-101(Cr)and MIL-101(Cr)-2OH
2.1.3 XPS
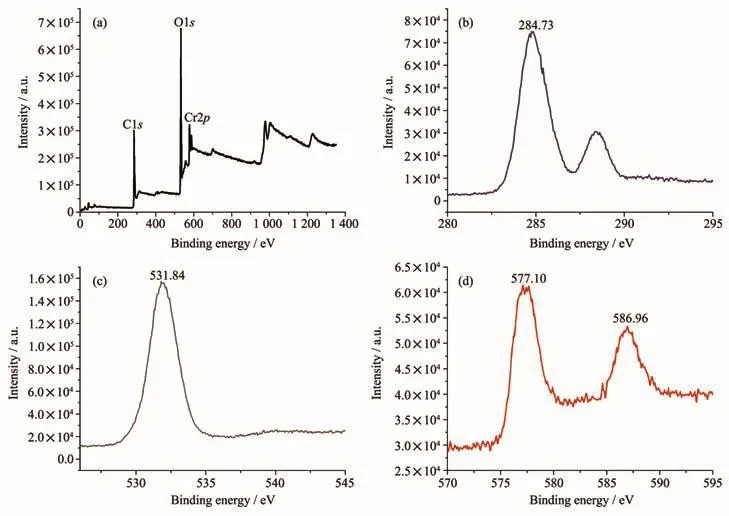
Fig.4 XPS spectra of MIL-101(Cr):(a)Survey spectrum;(b)C1s;(c)O1s;(d)Cr3d
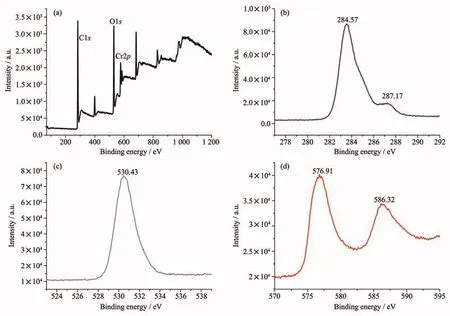
Fig.5 XPS spectra of MIL-101(Cr)-2OH:(a)Survey spetrum;(b)C1s;(c)O1s;(d)Cr3d
XPS can be used to characterize the elemental composition and elemental valence on the surface of the material.The XPS survey spectrum of MIL-101(Cr)(Fig.4a)shows that it mainly composed of C,O and Cr elements,which are consistent with those reported in the literature.The characteristic peaks of 284.73 and 288.52 eV are attributed to the C1sorbital in MIL-101(Cr)(Fig.4b);The characteristic peak of 531.84 eV is attributedtotheO1sorbitalinMIL-101(Cr)(Fig.4c);The characteristic peaks of 577.10 and 586.96 eV are attributed to the Cr3d5/2and Cr3d3/2orbitals of MIL-101(Cr)(Fig.4d),respectively.These characteristic peaks of MIL-101(Cr)are the same as those of the literature[45].
The XPS pattern of MIL-101(Cr)-2OH was similar to that of MIL-101(Cr).The XPS survey spectrum of MIL-101(Cr)-2OH(Fig.5a)shows that it mainly composed of C,O and Cr elements.The characteristic peaks of 284.57 and 287.17 eV are attributed to the C1sorbital in MIL-101(Cr)-2OH(Fig.5b).The characteristic peak of 530.43 eV is attributed to the O1sorbital in MIL-101(Cr)-2OH(Fig.5c).The characteristic peaks of 576.91 and 586.32 eV are respectively attributed to the Cr3d5/2and Cr3d3/2orbitals of MIL-101(Cr)-2OH(Fig.5d).
2.1.4 N2adsorption-desorption isotherm
The N2adsorption-desorption experiments at 77 K can be used to analyze materials,specific surface size and pore size distribution.The N2adsorptiondesorption experimental results of MIL-101(Cr)and MIL-101(Cr)-2OH(Fig.6)indicate that they were typical typeⅠadsorption isotherms,which are consistent with the literature[47].It can be seen from Fig.6 that the specific surface area of MIL-101(Cr)was larger than MIL-101(Cr)-2OH,and it can reach 3 690 m2·g−1.Since the MIL-101(Cr)-2OH has two-OH functional groups,its specific surface area still can reach 2 515 m2·g−1.The results of N2adsorption-desorption experiments of MIL-101(Cr)and MIL-101(Cr)-2OH also indirectly indicate that they have been synthesized.
