Effect of Thermal Ageing of Pd/Al2O3 Catalyst on the Selectivity of NH3 Formation
2020-07-08YAOLipengWANGChengxiongTANJianweiRENDezhiZHENGTingtingZHAOYunkun
YAO Li-peng, WANG Cheng-xiong, , TAN Jian-wei, REN De-zhi, ZHENG Ting-ting, ZHAO Yun-kun, *
Effect of Thermal Ageing of Pd/Al2O3Catalyst on the Selectivity of NH3Formation
YAO Li-peng1, WANG Cheng-xiong1, 2, TAN Jian-wei3, REN De-zhi1, ZHENG Ting-ting2, ZHAO Yun-kun1, 2 *
(1. State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals, Kunming Institute of Precious Metals, Kunming 650106, China;2. State-Local Joint Engineering Laboratory of Precious Metal Catalytic Technology and Application, Kunming Sino-platinum Metals Catalysts Co. Ltd., Kunming 650106, China; 3. School of Mechanical Engineering, Beijing Institute of Technology, Beijing 100081, China)
The effect of thermal aging on the selectivity of NH3products inthree-way catalytic reaction was studied. Steady-state experiments were carried out in dilute/rich circulating flow. X-ray diffraction (XRD), BET specific surface area, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy (Raman) and transmission electron microscopy (TEM) were used to characterize the physicochemical properties of the catalysts. The results showed that high temperature aging led to a decrease in the dispersion of palladium, an increase in particle size and a decrease in the relative proportion of the Pd2+active species. These microstructural changes contribute to the reduction of the three-way activity of catalysts, resulting in the increase in ammonia selectivity. Thermal ageing induces a significant increase in active palladium particles on the catalyst surface, and larger particles promotes NO to dissociate to form active nitrogen species, which in turn react with hydrogen to form NH3.
Pd/Al2O3catalyst; thermal ageing; NH3formation; three-way catalytic reactions(TWC); Pd particle
Automotive exhaust gases comprising of carbon monoxide (CO), nitrogen oxides (NO), and unburned hydrocarbons (HCs) contribute to global warming, ozone layer depletion, acid rain and environmental toxicity[1-2]. Three-way catalysts (TWCs), up to now, are the most satisfactory and efficient solution to diminish CO, NO, and HCs emissions from gasoline engines. The use of Pd as the only active metal component in TWC has received considerable attention on the basis of economical aspects (the high cost and scarcity of Rh). Pd-only three-way catalyst is used in the automotive industry to reduce harmful emissions that contain CO, NOand HCs[3-5], but leading to an increase in undesired NH3and N2O emissions[6-8]. Durbin et al.[9]reported that emission rates of NH3for a fleet consisted of light-duty trucks and light-duty passenger vehicles averaged 34 mg/km with a range of 2.5 to 110 mg/km, although the fleet of vehicles was certified at the ULEV standard for California. Heeb et al.[10]found that the average NH3emission rates were 16±12 mg/km and 10±7 mg/km for gasoline-fueled Euro-3- and Euro-4-passenger cars, respectively. NH3acts as a precursor of NH4+ion to form secondary inorganic aerosols, which reacts with nitrogen to form particulate matter (NH4NO3)[11-12].
Although the mechanisms of NH3formation during three-way catalytic reactions are complex, there are still some related mechanisms reported. For example, Mejía-Centeno et al.[5]suggested that hydrogen produced in the steam reforming (SR) and water gas-shift (WGS) reactions is a major contributor to NH3formation through the overall reaction of 2NO+2CO+3H2→2NH3+2CO2or NO+5H2→2NH3+ 2H2O. In addition, Adams et al.[13]found that Pd/Ce/Al2O3catalyst showed a higher selectivity of NH3formation, compared to that of Pd/Al2O3catalyst, owing to the promotional effects of ceria on SR and WGS reactions. As reported by Durbin et al.[14], bench rapid ageing lead to an increase in the amount of NH3emissions, according to the vehicle testing results. Our group[11]also proposed the mechanisms of hydroxyl- mediated NH3formation on the Rh-CeO2catalyst surface. In the presence of water, water-induced hydroxylation could promote NH3formation, whereas the competitive adsorption of H2O and NO on the same sites would inhibit the reactivity of NO reduction by NH3, leading to an increase in NH3emission.
