Fundamentals about onset and progressive disease character of type 2 diabetes mellitus
2020-07-06RobNMWeijers
Rob NM Weijers
Rob NM Weijers, Teaching Hospital, Onze Lieve Vrouwe Gasthuis, Amsterdam 1090,Netherlands
Abstract
Key words: ATP; Free fatty acid; Glucose transporter; Membrane flexibility; Metformin;Slow-down principle; Type 2 diabetes; Unsaturation index
INTRODUCTION
Nine years after Bantinget al[1]extracted insulin from a dog's pancreas, Faltaet al[2]introduced the term “insulin resistance” in the medical literature. Based on clinical and biochemical observations, they differentiated hyperglycemia in two distinct types: A form where a relative modest dose of insulin the metabolic disorder completely compensated, referred to as “Insular Diabetes”, and a form where a vast amount of insulin is well tolerated without causing a hypoglyceamia, referred to as“Insulinresistenter Diabetes”. They defined the concept that insulin resistance may be one of the main causes of type 2 diabetes[2]. Five years later Himsworth[3]confirmed the principle of insulin resistance.
Since then, insulin resistance as a term describing the relative ineffectiveness of insulin was commonly used. However, MacBryde[4]wrote already in 1933: “There is as yet no general agreement as to its definition”, but this remark did not come into general acceptance not even after almost 90 years. MacBryde is still a voice crying in the wilderness. A short, incomplete summary proves that MacBryde's remark is right up to the present day (Table 1)[5-12]. One of the aims of this study is to demonstrate that the recurrent phrase “insulin resistance” is an imaginary reality,i.e. a reality in which everyone believes, and as long as this collective belief exists this imaginary reality exerts power in the world.
GLUCOSE EFFECTIVENESS AND INSULIN SENSITIVITY
From the late 1990s, the advent of the minimal model for estimating the glucose metabolism in man has opened a new chapter to evaluate MacBryde's remark. The minimal model approach analyses the relationship between the pattern of insulin response and the rate of glucose decline to infer the sensitivity of tissues to insulin.Additionally, the model measures a relevant, less well recognized, factor called glucose effectiveness. This term describes the ability of glucose,per se, independent of changes in insulin concentration, to stimulate its own up-take by a mass action effect and to suppress its own release. Despite the one-compartment optimized minimal model overestimates the net glucose effectiveness, the results of this model as well as the two-compartment minimal model, which use computer modeling of glucose and insulin kinetics after intravenous glucose challenge, indicated that individuals with type 2 diabetes and individuals in the prediabetic phase had significantly lower values of both glucose effectiveness and insulin sensitivity compared to healthy controls (Table 2)[13-16]. So what this means is that insulin sensitivity (SI) is positively related to glucose effectiveness (SG). Moreover, a prospective study on the development of type 2 diabetes in normoglycaemic offspring of couples, who both had type 2 diabetes, showed significant defects in both SGand SI,i.e., more than 10years before the development of the disease, participants who developed the disease had lower values, compared to controls, of SI[(3.2 ± 2.4vs8.1 ± 6.7) ×10-3·L·min-1·pmol-1insulin;P< 0.0001] and SG[(1.6 ± 0.9vs2.3 ± 1.2) × 10-2·min-1;P<0.0001][15].
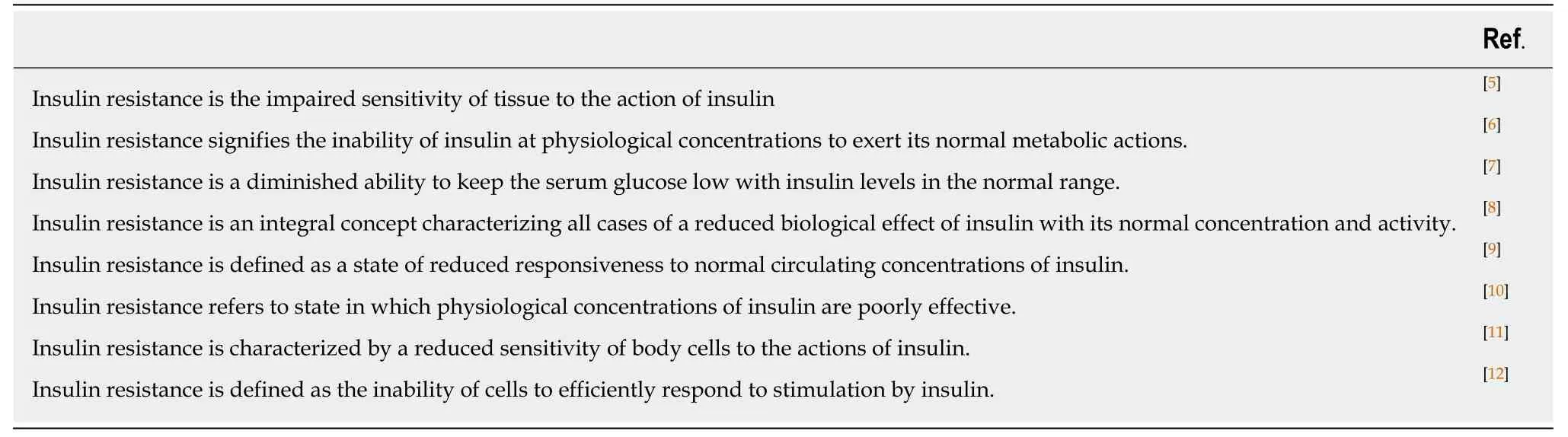
Table 1 Short summary of different definitions of the term: lnsulin resistance
To circumvent the limitations of the one-compartment minimal model, we used SGand SIvalues of stable isotope-labelled glucose data obtained from the twocompartment minimal model for estimation the relative contribution of SGto the glucose restoration rate during a basal state. In the basal state, healthy individuals have insulin oscillations with a regular 14-min periodicity of amplitude of 1.8 mU/L[17]. Hence, the relative contribution of SGto the glucose restoration rate during the basal state is given by SG/(SG+ SI× 10.8)[18]. The calculated data demonstrate that almost the total fractional glucose turnover of type 2 diabetes during the basal state results from the ability of glucose to stimulates its own uptake,i.e., 89.2% (Table 3).The essence of this outcome is the rather limited impact of insulin on the reduction in glucose effectiveness, which already appears in the prediabetic phase. Of note, the reduction in SIis essentially greater than the reduction in SGin type 2 diabetes and its prediabetic phase, independent of the assay methods (Table 2).
The results mentioned in Table 2 touch a fundamental problem: If the insulinsensitivity regulation system of an individual in his prediabetic or diabetic phase is unable to respond efficiently to an increase in the glucose level, what affects in this person thenon-insulin-sensitive regulation system,i.e., the reduction in the ability of glucose, per se, to stimulate its own up-take by a mass action effect and to suppress its own release? The question is: Is there a single, all-encompassing biochemical system between both the reduction in glucose effectiveness and insulin sensitivity? Up until now, the answer to this fundamental question cannot be found in any textbook, but is given in this review.