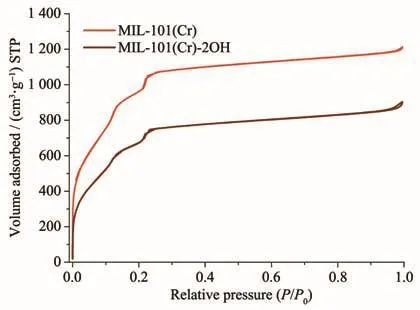
Fig.6 N2adsorption-desorption isotherms for MIL-101(Cr)and MIL-101(Cr)-2OH
2.2 UV-Vis DRS
The UV-Vis DRS of photocatalysts can be used to analysis its light absorption.The UV-Vis DRS of MIL-101(Cr)and MIL-101(Cr)-2OH showed that they had strong light absorption in both the UV and visible regions when the BaSO4was used as a blank control experiment(Fig.7).The MIL-101(Cr)-2OH absorbed more than 50%of light in the visible light range of 400~800 nm,but,the MIL-101(Cr)absorbed light less than 50%in the visible light range of 400~575 nm.So,the MIL-101(Cr)-2OH has stronger visible light absorption than MIL-101(Cr).Therefore,their photocatalytic properties can be studied under visible light conditions.
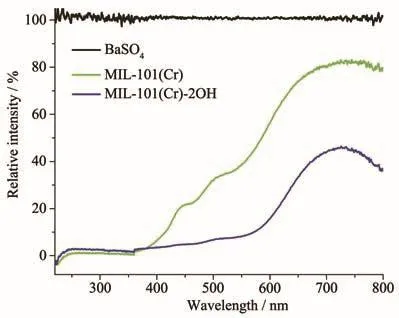
Fig.7 UV-Vis DRS of MIL-101(Cr)and MIL-101(Cr)-2OH
2.3 Photocatalytic performance
The UIO-66-NH2was constructed by Zr(Ⅳ)and 2-aminoterephthalic acid,which has been proven to be a very promising heterogeneous photocatalyst.It has stable structure,large specific surface area and visible light response.So,it has been extensively studied in hydrogen production and organic pollutants degradation[48-51].It can be used as a reference photocatalyst with P25.In this paper,the UIO-66-NH2and P25 are used as reference photocatalysts,and the MB is used as organic pollutants.The photocatalytic properties of MIL-101(Cr)and MIL-101(Cr)-2OH were studied in MB solution with pH=3~9.
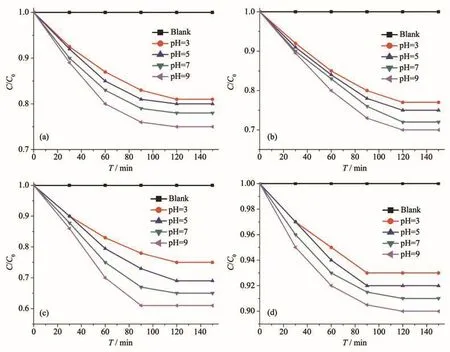
Fig.8 Photocatalysts adsorption of MB:(a)MIL-101(Cr);(b)MIL-101(Cr)-2OH;(c)UIO-66-NH2;(d)P25
However,since these MOFs photocatalysts have a large surface area,they can also remove MB in the solution by adsorption.Therefore,their photocatalytic properties were further evaluated by using adsorption control experiments(Fig.8).The adsorption experiments of MIL-101(Cr),MIL-101(Cr)-2OH,UIO-66-NH2,and P25 showed that their adsorption reached equilibrium after 120 min.The results of the adsorption experiments showed that the amount of MB adsorbed by these photocatalysts at pH=9 was greater than that at pH=3.And the adsorption amount follows UIO-66-NH2>MIL-101(Cr)-2OH>MIL-101(Cr)>P25.The results of photocatalytic degradation of MB by MIL-101(Cr),MIL-101(Cr)-2OH,UIO-66-NH2,and P25 indicate that they all have good properties(Fig.9).It can be seen from the results of photocatalytic MB that the photocatalytic reaction wasmore favorable underacidic conditions(Fig.9).Among them,at pH=3,the MIL-101(Cr)-2OH photocatalytic could degraded 50 mL of 5 m·L−1MB solution in 90 min,while the MIL-101(Cr)and UIO-66-NH2need 120 min.The inorganic semiconductor photocatalyst P25 just can only degrade 48%of MB under the above conditions.These photocatalysts photocatalytically degrade MB with pseudo first order kinetics under visible light illumination.This pseudo first-order kinetics is calculated as ln(C0/Ct)=kt(kis the reaction rate constant,Ctis the concentration of MB at timetin the photocatalytic reaction,andC0is the initial concentration of MB).According to the pseudo first-order kinetic model,at pH=3,the rate constant of photocatalytic degradation MB by MIL-101(Cr)-2OH,MIL-101(Cr),UIO-66-NH2,and P25 were 0.088 10,0.055 71,0.031 89,and 0.004 09 min−1,respectively(Fig.10).So,the photocatalytic degradation MB rate of MIL-101(Cr)-2OH was 1.6,2.8,and 21.5 times of MIL-101(Cr),UIO-66-NH2and P25,respectively.It can be seen that the photocatalytic performance of hydroxyl modified MIL-101(Cr)-2OH has significantly improved.The performance of MIL-101(Cr)-2OH photocatalytic degradation of MB was better than that reported in the literature of MIL-101(Cr)-NH2[52].Therefore,the functional groups on the organic ligands in the MOFs can be simply modified to improve the photocatalytic performance.