In this study, we used Pd-only catalyst supported by-Al2O3. This is because the decreased oxygen storage capacity (OSC) of TWC induced by ageing will result in activity loss of NH3oxidation under rich conditions[12]. In order to study the influence of OSC-free catalyst structure changes caused by ageing on the selectivity of ammonia, the generation and structure characterization of Pd/Al2O3catalyst after ageing in the three-way catalytic reaction process were carried out, providing a basis for NH3emission control.
1 Experimental
1.1 Catalyst preparation
Pd/Al2O3(1.0%, by weight) catalyst was prepared on incipient wetness impregnation method. Pd(NO3)2(Kunming Institute of Precious Metals 24%) and γ-Al2O3(Aladdin Industrial Corporation, 20 nm) were used as the starting materials. Al2O3powder was dispersed in 450 mL demonized water. Then Pd(NO3)2solution was added under continuous agitation. Dipping mixture and stirring at room temperature for 5 h, then drying overnight at 140°C. After calcination at 600°C in static air for 5 h, the fresh catalyst was obtained, followed by aged at 800°C and 1000°C in static air for 5 h, respectively.
1.2 Catalyst characterization
The specific surface areas of samples were measured using the N2adsorption at -196°C by the Brunauer-Emmett-Teller(BET) method on an automatic surface analyzer (Quantachrome Nova- 2200e). Prior to the measurements, the samples were degassed under vacuum at 200°C for 2 h.
The Pd dispersion on Pd-containing catalyst was determined by CO chemisorption at 100°C in a ChemBET Pulsar. Prior to each CO measurement, the catalyst was heated to 450°C in Argon for 60 min. After cooling to 100°C, the CO adsorption experiment was performed using CO pulse method. A ratio of CO/Pd was used for the amount calculation of Pd atoms on the surface. Actual content of Pd was analyzed on PerkinElmer Optima 8000 ICP-OES instrument. The total Pd atoms were calculated from actual content of Pd in catalyst.
The X-ray diffraction (XRD) analysis of sample was performed on an X’Pert Pro diffractmeter (Japan) using Cu Kradiation. The operating voltage is 36 kV. And the working current is 30 mA. The data for step scanning XRD were recorded at 0.02ointervals in the range 10o≤2≤80o.
The XPS experiments were performed on a spectrometer (Thermo Fisher Scientific) equipped with a monochromatic Al KX-rays under ultra-high vacuum (2×10-7mba). Sample charging during the measurement was compensated by an electron flood gun. The binding energy was calculated internally by the carbon deposit C1s binding energy (BE) at 284.8 eV.
Transmission electron microscopy (TEM) images were taken on a JEM-2100 transmission electron microscope with an accelerating voltage of 200 kV.
Raman measurements were carried out on a Renishaw Raman 2000 system spectrometer with a spectral resolution of 1 cm-1. The acquisition was run for a 10 s exposure time for collection of a single spectrum with a 532.5 nm laser excitation source after 30 min of stabilization at each temperature step. Before the measurement, the Raman tool was calibrated with a crystalline Si wafer with the characteristic peak observed at 519.6 cm-1. A low laser power of 0.1 mW (power density of 8 μw/μm2) on the sample was used in all measurements to avoid laser heating without using any neutral density filters. The experimental spectra were analyzed with theoretical models that include phonon interactions and thermal expansion effects and possible phonon decay channels identified.
Temperature-programmed reduction of the samples in hydrogen was carried out in a flow installation with a thermal conductivity detector (TCD) using the powder catalyst fraction. Prior to the TPR experiments, the samples were treated in an Ar steam for 1 h at 350°C and cooled down to 10°C in this condition. The mass of the samples was 100 mg. TPR was carried out in a 75 mL/min flow of the reducing mixture containing 10% H2in Ar. The samples were heated in this flow from 10°C to 800°C with 10°C/min of heating rate. The consumption of H2was detected by TCD.