CELL MEMBRANES
Shulmanet al[19]resolved in part the questions raised in the previous section by studying muscle glycogen synthesis in subjects with type 2 diabetes and matched controls by means ofin vivocarbon-13 nuclear magnetic resonance spectroscopy[19-21].They demonstrated that the muscle glycogen synthesis rate in subjects with type 2 diabetes was about 50% of the rate observed in healthy controls. The same group also investigated, under hyperglycaemic-hyperinsulinaemic conditions, the pathway:Transmembrane glucose transport into the muscle cell, conversion of intracellular glucose into glucose-6-phosphate, andviatwo more intermediates to glycogen synthase, which adds glucose to the glycogen polymer. They concluded that the results are consistent with the hypothesis that transmembrane glucose transport is the rate-controlling step in insulin-stimulated muscle glycogen synthesis in patients with type 2 diabetes and the delivery of insulin is not responsible for the insulin resistance.Based on these results, two options arise for explaining the significantly reduced glycogen synthesis rate in subjects with type 2 diabetes relative to healthy controls:First, type 2 diabetes is characterized by a reduction in the amount of glucose transporter 4 (GLUT4) per cell surface area, or second, a change in the threedimensional (3D) structure of GLUT4, which affects the amount of transmembrane glucose-transport. Northern blot and slot blot study results of biopsies of skeletal muscles obtained from individuals with type 2 diabetes and age-matched and bodyweight-matched healthy controls indicated that there was no significant alteration in the level of GLUT4 mRNA and GLUT4 protein in individuals with type 2 diabetescompared to healthy controls. GLUT1 mRNA and protein concentrations were also not significantly different in individuals with type 2 diabetes compared to control subjects[22,23]. This excludes the first option. To demonstrate the second option plays a pivotal role in the onset of type 2 diabetes, we must enter the area of cell membranes.
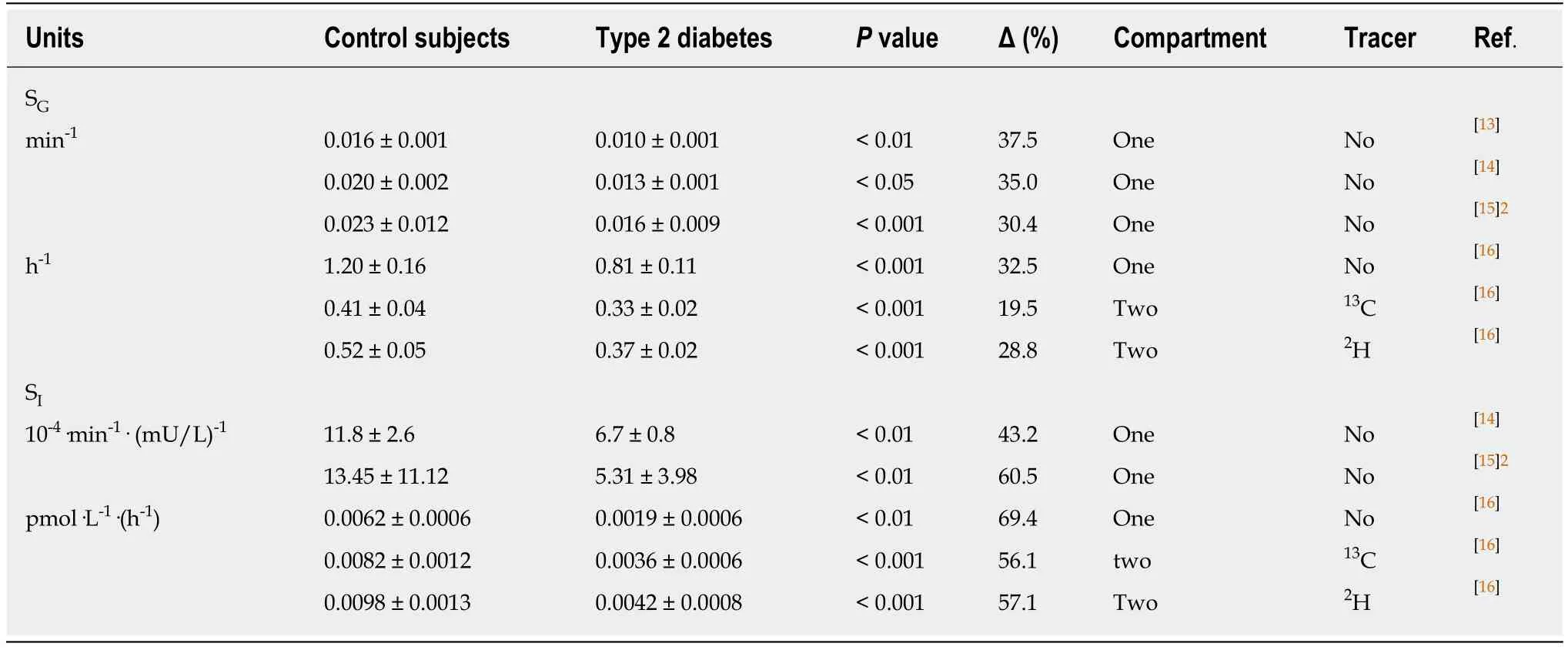
Table 2 Values of glucose effectiveness and insulin sensitivity1 for minimal models
Phospholipid bilayers form rapidly and spontaneously when phospholipids are added to water. Mammalian phospholipids contain a 1,2-diacylglycerol backbone that has a phosphate group esterified at carbon atom 3, and generally a saturated fatty acid(FA) esterified at carbon atom 1, and a saturated, monounsaturated or polyunsaturated FA esterified at carbon atom 2. The two acyl chains yield a roughly cylindrical molecule that can easily pack in parallel arrays to form extended sheets of membranes composed of a mosaic of proteins and phospholipids in a fluid phospholipid matrix[24]. The driving force of this aggregation is the weak, noncovalent bond (van der Waals force) between pairs of carbon atoms, lying next to each other in the carbon 1 and the carbon 2 acyl chains. The most structural result obtained from Xray scattering analyses of oriented bilayers in artificial phospholipid membrane systems is the area (A) per lipid molecule. This area denotes the cross-section of the cylindrical space occupied by a phospholipid. Various studies of fully hydrated, fluid phase, model phosphatidylcholine bilayers have demonstrated that introducing one or more carbon-carboncisdouble bonds into saturated acyl chains will increase the cross-section area A (Table 4)[25-28]. The advantage of this type of artificial bilayer model is its flexibility, the ability to bend or to be bent easily without breaking.
Based on the published data, we are able using the Lennard-Jones equation: U =(11.5 × 10-6)/r12- (5.96 × 10-3) /r6to estimate roughly the interaction energy between a pair of carbon atoms, which lie next to each other in the phospholipid acyl chains,esterified at the 1- and 2-positions of glycerol (Table 4)[29]. For instance, a comparison of the cross-section area (A) of an artificial bilayer consisting of dimyristoylphosphatidylcholine [(C14:0;C14:0)PC; area (A) = 60.6 (Å)2] with the crosssection area (A) of an artificial bilayer consisting of palmitoyl-docosahexaenoic phosphatidylcholine [(C16:0; C22:6)PC; area (A) = 74.8 (Å)2] reveals that the crosssection area (A) of the latter increases by 23.4%, which results in an increase in the interchain carbon-carbon distance of 11.2%, and thereby reduces with 37.6% the interaction energy per pair of acyl chain carbon atoms. In other words, a reduction in carbon-carbon double bonds in phospholipid acyl chains results in a reduction in membrane flexibility[30]. More generally, the flexibility of polyunsaturated phospholipids along the membrane normal (zdirection) might soften various mechanical stresses in the membrane[31]. In other words, the number of double bonds(= unsaturation) in the acyl chains of phospholipids influences the physical properties of cellular membranes.
The degree of membrane flexibility is expressed in unsaturation index (UI) (number of carbon-carbon double bonds per 100 acyl chains) of membrane phospholipids, as observed in erythrocytes. For information about the analytical details of the lipid extraction from erythrocytes, the fatty acid analysis by gas chromatography and the calculation of UI see section Supplementary material. The UI is a variable unit; a valueon the right of the reference interval (the range of values that is deemed normal for a physiologic measurement in healthy persons) means an acyl composition of phospholipids with an increased number of carbon-carbon double bonds, whereas a value on the left of the reference interval means an acyl composition of phospholipids with a decreased number of carbon-carbon double bonds. Borkmanet al[5]demonstrated in the phospholipid fraction of avastus lateralismuscle biopsy of healthy man that insulin sensitivity was positively correlated with the percentage of arachidonic acid in muscle (r= 0.76,P< 0.01), the total percentage of C20-C22 polyunsaturated fatty acids (r= 0.76,P< 0.01), and the unsaturation index (r= 0.62,P< 0.05).