2.4 Recycling experiments
In order to evaluate the stability of hydroxyl-modified MOF MIL-101(Cr)-2OH,the photocatalytic degradation MB cycle experiment was carried out at pH=7.The efficiency of photocatalytic degradation of one cycle can still reach 100%(Fig.11).The efficiency of photocatalytic degradation of five cycles can still reach 98.5%.The photocatalysts after five cycles were col-lected by centrifugation,and were characterized by XRD(Fig.12).It was found that the structure of MIL-101(Cr)-2OH remained unchanged after five cycles.It can be seen that the hydroxyl modified MOFs MIL-101(Cr)-2OH also has good stability.

Fig.9 Photocatalytic degradation of MB by photocatalysts:(a)MIL-101(Cr);(b)MIL-101(Cr)-2OH;(c)UIO-66-NH2;(d)P25
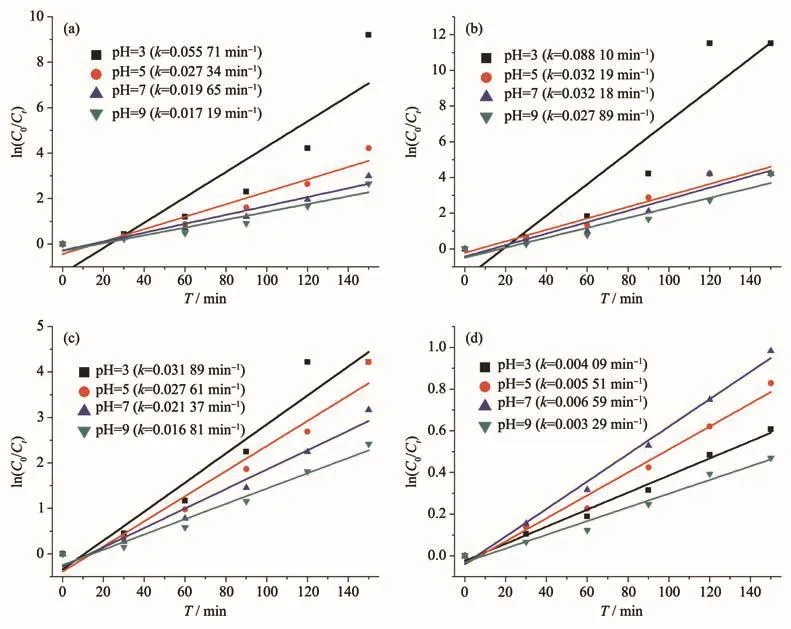
Fig.10 Kinetics of photocatalytic degradation of MB by photocatalyst:(a)MIL-101(Cr);(b)MIL-101(Cr)-2OH;(c)UIO-66-NH2;(d)P25
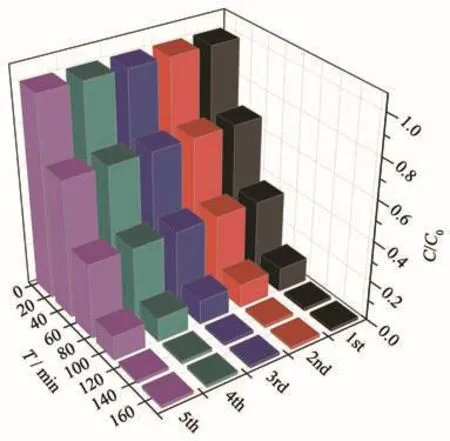
Fig.11 Circulation experiment of photocatalytic degradation of MB by MIL-101(Cr)-2OH at pH=7
2.5 Hydroxyl regulation mechanism
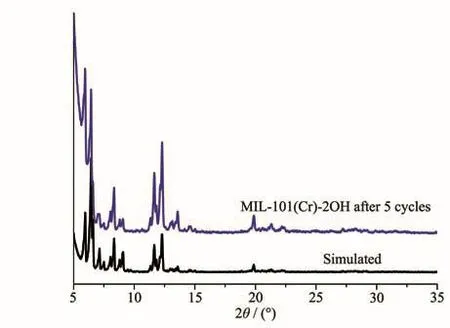
Fig.12 XRD patterns of MIL-101(Cr)-2OH after 5 cycles
The ability of photocatalysts to absorb light directly affects their photocatalytic efficiency.It is known from the synthesis of MIL-101(Cr)and MIL-101(Cr)-2OH that their structures are self-assembled by terephthalic acid,2,5-dihydroxyterephthalic acid and Cr(III),respectively.The UV-Vis DRS indicates that MIL-101(Cr)-2OH absorbed light more strongly than MIL-101(Cr).In addition to this,the rate of photocatalytic degradation MB by MIL-101(Cr)-2OH was 1.6 times that of MIL-101(Cr).Therefore,the MIL-101(Cr)-2OH,which is modified by hydroxyl groups can effectively improve the photocatalytic efficiency.This phenomenon can be attributed to the result of the transfer of electrons from the ligand to the metal(LMCT)[49,53].This is because the organic ligand of terephthalic acid and 2,5-dihydroxyterephthalic acid are composed of benzene ring,carboxyl groups,and hydroxyl groups,respectively.It is well known that there is a largeπbond on the benzene ring.Since the 2,5-dihydroxyterephthalic acid ligand has two hydroxyl groups,the hydroxyl group contains relatively high energy lone pair of electron pairs.Therefore,in MIL-101(Cr)-2OH,the isolated