1.3 Catalytic activity measurement
The three-way catalytic reactions on Pd/Al2O3catalyst were employed in a continuous flow fixed-bed micro-reactor. 500 mg catalysts with an average diameter 40~60 mesh were placed in a stainless-steel tube with an inner diameter of 5 mm. The catalysts were activated at 550°C in 21% O2/N2stream for 30 min. Steady-state experiments were conducted in a lean/rich cycling (1.67×10-2Hz) stream with total flow rate of 500 mL/min and the space velocity (Sv = 60000 mL·g-1·h-1) at every 25°C from 175°C to 550°C. Rich condition: 0.1%CO, 0.05%NO, 0.1%C3H6, 8%CO2, 5%H2O, 0.1%O2, N2balance. Lean condition: 0.1%CO, 0.05%NO, 0.1%C3H6, 8%CO2, 5%H2O, 0.86%O2, N2balance. The composition of the effluent gas was analyzed online using a 2030DBG2EZKS13T MultiGas FTIR Analyzer. The conversion rate of CO,(CO, %) was calculated by following Eq. (1):
(CO, %) ={[CO]in-[CO]out}/[CO]in×100% (1)
Where [CO]inand [CO]outrepresent the concentration of the injected and effluent CO gas, respectively. The values of NO and C3H6conversion rate were calculated by the same method. The selectivity of NH3and N2O,(N2O, %),(NH3, %), were calculated by the Eqs. (2)~(3) based on the con- centration of NH3and N2O products, respectively[15].
(N2O, %)={[NO]in-[NO]out}/2[N2O]out×100% (2)
(NH3, %)={[NO]in-[NO]out}/[NH3]out×100% (3)
(N2, %)=100-SN2O-SNH3(4)
2 Results and discussion
2.1 XRD and BET surface area
Fig.1 shows XRD patterns of different catalysts. The XRD pattern of the fresh catalyst exhibits the characteristic peaks of-Al2O3and there is no obvious diffraction peaks of PdO and Pd. However, the diffraction peak of PdO becomes sharpened clearly after ageing at 800°C, which indicates that the size of Pd particles increased after ageing[16]. Increasing ageing temperature to 1000°C results in a slight decomposition of PdO to metallic Pd, evidence in line at 2= 40.1°[17-19], and a sharper diffraction peak of PdO than that of 800°C-aged catalyst, which illustrates occurrence of Pd particles with increased size . And it shows a poor Al2O3phase transformation from γ-Al2O3to θ-Al2O3according to the XRD pattern of the 800°C-aged samples. However, the clear diffraction peaks of α-Al2O3and θ-Al2O3phase can be seen after ageing at 1000°C.

(1). Fresh(新制); (2). 800°C-aged (800°C老化); (3). 1000°C-aged(1000°C老化)
The values of BET surface area (BET) of the samples are displayed in Tab.1. As listed in Tab.1, the surface area of fresh catalyst is 187.7 m2/g, while the catalysts aged at 800°C and 1000°C have lower surface area, and their surface area are 157.1 m2/g and 112.7 m2/g, respectively. The decrease in specific surface area induced by high ageing temperature is related to alumina transformation[20]. The crystal structure of γ-Al2O3will change at higher ageing temperature. The inner reticular structure is destroyed, resulting in the internal voids collapse or close, an increase in the crystal particles, and smaller specific surface area. At this time, the γ-Al2O3will produce phase trans- formation, form θ-Al2O3or α-Al2O3. This result is consistent with XRD patterns. The Pd metals are dispersed on the surface of γ-Al2O3and γ-Al2O3is converted into θ-Al2O3or α-Al2O3at high temperature, so most of the catalysts are covered by a thin Al2O3layer in the crystal structure and can not be contacted and utilized by gas, and the catalysts are basically deactivated.
Tab.1 Textual properties obtained from N2adsorption and Pd dispersion of catalysts

表1 不同催化剂的N2吸附和Pd分散度性质
Detected by ICP-OES analysis;
Calculated by assuming that CO adsorbs stoichiometrically to surface Pd atoms;Based on formulap= 1.12/(nm), whererepresents Pd dispersion.