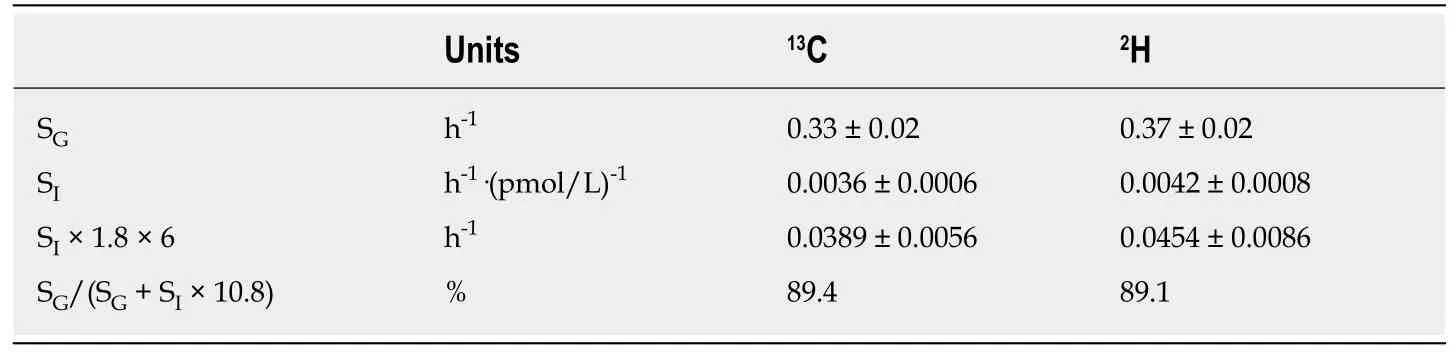
Table 3 Two-compartment minimal model analysis of the relative contribution of glucose effectiveness to the glucose restoration rate of type 2 diabetes during basal state
The consequence of the aforementioned data is that a reduction in UI is associated with a reduction in insulin sensitivity, which suggests that changes in the acyl composition of tissue phospholipid membranes modulates the action of insulin.Borkmanet al[5]suggested that if the action of insulin depends on the acyl composition of muscle membranes, it may be due to interactions within membranes specifically involved in the action of insulin, although a more general effect of membranes cannot be excluded. However, not only a reduction in insulin-mediated glucose disposal is a marker of type 2 diabetes, but also a reduction in non-insulin-mediated glucose disposal (Table 2).
It is interesting to note that the experimental results of Minet al[32,33]demonstrated that a key feature of the prediabetic phase, which appears in individuals with impaired glucose tolerance and women with gestational diabetes mellitus, is an essential reduction, compared to healthy controls, in the percentage of phospholipid poly-unsaturated acyl chains, including UI (Table 5, Supplementary Tables 1 and 2)[32,33]. Because phospholipid bilayers with an acyl composition of more carbon-carbon double bonds are more flexible than those with less carbon-carbon double bonds(Table 4), a reduction in membrane flexibility is a key factor regarding the increase in the plasma glucose concentration in type 2 diabetes and its prediabetic phase[34,35].
Variations in the acyl composition of phospholipid membranes can strongly influence the function of proteins embedded therein[36]. The biochemical and physical background of this mechanism is a reduction in UI, which is equivalent to a reduction in carbon-carbon double bonds of membrane phospholipids and results in a reduction of area A of the lipid molecules (Table 4). A reduction in area A translates into increased attractive forces between the mutual phospholipid acyl chains, which forms a redistribution of the lateral pressure in cell membranes. As a consequence, the redistribution induces a cross-sectional contraction of all Class I GLUT proteins,which in turn, causes a reduction in the amount of transmembrane transported glucose (Figure 1). This important hypothesis is in line with observations presented in biophysical and structural studies, which indicate that interactions of membrane proteins with lipid molecules are critical to their folding and stability[37-39]. This is what evolution is all about: Communicate with the environment and react to changes in the most efficient way[40].
The high glucose environment in women with gestational diabetes mellitus disappears after birth, in conjunction with the raised levels of free fatty acids (FFAs).So, the reduction in membrane flexibility is reversible and the restoration of UI will repair the amount of transmembrane glucose-transport. This is a nice example of how science works in unexpected ways. This generally unknown system of glucose regulation through changes in the acyl composition of phospholipids works fully independent of the insulin level and represents a beautiful unification of insulin sensitivity with glucose effectiveness. I will argue later on that this glucose-regulation system is effective in type 2 diabetes.
Several studies published UI values and supported its utility as a crucial parameter for membrane flexibility, even if still not properly considered by the scientific medical community[41]. It is interesting to note that the elegant study of Borkmanet al[5]sumsup the idea that the acyl composition of skeletal-muscle phospholipids may influence the action of insulin; unfortunately, this idea turns out not to be true. The author's idea is that in type 2 diabetes a redistribution of the lateral pressure in cell membranes results in a reduction in the cross-sectional area of all class I GLUTs, and thereby reduces the transmembrane glucose-transport.
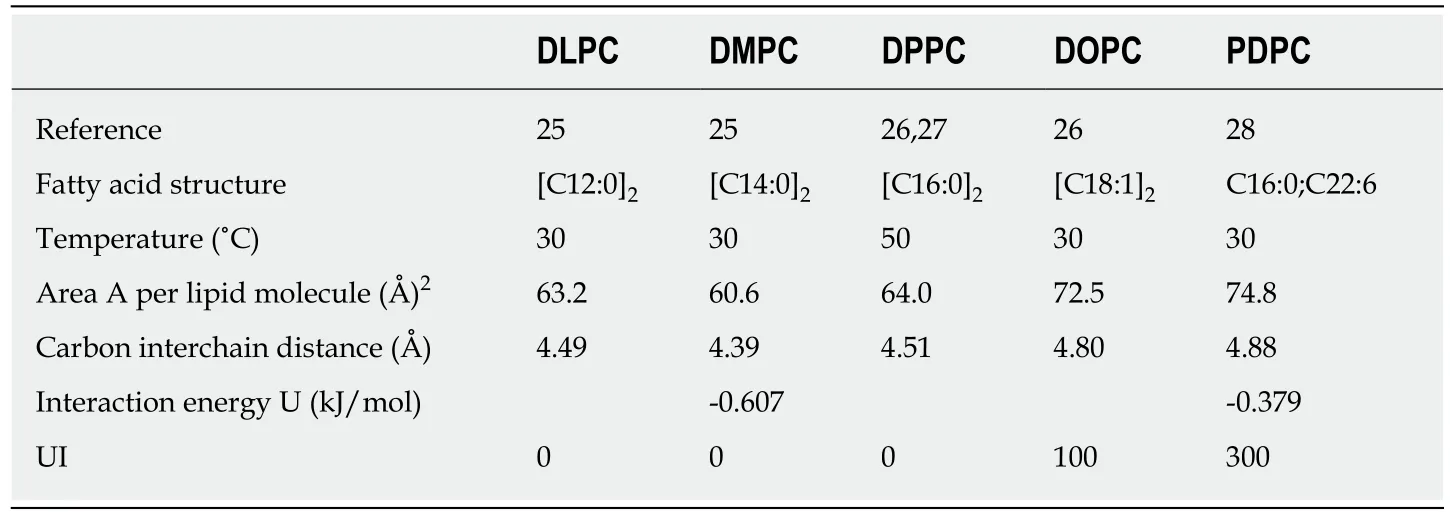
Table 4 Experimental data of fully hydrated fluid phase phosphatidylcholine lipid bilayers
MEMBRANE FLEXIBILITY
Membrane flexibility plays an important role in the scientific understanding of the molecular nature of life. Its role is evident in type 2 diabetes for at least three reasons.