hydroxyl groups have relatively high energy lone pair of electrons,while Cr(Ⅲ)has lower energy empty orbit.The electrons on the hydroxyl group in 2,5-dihydroxyterephthalic acid can transfer part of the electrons to the benzene ring in the ligand byp-πconjugation,which leads to an increase in electronegativity on the benzene ring.Moreover,since the Cr(Ⅲ)in MIL-101(Cr)-2OH has a lower energy orbit,the electrons added to the benzene ring are transferred to the empty orbit of Cr(Ⅲ)(Fig.13).This result causes the charge transport spectrum to appear in the visible region and the complex can have distinct color change.
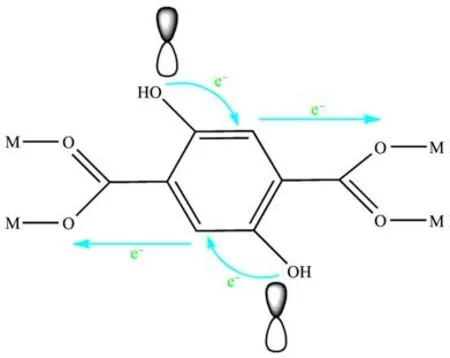
Fig.13 Hydroxylation regulates the electronegativity of MIL-101(Cr)-2OH
Theζpotential can be used to determine the stability of photocatalysts at a certain pH value and indirectly reflect the amount of charge on their surface.Theζpotentials of MIL-101(Cr)-2OH,MIL-101(Cr),UIO-66-NH2,and P25(Table 1)indicate MIL-101(Cr)-2OH had higherζpotential than MIL-101(Cr),UIO-66-NH2and P25 under the same conditions.This is because the MIL-101(Cr)-2OH had high specific surface area and contain carboxyl groups and hydroxyl groups.The carboxyl groups and hydroxyl groups are facilitate adsorption and enrichment of H+.Therefore,it can adsorb more H+on the surface than MIL-101(Cr),UIO-66-NH2,and P25 in solution with pH=3~9.Theζpotentials of MIL-101(Cr)-2OH,MIL-101(Cr),UIO-66-NH2,and P25 in pH=3 were 43.51,36.76,27.50,and 18.84 mV,respectively.The quantum efficiency of photocatalytic reactions was mainly related to the generation and capture process of photogenerated electrons.Therefore,an increase in the acidity of the surface of the photocatalyst,which will accelerates the transfer of photogenerated electrons from the conduction band to the surface.Therefore,under acidic conditions,the separation of photogenerated electrons and the suppression of electron-hole recombination are accelerated,which is advantageous for the photocatalytic reaction.Theζpotential of MIL-101(Cr)-2OH indirectly reveals that its rate of photocatalytic degradation of MB is faster than that of MIL-101(Cr),UIO-66-NH2,and P25.At the same time,it is also strongly proved that the introduction of hydroxyl groups into MOFs can effectively regulate the electronegativity of new MOFs.