2.2 XPS and Raman
XPS was used to determine the chemical states of the active Pd species in catalysts. Fig.2 shows Pd 3d XPS spectra. The peaks centered at 341.0 eV, 335.9 eV, 342.2 eV and 337.0 eV in Pd 3d XPS spectra of catalyst samples can be assigned to Pd03d3/2, Pd03d5/2,Pd2+3d3/2and Pd2+3d5/2, respectively[21-23].
The values of surface Pd2+/(Pd0+Pd2+) in different catalysts were obtained from the integral area of the spectral components in Pd03d5/2and Pd2+3d5/2and given in Tab.2. A slight decrease in surface Pd2+/(Pd0+Pd2+) of 1000°C-aged catalyst indicates the formation of metallic Pd, which is well agreement with XRD results.

(1). Fresh(新制); (2). 800°C-aged (800°C老化); (3). 1000°C-aged(1000°C老化)
Tab.2 XPS data of various samples

表2 不同样品的XPS数据
Obtained from the integral area of the spectral components in Pd03d5/2and Pd2+3d5/2.
Structural information of the catalysts is also measured by Raman. The Raman spectra are given in Fig.3. All samples shows a peak centered at 645 cm-1, illustrating the existence of PdO phase[24-26]. Moreover, the additional weak peaks at about 277, 339 and 567 cm-1are also derived from the PdO phase[26]. The result is well consistent with the results of XRD and XPS.

(1). Fresh(新制); (2). 800°C-aged (800°C老化);(3). 1000°C-aged(1000°C老化)
2.3 TEM and Pd dispersion
Fig.4 shows TEM images of catalysts. The size of Pd particle in fresh catalyst is with average dimensions of 3 nm. As the ageing temperature increased to 800°C, the Pd particle size slightly increased, reaching ~4 nm. It can be seen that the size of Pd particle in 1000°C- aged sample is about 18 nm, indicating significant sintering and agglomeration of Pd particle induced by ageing at 1000°C. As displayed in Tab.1, the Pd dispersion of fresh and 800°C-aged samples is 37.4% and 28.0%, respectively, and the corresponding Pd particle sizes are 3.0 nm and 4.0 nm. While the Pd dispersion of 1000°C-aged sample is 6.1% and the particle size is 18.4 nm. The calculation results coincide with the TEM results. The results show that after high thermal ageing treatment the Pd particle has a significant growth.
2.4 H2-TPR
The reducibility of the catalysts was investigated by means of H2-TPR and the results are displayed in Fig.5. As is well known to us all is that the reduction of PdO species usually occurs at relatively low temperature[27], which is below 200°C as shown in Fig.5. All catalysts exhibit TPR profiles with one main reduction peak which can be assigned to the reduction of PdO to Pd metal. However, the formation of palladium hydrides on the high dispersed Pd is considerably suppressed. It indicates that larger crystallites of metallic Pd appears on the aging catalyst and the dispersed of active PdO decreases during ageing. As shown in Fig.5, the reduction peaks shift to higher temperature and the H2consumption values show obvious increase for 1000°C-aged sample. It demonstrates that the amount of active PdO species on the catalyst decreases at high ageing temperature.
2.5 Catalytic performance
Three-way catalytic reactions are of great importance in automobile exhaust purification. In this work, three-way catalysis is carried out using a typical fixed-bed flow reactor in a lean/rich cycling (1.67× 10-2Hz) stream. The typical steady state C3H6, NO and CO conversion curves as a function of temperature under the switch of lean and rich feed conditions in three-way catalytic reactions are shown in Fig.6. It can be seen that fresh and 800°C-aged catalysts have almost similar conversion trend of C3H6, NO and CO. At low temperature, CO and NO are not converted due to self-inhibition[28]. As the temperature increases from ~200 to 275°C, the CO and NO conversions sharply increase to maximum. The NO and CO conversion rates decrease with further increments in reaction temperature, owing to the fluctuation of O2concentration and the partial oxidation by C3H6[15].