It first affects the insertion of GLUTs into a plasma membrane. GLUT1 is a monomeric protein with 12 transmembrane helical segments[42]. The transporter protein traverses the plasma membrane 12 times in a zigzag fashion before initiating the folding, which is essential for creating its final 3D structure. A central channel across the protein communicates the extracellular and intracellular environments.Several amino acid residues of GLUT1, crucial for transport of β-D-glucose, bound a channel segment of approximately 15 Å long and 7 Å wide. In the proposed structure of GLUT3 the 12 transmembrane helices form a right-hand barrel with a central pore,which is shaped like a funnel with dimensions of approximately 5-6 Å by 8 Å at its narrowest point[43]. To get an idea of the dimensions: The dimensions of an orthorhombic bisphenoidal α-glucose crystal are: a = 10.36 Å, b = 14.84 Å, and c = 4.97 Å[44]. One GLUT1 molecule with a mean cross-section area of about 1100 Å2covers an area of about 17 phospholipid molecules of a phosphatidylcholine bilayer with saturated acyl chains[27]. So the folding mechanism of a GLUT1 molecule requires flexibility of the cell membrane for achieving a correct 3D structure. In contrast,GLUT4 is inserted into membranes of intracellular vesicles, which demands flexibility of the vesicular membrane. After the insulin-stimulated translocation of the intracellular GLUT4-containing vesicles to the plasma membrane, the GLUT4 containing vesicles take part in a fusion process with the plasma membrane. In the final stage of this process, fusion proteins induce bending of the plasma membrane bilayer to drive fusion pore formation. This includes that in type 2 diabetes the transmembrane glucose transport through non-insulin sensitive glucose transporters is being hampered solely by a reduction in the plasma cell membrane flexibility,whereas the transmembrane glucose transport through the insulin sensitive glucose transporter GLUT4 is being hampered by a reduction in the flexibility of two membranes,i.e. the vesicular membrane and the plasma membrane[45,46]. For this reason, the reduction in SIis essentially greater than the reduction in SGat type 2 diabetes (Table 2).
Next, membrane flexibility is associated with atherosclerosis, a condition where arteries become narrowed and showed an increase in vascular stiffness[47]. The erythrocyte membrane is compositionally very similar to the vascular endothelium, a thin layer of cells that keeps arteries smooth and allows blood to flow easily[48]. In support of this, the UI of red cell membrane phospholipids of healthy controls was found to be 155.4[49], and the reported UI of cultured endothelial cells from human umbilical cord veins was found to be 148.2 ± 6.3[50]. So we may suggest that the UI is a highly sensitive sensor for cellular membrane functionality,i.e. if the erythrocyte membrane is affected in type 2 diabetes, then the endothelium may also be affected[51,52]. An amazing example of endothelial dysfunction is presented in a WatchWebMD Video, entitled “How atherosclerosis plaque forms”[53]. This video underlines that although the exact causes of atherosclerosis are not yet clear, many scientists think that plaque formation begins with damage of the endothelium. The author's idea is that individuals with a low UI (increased membrane stiffness) are more prone to develop atherosclerotic cardiovascular disease, compared to healthy controls.
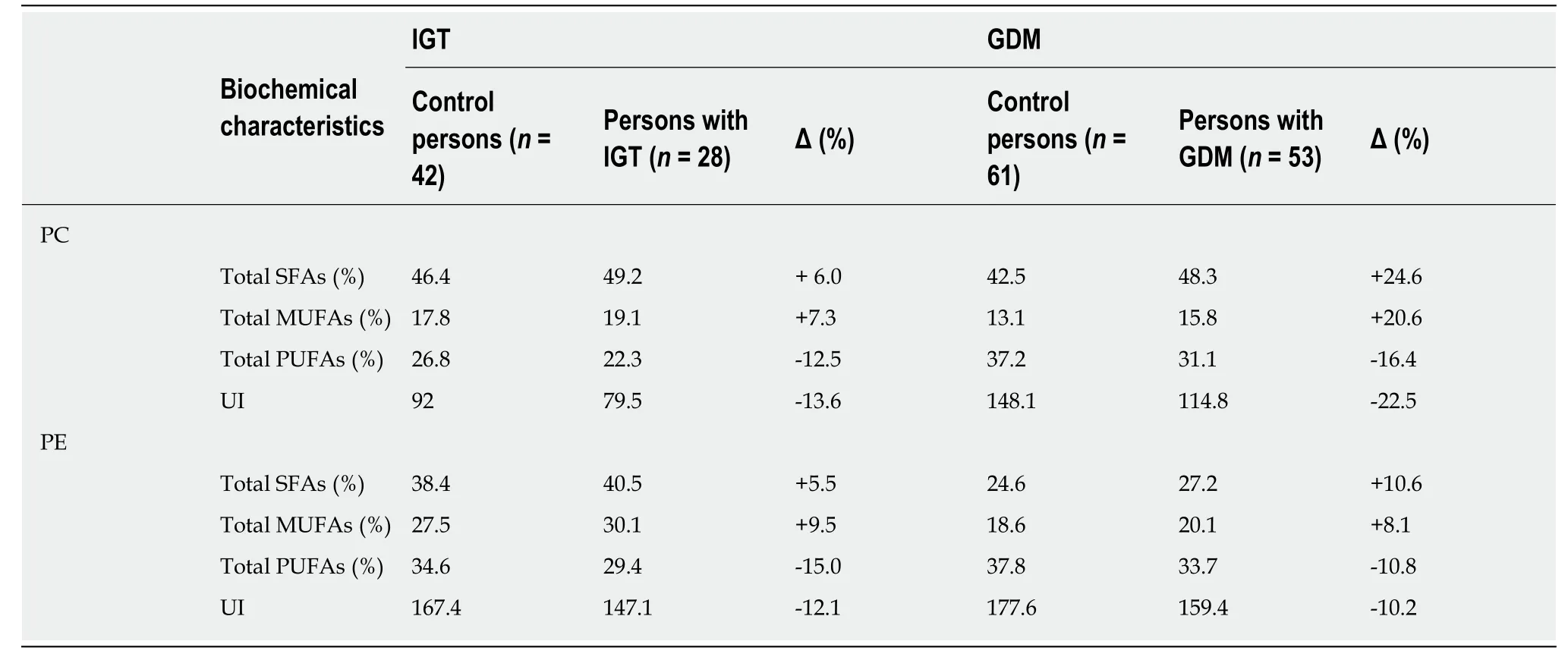
Table 5 Erythrocyte acyl composition of phospholipids and unsaturation index of control individuals, individuals with impaired glucose tolerance, and individuals with gestational diabetes mellitus1
Finally, membrane stiffness induces tissue hypoxia[54]. The minimum lumen of capillary vessels is about 4-9 μm, and the size of erythrocytes is approximately 8 μm.In the case of type 2 diabetes, the reduction in flexibility of both the erythrocyte membrane and the endothelium has a profound impact on the microcirculation[55]. The resulting decrease in blood flow leads to a reduction in oxygen supply of the surrounding tissues. Because the electrons of the respiratory chain are finally donated to molecular oxygen to form H2O, a status of hypoxia results in an accumulation of electrons in the respiratory complexes, which finally results in the production of the negatively charged superoxide radical O2·-(the dot means a single unpaired electron), and thereby reduces, among other things, ATP synthesis[56]. Life can only exist by the grace of ATP synthesis. The aforementioned reduced ATP synthesis could be the principle reason that type 2 diabetes is linked to lower life expectancy[57]. The paragraph entitled “Energy transport” later on goes into more detail about the influence of type 2 diabetes on the production of ATP.