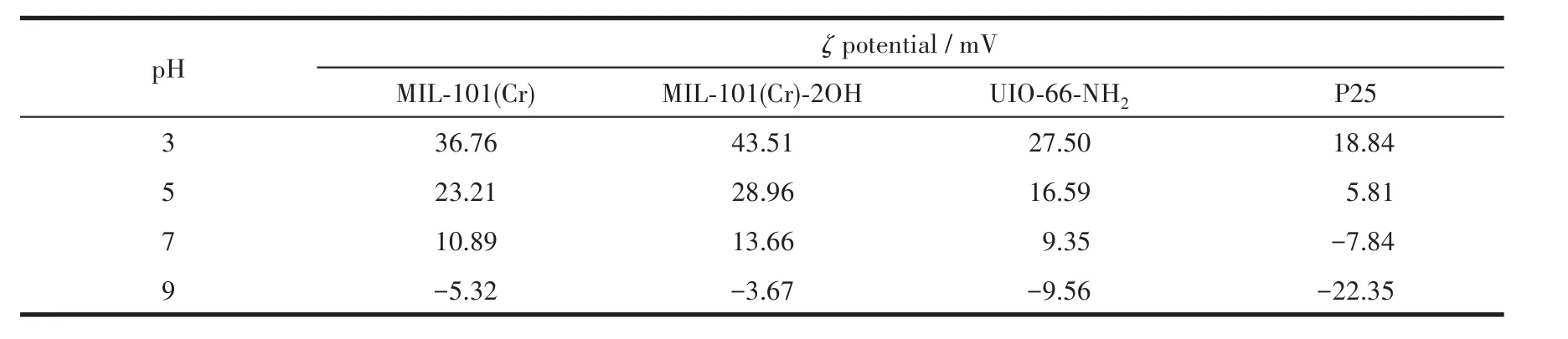
Table 1 ζ potential of MIL-101(Cr),MIL-101(Cr)-2OH,UIO-66-NH2,and P25
2.6 Photocatalytic degradation mechanism
At pH=3,the photocatalyst was removed by centrifugation after the MIL-101(Cr)-2OH photocatalyzed MB for 30 min,and the reaction solution was concen-trated in a sample vial.The concentrate solution was characterized by electrospray ionization mass spectrometry(ESI-MS).The ESI-MS spectrum results(Fig.14)show that there are four strong characteristic signals for[C16H18N3S]+,[C16H22N3SO]+,[C6H9N2SO8]+,[C4H6NO6]+.As can be seen from Fig.14 that the decomposition of the MB is performed in multiple steps(Fig.15).Firstly,the amide bond in the MB was broken to form an amino group,and the S obtained an O atom to from S=O bond;the methyl group,which attached to an N atom is oxidized to form a small molecular acid;subsequently,a small molecular acid of six C chains is formed by the electron attack the benzene ring;then,the six C chains are oxidized to 2-nitrosuccinic acid;finally,all organic molecules are completely mineralized to CO2and H2O.
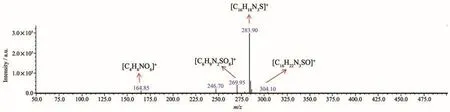
Fig.14 MB decomposition products characterizated by ESI-MS
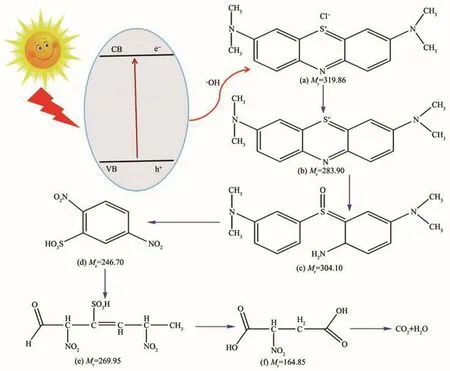
Fig.15 Mechanism of photocatalytic degradation MB by MIL-101(Cr)-2OH
3 Conclusions
In summary,the metalorganic frameworks(MOFs)photocatalysts,MIL-101(Cr)and MIL-101(Cr)-2OH,were synthesized and characterized by FT-IR,XRD and XPS.The effects of isolated hydroxyl groups in MOFs were systematically investigated by UV-Vis DRS,ζpotential and ESI-MS.The results show that the isolated electron-donating groups(-OH)can be introduced to modify the MOFs photocatalyst and improve its photocatalytic performance.The results of this study help to elucidate the mechanism of electron-donating groups regulate MOFs,and guide the synthesis of highly efficient photocatalysts.