Fig.4 TEM images of different samples

Fig.5 Temperature programmed reduction of various Pd/Al2O3 catalysts
C3H6+4.5O2→3CO2+3H2O (5)
C3H6+3O2→3CO+3H2O (6)
In contrast, the C3H6conversion reaches a 100% maximum at ≈325°C. However, the great differences are reflected in the 1000°C-aged catalyst. The conversion rates of C3H6, NO and CO on 1000°C-aged catalyst peaks at 425°C (100%), 390°C (75%) and 275°C (95%), respectively.
As the three-way catalysis, NO is reduced to N2, as well as the undesirable secondary products, N2O and NH3[5].
NO+H2→0.5N2+H2O (7)
NO+CO→0.5N2+CO2(8)
NO+2.5H2→NH3+H2O (9)
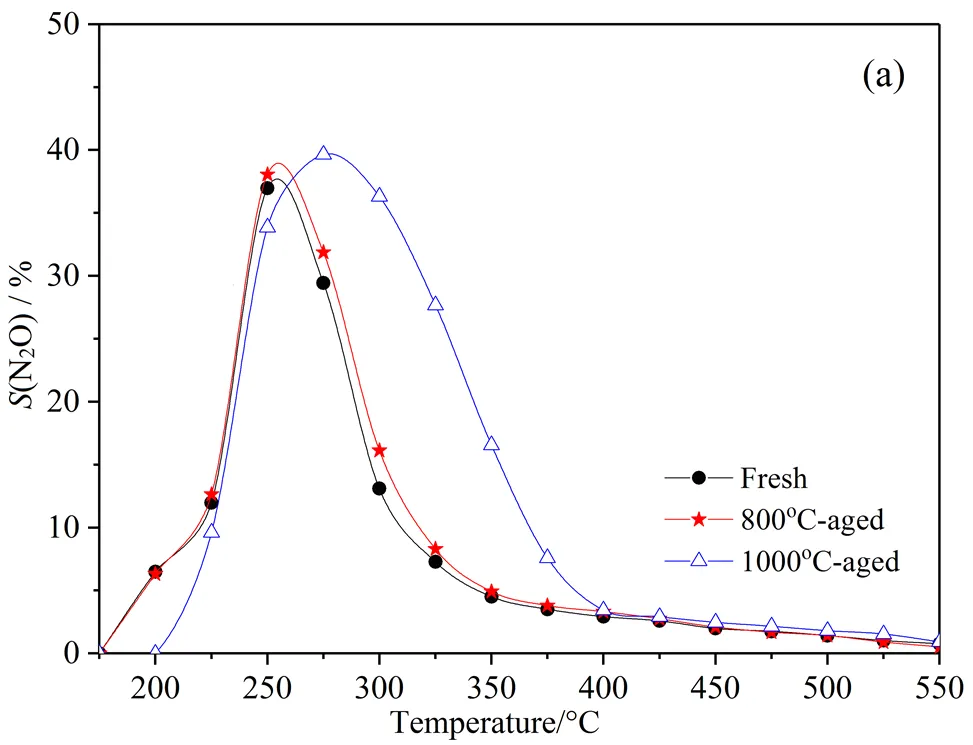
Fig.7 shows the selectivity curves of N2O and NH3formed on the fresh and aged catalysts. It can be seen that the proportion of N2O and NH3is a strong function of the temperature and the ageing temperature. The decreasing N2O selectivity (Fig.7(a)) is due in part to a declining availability of NO because of the increase of NO desorption rate. That N2O is the highest at low temperature is a result of the lower rate of N-O bond scission, making NO available to react through N-NO or NO-NO coupling[29].

N2O formation sharply decreases at higher temperatures due to the increased selectivity to N2(Fig.7(c)), a result of an increased rate of N-O bond scission at higher temperatures, minimizing the presence of NO availablility for N-NO coupling, and yielding N-N coupling instead. Other oxidation reactions can occur, including NH3oxidation to N2O and NO:
NH3+O2→0.5N2O+1.5H2O (10)
NH3+1.25O2→NO+1.5H2O (11)
In this study, N2O is mainly detected below 400°C and the selectivity of N2O rises to ≈38% approximately at 260°C for fresh and 800°C-aged catalysts and then decreases with further reaction temperature increments, whereas the selectivity curve of N2O formed 1000°C-aged catalyst shows maximum of ≈42% at 275°C and higher selectivity with the increased reaction temperature compared to that of other catalysts. N2O formswithin the same temperature range for cars under standard driving conditions, observing N2O emissions between 250 and 450ºC[9, 14]. Under lean steady state conditions, the precious metal sites are occupied by an admixture of O, NO and N species. N2O can form through the reaction[30].