EFFECTS OF TEMPERATURE ACCLIMATION ON PHOSPHOLIPID MEMBRANE COMPOSITION
The adaptation of an integrated bilayer system to an environmental factor such as temperature is referred to as homeoviscous adaptation. Studies with regard to this theme consistently reported that cold acclimation generates an increase in polyunsaturation of cell membranes of aquatic organisms[58,59]. To keep a flexible and effective membrane at low temperatures, aquatic organisms actualize an increased concentration of unsaturated acyl chains in phospholipids relative to those at warmer temperatures (Table 6, Supplementary Table 3)[60]. It is important to note that the homeoviscous adaptation is a reversible process and exists as early as the beginning of the Ordovicium, about 500 to 400 million years ago.
Hanssenet al[61]reported that 10 d of cold acclimation (14-15 ˚C) markedly increased peripheral insulin sensitivity by about 43% in eight type 2 diabetes subjects. Cold acclimation resulted in an enrichment of GLUT4 at sarcolemma, which facilitated the uptake of glucose. The GLUT4 translocation could not be explained by AMPK activation or improved insulin signaling. Presumably, the authors were unaware that cold acclimation generates an increase in polyunsaturation of cell membranes, which increases membrane flexibility and reduces the cross-sectional contraction of the area A of all Class I GLUT proteins.
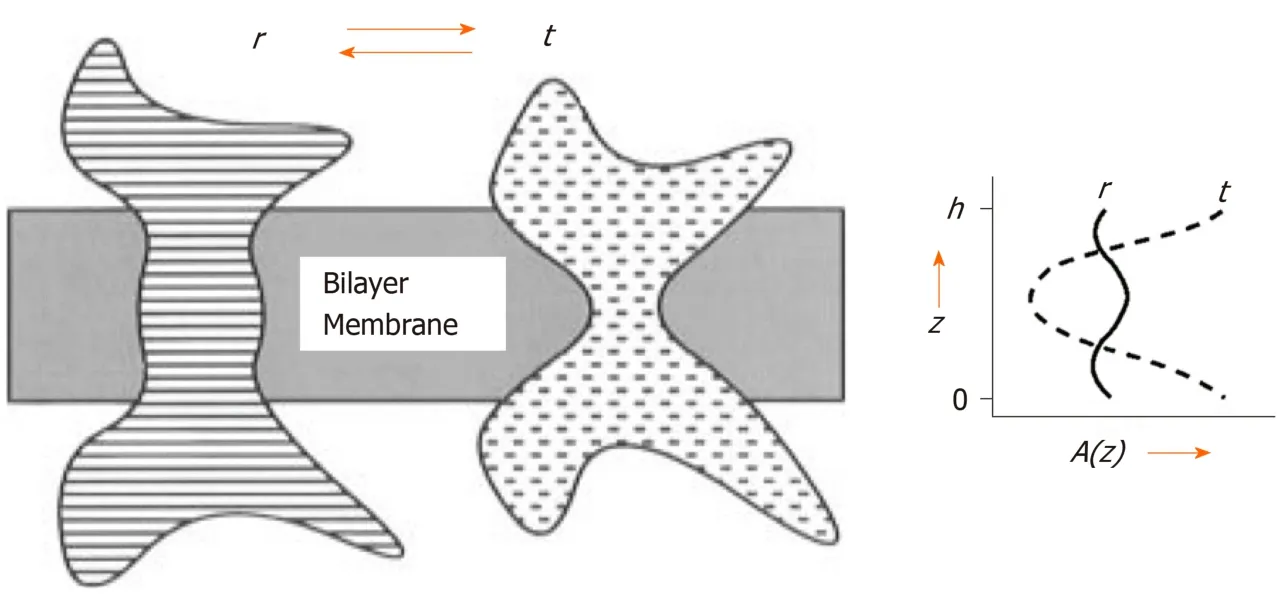
Figure 1 Slice through a bilayer membrane containing an intrinsic protein viewed in two different conformational states, r and t.
A telling example of homeoviscous adaptation is a form of evolutionary heat acclimation, which lies hidden in the relationship between the mammal body mass(M, g) and the basal metabolic rate (BMR; mL of O2per hour) expressed in the allometric equation of the form: BMR = 4.12 × M0.69[62]. This relationship, with an allometric coefficient of 0.69, means that the BMR grows at a slower rate than the body mass, referred to as the slow-down principle[63]. To understand the consequences of the slow-down principle, imagine a multi-celled development of a single-celled,cube-shaped eukaryote, which grows through cell division with a same speed in all three directions of a Cartesian coordinate system. This type of growth creates a generation sequence of cube-shaped eukaryotic cells with a one cell extension in each three dimensions, per new generation (Table 7). Moreover, each unit cell burns continuously food in oxygen and the molecular remains of the food are finally converted into ATP and heat. To maintain an adequate cell temperature, each of the one-unit cells exchanges one metabolic heat unit per time unit with the environment.The first eukaryotic cell thus exchanges with the environment one heat unit per time unit through 6 identical surface planes. The next generation with 8 (23) unit cells and a total of 24 (6 × 22) unit surface planes exchanges 8 (23) heat units per time unit. The subsequent generation with 27 (33) unit cells and a total of 54 (6 × 32) unit surface planes exchanges 27 (33) heat units per time unit, and so on (Table 7).
The important outcome of this model with the growing cube-shaped eukaryotic cells is that the number of heat units to be exchanged per time unit increases by its cube, and the number of unit surface planes increases by its square (Table 7)[63]. This means that the relative rate of heat production must fall as the number of cells gets larger. How did the evolutionary trajectory of live resolve this problem? Well, over evolutionary time, the slower rate of heat production was achieved by a reduction in UI of membrane phospholipids. Hulbertet al[64]reported that the UI of phospholipids from mammalian species significantly decreased as species body size increased whilst the percentage of total unsaturated acyl chains was relatively constant in mammalian species of very different body mass. So the membrane bilayers of small mammals were generally high in docosahexaenoyl (C22:6 n-3) chains and low in oleyl (C18:1 n-9) chains, and the opposite was observed in large mammals[64]. A telling example of body core temperature regulation during the evolution period from mouse toHomo sapiensis the reduction in skeletal muscle percentage of docosahexaenoyl (C22:6 n-3)chains from approximately 30% to 2% in parallel with a body mass increase from approximately 10 g to 85.000 g[64]. Apart from the brain, the phospholipids of heart,skeletal muscle, kidney, and liver tissues showed a significant negative relationship between the body mass of the species and the docosahexaenoate (C22:6 n-3) content of tissue phospholipids. The brain phospholipids from mammals have a high and relatively constant docosahexaenoate content, irrespectively of the body size of the species[65]. Also the mass-specific metabolic rate of birds depends on the relative balance between mono-unsaturated and poly-unsaturated acyl chains. These data suggest that the biochemical translation of the slow-down principle is a replacement of polyunsaturated acyl chains by monounsaturated acyl chains in membrane phospholipids, which means that the number of unsaturated acyl chains remains the same, whereas their number of carbon-carbon double bonds decreases, with aconsequent reduction of the area A of lipid molecules with all the consequences that entails (see previous section). Instead of insulin action, heat production may cause a reduction in the amount of transmembrane glucose-transport.
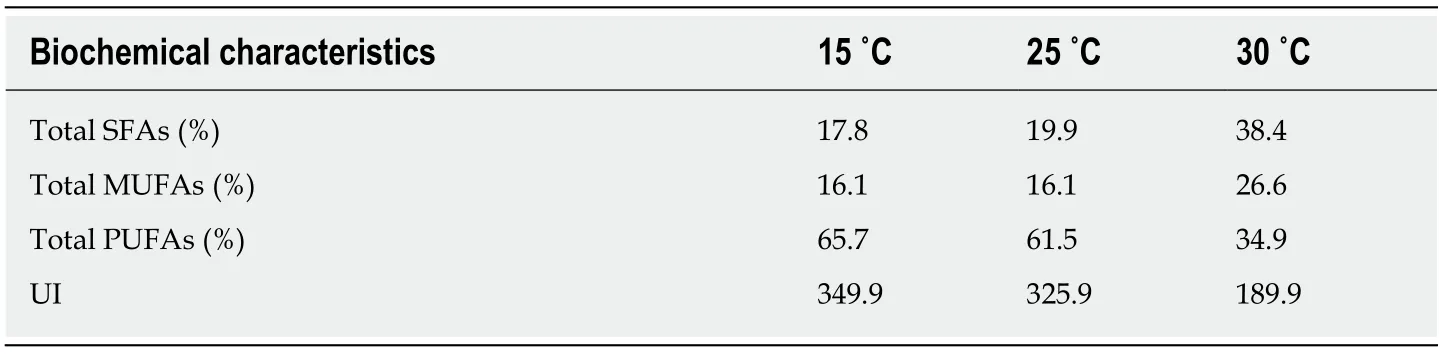
Table 6 Acyl composition (% of total acyl chains) of membrane phospholipids and unsaturation index in fathead minnow (Pimephales promelas) muscle1
Today, a human parallel of this evolutionary principle still occurs during a pregnancy,i.e. an increase in maternal mass may generate a reduction in UI of the acyl composition of maternal phospholipids, which in turn, lowers the maternal membrane flexibility, and thereby reduces the maternal transmembrane glucosetransport. In this way, the maternal plasma glucose concentration and insulin level increase during pregnancy without any impaired sensitivity of tissue to the action of insulin[66].
FREE FATTY ACIDS
Type 2 diabetes and its prediabetic phase are characterized, among other things, by an increase in the plasma FFA concentration[67,68]. The cause of this phenomenon is a reduction in the transmembrane glucose-transport of all Class 1 GLUTs, which leads to a reduction in the glucose-mediated ATP production. An absolute requirement for ATP production necessitates cells to make a switch from glucose-mediated ATP production to FFA-mediated ATP production - remember that a single cell consumes around 10 million molecules of ATP every second, which means that in the human body the total turnover of ATP is around 60-100 kg/d[56]. So a reduction in glucosemediated ATP production promotes an increase in the level of essentially saturated FFAs for extra FFA-mediated ATP production, which will set up a vicious cycle of raising the levels of essentially saturated plasma FFAs and lowering the level of transmembrane glucose transport. After all, the released FFA-pool of human white cells showed approximately 110- and 9-fold decreased percentages of docosahexaenoic acid (C22:6) and arachidonic acid (C20:4), respectively, compared with the human serum pool. So, the UI of released FFAs from human white fat cells is substantially lower compared with the UI of serum FFAs in healthy controls (85.5 and 191.9, respectively; Supplementary Table 4)[69-71]. Thus, an increased release of FFAs from adipose tissue into the circulation elevates the plasma concentration of saturated fatty acids. This sequence of events forced a shift from unsaturated to saturated acyl chains in phospholipids of both the erythrocyte membrane and the vascular endothelium[68]. I am telling this matter, because I believe the existence of this vicious cycle decreases progressively the UI of plasma FFAs, which may be a root cause of the progressive character of type 2 diabetes.
ENERGY TRANSPORT
To understand the relationship between heat production and the onset of type 2 diabetes and gestational diabetes, it is time to address briefly the concept of eukaryotic cellular energy transport. Hydrogen is the energy carrier par excellence for the energy saved in our food. After absorption of food, hydrogen is being stripped from the molecular remains of the nutrients, and passedviathe citric cycle into the mitochondrial electron-transport chain. Mitochondria are surrounded by a simple outer membrane and a more complex inner membrane. The space between these two membranes is referred to as the intermembrane space and the space surrounded by the inner membrane as the matrix. The four separate protein complexes of the electron-transport chain are located in the inner membrane. The first two complexesseparates the electrons from the hydrogen energy-carriers, after which the electrons are finally donated to molecular oxygen, the ultimate electron acceptor in complex IV of the electron-transport chain, to form H2O. In concert with these processes,respiration pushes the remaining protons (H+-ions) across the mitochondrial inner membrane into the intermembrane space, against a concentration gradient. These protons could re-enter the matrix through the inner mitochondrial membrane in two different ways, firstviathe channel of the ATP synthase protein complex for driving ATP synthesis, and secondviathe uncoupling protein1 (UCP1) without using energy for any purpose. In the last way, the proton potential energy is released as heat[72]. It is therefore concluded that UCP1 may play a crucial role in thermogenesis.
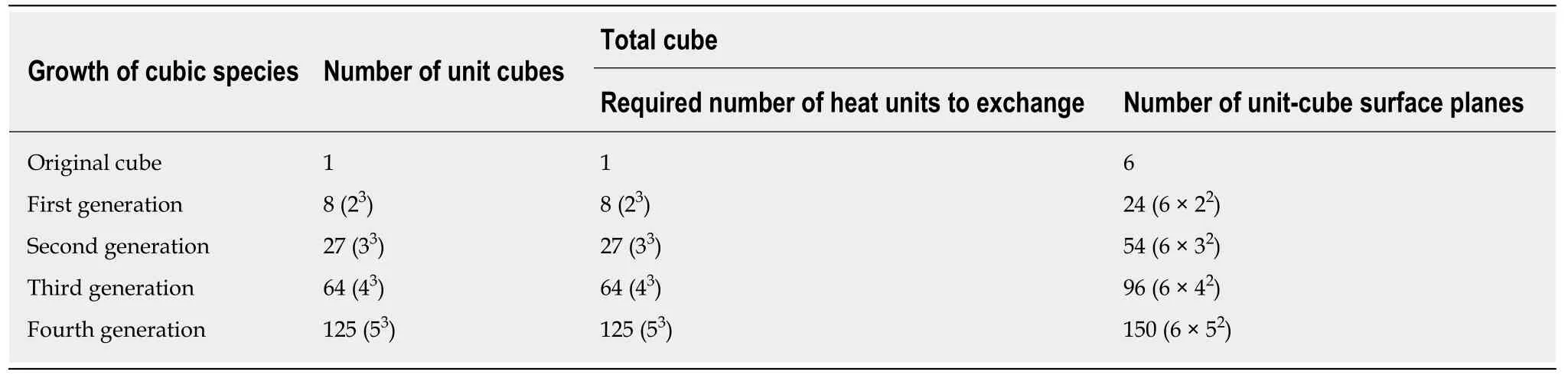
Table 7 Thought experiment of multi-celled development of a single-celled, cube-shaped eukaryotic cell, which grows through cell division in a Cartesian coordinate system with the same speed in all three directions, and exchanges with its environment per unit-cell one heat unit per time unit[63]
HEAT PRODUCTION AND THE ONSET OF TYPE 2 DIABETES
Experimental data of intracellular temperature mapping, based on a novel fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy, demonstrated clearly the existence of mitochondrial-mediated heat production[73]. This heat production was observed as a proximal local temperature increase. It could be concluded that the local temperature near the mitochondria was higher than the temperature of the rest of the space in the cytosol (aside from the centrosome).Furthermore, this local heat release from mitochondria is accelerated when ATP synthesis is stalled by an uncoupling reagent[74]. Despite incomplete understanding of the uncoupling functions for maintaining energy homeostasis, all these data suggest that, in healthy subjects, a balance exists between the amount of protons, which reenter the matrix through ATP synthase on the one hand, and the amount of protons,which re-enter the matrix through UCP1 on the other.