NH3starts to form at 200ºC and shows a peaks centered at 225ºC, due to hydroxyl-mediated NH3formation[11]. As shown in Fig.6(b), the increasing conversions of CO, NO and C3H6result in a shift to NH3as a main N containing product. And NH3is main secondary product as the temperature is raised above 300ºC, showing the similar results with literature reported by Mejía-Centeno et al.[5]in this case by reduction of NO by C3H6as well as hydrogen generated from SR and WGS reactions[15].
CO+H2O→CO2+H2(12)
C3H6+6H2O→3CO2+9H2(13)
A significant difference in selectivity NH3formed on different catalysts starts to be observed above 275ºC, and the NH3selectivity increases in following order: fresh catalyst <800ºC-aged catalyst <1000ºC- aged catalyst. Adams et al.[13]found that addition of ceria promotes WGS activity to form more hydrogen, which makes higher amount of NH3formed. Nevalainen et al.[12]emphasized that reduced oxygen storage capacity induced by thermal ageing will lead to an increase in amount of formed NH3, due to the activity loss of NH3oxidation.
However, in this case, the increase in the selectivity of NH3formed on Pd/Al2O3catalyst caused by thermal ageing is closely related to significant growth of Pd particle. On the basis of the results of XRD, BET, XPS, Raman and Pd dispersion, it demonstrates that the amount of active PdO species on the supported Pd catalyst decreases after calcination at high ageing temperature. NO dissociation (elementary reaction (14)) is thought as the rate-determining step for NO reduction and occurs more readily on larger Pd particle to generate active ·N species (so-called “structure-insensitive reaction”)[31]. As-generated ·N species will react with ·H species to form NH3, as the following elementary reaction (15).
NO→·N+·O (14)
·N+3·H→NH3(15)
3 Conclusions
The study showed that, compared with the fresh catalyst, the Pd particle size of 800°C-aged catalyst grew from 3 nm to 4 nm, and reached 18 nm when it was aged at 1000°C. Raman diagram is also clearly illustrated that the growth of Pd particle size. It also can be obtained from the analysis of the integral area of the optical component, the ratios of Pd2+were 80.8%, 79.4% and 76.5%, respectively. Thus, the number of catalytic active sites decreased. The XRD figure shows that the phase change of γ-Al2O3.The transformation leads to the decrease of the BET surface area, which has effect on the catalytic performance of the catalyst. NH3as the main toxic by-products, with the increase of aging temperature, the selectivity of NH3increased significantly. After aged at 1000°C, the selectivity of NH3increased to 53%.
[1] WILLIAMSON W B, PERRY J, GANDHI H S, et al. Effects of oil phosphorus on deactivation of monolithic three-way catalysts[J]. Applied catalysis, 1985, 15(2): 277-292.
[2] BRANDENBERGER S, KROCHER O, TISSLER A, et al. The state of the art in selective catalytic reduction of NOby ammonia using metalaexchanged zeolite catalysts[J]. Catalysis reviews, 2008, 50(4): 492-531.
[3] LAN L, CHEN S, LI H J. et al. Optimized synthesis of highly thermal stable CeO2-ZrO2/Al2O3composite for improved Pd-only three-way catalyst[J]. Materal design, 2018, 147: 191-199.
[4] LAN L, LI H, CHEN S, et al. CeO2-ZrO2-Al2O3modified by selective doping with SrO for improved Pd-only three-way catalyst[J]. Russia journal of physics chemistry, 2018, 92(4): 696-705.
[5] MEJIA-CENTENO I, CASTILLO S, FUENTES G A. Enhanced emissions of NH3, N2O and H2from Pd-only TWC and supported model catalysts: Light-off and sulfur level studies[J]. Applied catalysis B: Environmental, 2012(119/120): 234-240.