The reviewed data strongly support an alternative model with proposed steps in the development of type 2 diabetes, a disease arising as a result of a hypothetical hereditary anomaly (Figure 2)[63]. The author's hypothesis proposes that the final result of this anomaly, which already appears in the prediabetic phase, is a status of an increased flux, compared to healthy controls, of intermembrane-space protons,which re-enter the matrixviaUCP1, and thereby causes hyperthermia in and around the mitochondria[63]. To keep the mitochondrial temperature within the narrow range compatible with live, the slow-down principle enters into force, which results in an appreciable reduction in UI. This process leads to a marked reduction in membrane flexibility, and thereby reduces the transmembrane glucose-transport, which generates a reduction in glucose-mediated heat production. However, the concomitant disadvantage of this sequence of events is also a reduction in glucosemediated ATP production. To compensate for the extra loss of glucose-mediated ATP,lipolysis increases to raise the levels of circulating, essentially saturated FFAs, which are needed to generate extra ATP energy for sustaining life. The progressive reduction in UI will set up a vicious cycle of raising the levels of essentially saturated plasma FFAs and lowering the level of transmembrane glucose transport. These phenomena represent a blueprint of the presence of type 2 diabetes and its progressive character in human individuals.
Remember that Kelleyet al[75]reported the presence of impaired functional capacity and morphological alterations of mitochondria, which were obtained from the vastus lateralis muscle of volunteers with type 2 diabetes. A status of long-term heat acclimation may be the cause of the reported reduced activity of NADH oxidation by the respiratory chain, the smaller mean size of mitochondria with a less clearly defined internal membrane structure, and smaller cristae.
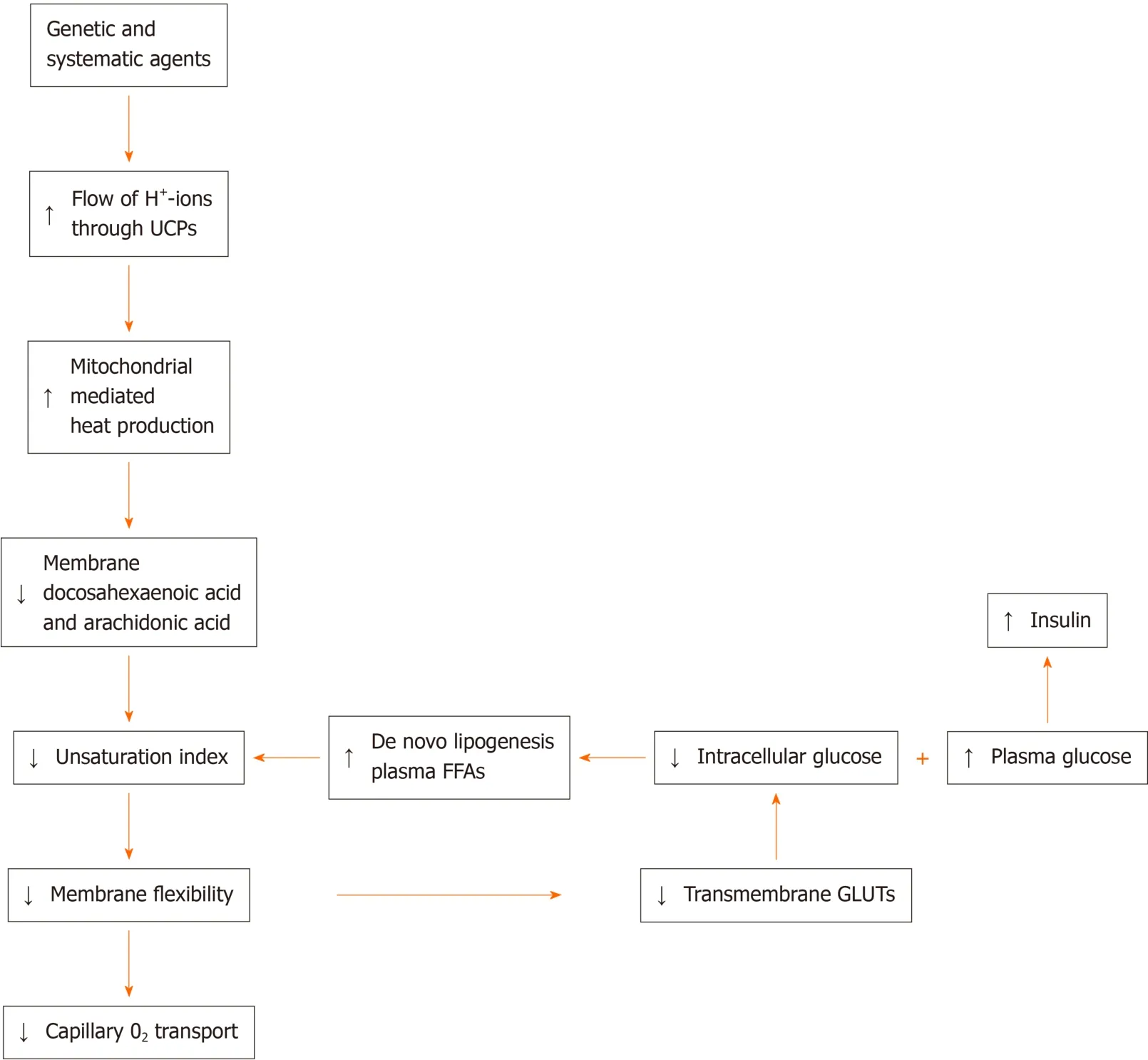
Figure 2 Although the results of genome-wide screen for type 2 diabetes susceptibility genes are still under debate, a refined working hypothesis proposes that the primary effect of the involved genes generates an increased flux of mitochondrial intermembrane-space protons through UCP1 into the matrix, which causes an increase of extra heat.
We can now explain why the classical research results of laboratory animals mimicking the human type 2 diabetes has not given any indication regarding the onset of human type 2 diabetes[76,77]. All in all, the characteristics of the animal model should mirror the pathophysiology and natural history of type 2. By contrast, no experimental results are given so far based on animal models with type 2 diabetes,caused by a status of long-term heat acclimation.
METFORMIN
The proposed steps in the development of type 2 diabetes model improves our knowledge of the metformin-medication effects[63]. The most widely accepted model of the metformin antihyperglycemic action is its suppression of gluconeogenesis with the amino acid alanine as glucogenic substrate, which principally occurs as a consequence of mitochondrial inhibition[78]. In this context it is interesting to note that the type 2 diabetes specific reduction in the transmembrane glucose-transport promotes a significantly increased hepatic efficiency, compared to healthy controls, in converting alanine to glucose[79,80]. The suppression of hepatic gluconeogenesis creates a disturbed participation of glucose in the supply of ATP energy to the body and includes, among other things, stimulation of lipolysis. This process increases the amount of circulating, essentially saturated FFAs, referred to as metformin-mediated FFA increase. Besides the increase of essentially saturated FFAs due to the FFAmediated ATP production (see section: Free fatty acids), the metformin-mediated FFA-increase could also have a deleterious effect on the membrane flexibility, which accelerates the onset of vascular and neurological complications over the long term[81].The results of two studies are in line with this idea. First, the Diabetes Prevention Program (DPP) study results indicated that metformin therapy was not as beneficial as life style modification for delaying the development of type 2 diabetes in individuals at high risk of type 2 diabetes,i.e. the DPP study results demonstrated that lifestyle intervention was twice as good as metformin therapy for delaying the development of type 2 diabetes, and at least as effective in older participants as it was in younger participants[82]. Second, the saturated acyl chain percentages in erythrocyte membrane phospholipids of control individuals, people with type 2 diabetes without retinopathy, and people with type 2 diabetes with retinopathy increased within a lifetime from 42.0%via44.2% to 46.9%, respectively, (Table 8, Supplementary Tables 5 and 6)[83]. However, the percentage of the saturated acyl content of membrane phospholipids was almost constant in mammalian species, independent of their body mass, during the last 600 million years of evolution, which means that a constant percentage of membrane saturated acyl chains is important for biological and biochemical processes[62]. Basically, metformin reduces the plasma glucose concentration, but tends towards an increase in the FFA concentration. This side-effect of metformin is not limited to metformin, but also applies to another class of drugs,which are used for lowering the plasma glucose concentrationviainhibiting the glucose reabsorption by inhibiting the sodium-glucose co-transporter[84].