[6] KEAN A J, BAN-WEISS D L A, HARLEY R A, et al. Trends in on-road vehicle emissions of ammonia[J]. Atmospheric environment, 2009, 43(8): 1565-1570.
[7] ISIDRO MEJIA-CENTENO, ANGEL MARTINES- HERNANDEZ G A. Effect of low-sulfur fuels upon NH3and NO emission during operation of commercial three-way catalytic converters[J]. Topics in catalysis, 2007, 43(1/4): 381-385.
[8] SUTTON M A, DRAGOSITS U, TANG Y S, et al. Ammonia emissions from non-agricultural sources in the UK-source testing results[J]. Atmospheric environment, 2000, 34(6): 855-869.
[9] DURBIN T D, WILSON R D, NORBECK J M, et al. Estimates of the emission rates of ammonia from light-duty vehicles using standard chassis dynamometer test cycles[J]. Atmospheric environment, 2002, 36(9): 1475-1482.
[10] HEEB N V, BRIIHLMANN S. Trends of NO, NO2, and NH3emissions from gasoline-fueled Euro-3- to Euro-4-passenger cars[J]. Atmospheric environment, 2008, 42(10): 2543-2554.
[11] WANG C X, XIA W Z, ZHAO Y K. New insight into hydroxyl-mediated NH3formation on the Rh-CeO2catalyst surface during catalytic reduction of NO by CO[J]. Chinese journal of catalysis, 2017, 38(8): 1399-1405.
[12] NEVALAINEN P, KINNUNEN N M, KIRVESLAHTI A, et al. Formation of NH3and N2O in a modern natural gas three-way catalyst designed for heavy-duty vehicles: The effects of simulated exhaust gas composition and ageing[J]. Applied catalysis A: General, 2017, 552: 30-37.
[13] ADAMS E C, SKOGLUNDH M, CARLSSON P A. Ammonia formation from nitric oxide over Pd-based catalysts in multicomponent feed gas compositions[J]. Catalysis communications, 2017, 95: 26-30.
[14] DURBIN T D, PISANO J T, YOUNGLOVE T, et al. The effect of fuel sulfur on NH3and other emissions from 2000-2001 model year vehicles[J]. Atmospheric Environment, 2004, 38(17): 2699-2708.
[15] WANG C X, ZHENG T T, LU J, et al. Three-way catalytic reactions on Rh-based catalyst: Effect of Rh/ceria interfaces[J]. Apply catalysts A: General, 2017, 544: 30-39.
[16] HIETIKKO M, LASSI U, KALLINEN K, et al. Effect of the ageing atmosphere on catalytic activity and textural properties of Pd/Rh exhaust gas catalysts studied by XRD[J]. Applied catalysis A: General, 2004, 277(1): 107-117.
[17] XU W, ZHAN Z, DI L, et al. Enhanced activity for CO oxidation over Pd/Al2O3catalysts prepared by atmospheric-pressure cold plasma[J]. Catalysis today, 2015, 256: 148-152.
[18] HAN Y, GU G, SUN J, et al. Selective hydro- dechlorination of 1,2-dichloroethane to ethylene over Pd-Ag/Al2O3catalysts prepared by surface reduction[J]. Applied surface science, 2015, 355: 183-190.
[19] LIU W W, FENG Y S, WANG G Y, et al. Characterization and reactivity of γ-Al2O3supported Pd-Cu bimetallic nanocatalysts for the selective oxygenization of cyclopentene[J]. Chinese chemical letters, 2016, 27(6): 905-909.
[20] KOLLI T, LASSI U, RAHKAMAA-TOLONEN K, et al. The effect of barium on the catalytic behavior of fresh and aged Pd-Ba-OSC/Al2O3catalysts[J]. Applied catalysis A: General, 2006, 298(1): 65-72.
[21] BRUN M, BERTHET A, BERTOLINI J C. XPS, AES and Auger parameter of Pd and PdO[J]. Journal of electron spectroscopy & related phenomena, 1999, 104(1/3): 55-60.
[22] PEUCKERT M. XPS study on surface and bulk palladium oxide, its thermal stability and a comparison with other noble metal oxides[J]. Cheminform, 1985, 16(39): 2481-2486.
[23] SU S C, CARSTENS J N, BELL A T. A study of the dynamics of Pd oxidation and PdO reduction by H2and CH4[J]. Journal of catalysis, 1998, 176(1): 125-135.
[24] CAO Y, RUI R, WU X, et al. Comparative study of ageing condition effects on Pd/Ce0.5Zr0.5O2and Pd/Al2O3catalysts: Catalytic activity, palladium nanoparticle structure and Pd-support interaction[J]. Applied catalysis A: General, 2013, 457(4): 52-61.
[25] GRAHAM G W, O'NEILL A E, UY D, et al. Observation of strained PdO in an aged Pd/ceria-zirconia catalyst[J]. Catalysis letters, 2002, 79(1/4): 99-105.
[26] IGLESIAS-JUEZ A, MARTINEZ-ARIAS A, FERNANDEZ-GARCIA M. Metal-promoter interface in Pd/(Ce,Zr)O/Al2O3catalysts: effect of thermal aging[J]. Journal of catalysis, 2004, 221(1): 148-161.
[27] YUN G, LU G, ZHANG Z, et al. Preparation of CeZr1-O2(=0.75, 0.62) solid solution and its application in Pd-only three-way catalysts[J]. Catalysis today, 2007, 126(3): 296-302.
[28] VOLTZ S E, MORGAN C R, LIEDERMAN D, et al. Kinetic study of carbon monoxide and propylene oxidation on platinum catalysts[J].Industrial & engineering chemistry product research and development, 1973, 12(4): 294-301.
[29] BURCH R, SHESTOV A A, SULLIVAN J A. A steady-state isotopic transient kinetic analysis of the NO/O2/H2reaction over Pt/SiO2catalysts[J]. Journal of catalysis, 1999, 188(1): 69-82.
[30] XU J, CLAYTON R, BALAKOTAIAH V, et al. Experimental and microkinetic modeling of steady-state NO reduction by H2on Pt/BaO/Al2O3monolith catalysts [J]. Applied catalysis B: Environmental, 2008, 77(3): 395-408.
[31] TANAKA K I, HIRANO H. Isolation of intermediate compounds of catalytic reactions on single crystal surfaces[J]. Catalysis letters, 1992, 12(1/3): 1-6.
热老化对Pd/Al2O3选择性催化NH3反应性能的影响
姚丽鹏1,王成雄1, 2,谭建伟3,任德志1,郑婷婷2,赵云昆1, 2 *
(1. 昆明贵金属研究所 稀贵金属综合利用国家重点实验室,昆明 650106;2. 昆明贵研催化剂有限责任公司 贵金属催化技术与应用国家地方联合工程实验室,昆明 650106;3. 北京理工大学 机械与车辆学院,北京 100081)
研究了在三效催化反应过程中热老化对Pd/Al2O3选择性催化NH3反应性能的影响。在稀/富循环流中进行稳态实验,采用X射线衍射(XRD)、BET比表面积、X射线光电子能谱(XPS)、拉曼光谱(Raman)和透射电镜(TEM)对催化剂的物理化学性质进行表征。结果表明,高温热老化导致活性金属钯的分散度降低、颗粒尺寸增大、Pd2+活性物种的相对比例下降,这些微结构变化促使催化剂的三效活性降低、氨选择性增加。热老化诱导催化剂表面活性钯颗粒明显增大,较大的钯颗粒有助于NO解离生成活性氮物种,进而与氢反应生成NH3。
Pd/Al2O3催化剂;热老化;NH3生成;三效催化反应(TWC);Pd颗粒
O643.3
A
1004-0676(2020)01-0001-09
2019-05-16
国家自然科学基金(51676017, 21862010)、云南省科技人才和平台计划项目(2018IC091)、云南省重大科技专项(2018ZE017)
姚丽鹏,男,硕士研究生,研究方向:贵金属催化剂。E-mail:ylpppp@163.com
赵云昆,男,研究员,研究方向:汽车尾气后处理技术。E-mail:yk.zhao@spmcatalyst.com