It is worth to note that the patients included in the “diabetic retinopathy” study were treated according to the recommendations of the French High Authority of Health, who published its last recommendations with metformin as the optimal firstline drug, for the optimal management of diabetes, on January 2013 (personal communication, Niyazi Acar)[85].
EXERCISE
Although the role of irisin in the conversion of white adipose tissue into brown adipose tissue is still under debate[86], acute exercise training showed direct effects on“browning” of white fat[87]. Hamiltonet al[88]demonstrated in excised murine adipose tissue samples lower levels of unsaturated triglycerides in brown adipose tissue compared with white adipose tissue, an observation consistent with previous results[89]. If the gene expression in the corresponding mouse model does not significantly differ from the human conditions[90], burning of human brown adipose tissue, due to physical activities, induces in phospholipids a shift from saturation into unsaturation, which promotes membrane flexibility. Also, muscular exercise increases the blood flow[91], which promotes oxygenation of cells, stimulates the electron flow down of the respiratory chain, and improves the proton (H+) flux through the ATP synthase driving ATP synthesis, which in turn, reduces the need for FFA-mediated ATP production, and thereby increases membrane flexibility. This may be the main cause, why the US DPP study results indicated that the incidence of type 2 diabetes was 58% lower in the lifestyle-intervention group than in the placebo group[82].Cellular flexibility is a critical factor for modulating blood flow in microcapillaries (see section: Membrane flexibility). So, an increase in both erythrocyte-membrane flexibility and microvascular endothelium flexibility is of unprecedented clinical relevance: It generates a beneficial reduction in microvascular and macrovascular complications,
Structured lifestyle intervention trials (including physical activity, dietary energy restriction and weight loss) demonstrated reductions of 28%-59% in the risk of developing type 2 diabetes in individuals with impaired glucose tolerance[82,92,93].However, it is important to note that all the exercise sessions in these randomized controlled trials were supervised, a situation that is not often met in daily practice.
CONCLUSION
This review highlights a deeper scientific understanding of the molecular nature about important factors underlying the pathophysiology of type 2 diabetes and its prediabetic phase. Work described in this review is designed to communicate some newly discovered fundamental biomolecular processes governing this disease to a broad audience including everyone who is involved in the complex field of this disease, which is one of the today's greatest unsolved medical mysteries.
The discovery of the existence of yet another blood glucose regulation mechanism is a milestone in the literature about type 2 diabetes. This overlooked transmembrane glucose transport mechanism, still not mentioned in the medical literature, is based on a redistribution of the lateral pressure in cell membranes, due to a reduction in membrane flexibility, which influences the 3D structure of all Class I GLUT proteins.Variations in the number of carbon-carbon double bounds of phospholipid acylchains of fluid lipid bilayers modulates the GLUT protein pore diameter of the transmembrane glucose transport channel. In short, impaired glucose tolerance,gestational diabetes mellitus and type 2 diabetes are characterized by an elevated concentration of essentially saturated FFAs, which creates less flexible membranes,narrows the glucose channels of all Class I GLUT proteins, and thereby lowers the amount of transmembrane glucose transport. Martinet al[15]reported that 10 years before the development of type 2 diabetes glucose effectiveness and insulin sensitivity in normoglycaemic offspring of couples, who both had type 2 diabetes, were 30% and 60%, respectively, lower compared to controls. This fact indicates that a redistribution of the lateral pressure in cell membranes, due to a reduction in membrane flexibility,influences the 3D structure of all Class I GLUT proteins early in live, which is an unprecedented discovery.
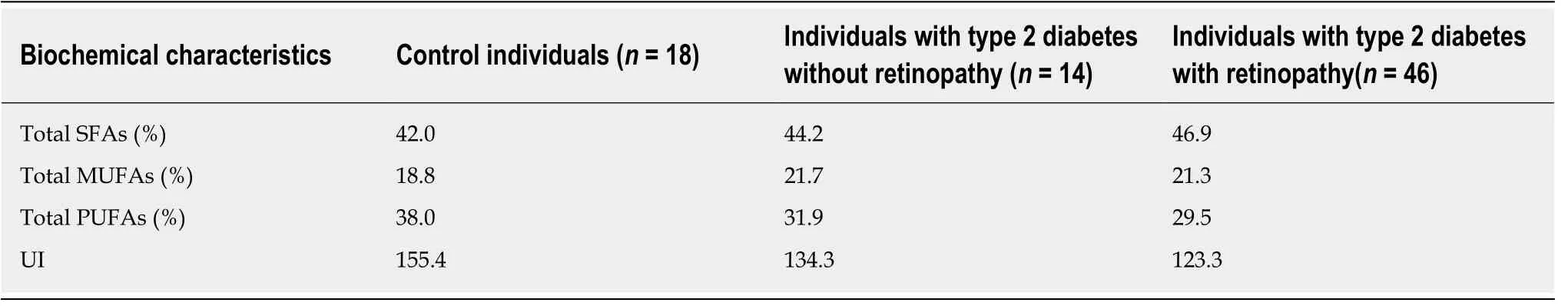
Table 8 Erythrocyte acyl chain composition in phospholipids and unsaturation index of control individuals, people with type 2 diabetes without retinopathy, and people with type 2 diabetes with retinopathy1
A second milestone is the observation that a reduction in UI, compared to a healthy status, is a characteristic of both the prediabetic phase and diabetic phase of type 2 diabetes. In a previous study I offered the clue that a raised leakage of H+-ionsviaUCP1 induces in and around mitochondria hyperthermia, which will lead to both a reduction in mitochondrial activity and content[94]. The proposed hyperthermia is countered by the slow-down principle already in the prediabetic phase and initiates a status of long-term heat acclimation, which generates a reduction in both insulin sensitivity and glucose effectiveness, and an increase in the percentage of the saturated acyl content of phospholipids. Both milestones degrades “insulin resistance” into an imaginary reality and requires a U-turn in thinking on some important issues of type 2 diabetes.
The genetic cause of hyperthermia remains incurable until today; however, there is a way forward. Metformin is useful to obtain a rapid reduction in (too) high glucose concentrations. Doctors who prescribe metformin should give clear information to patients about the metformin-mediated FFA increase and therefore point out the necessity of exercise and diet, which will minimize this metformin side effect. They should control the effect of exercise prescription on patients' wellbeing by the assessment of plasma FFA concentration for monitoring purposes and the assessment of UI for controlling the level of the total percentage saturated acyl chains of membrane phospholipids, as observed in erythrocytes. To prevent or delay the progressive character of type 2 diabetes, exercise is the medication to be preferred,because it improves blood circulation, micro-oxygenation of cells, and thereby reduces membrane stiffness. However, given the high degree of difficulty of structured lifestyle intervention, exercise sessions need counseling, just like with dietary energy restriction[95].
Remember that the complete oxidation of palmitic acid or stearic acid to CO2and H2O per hydrogen atom more molecules of ATP produces, compared to a complete oxidation of glucose[96], which logically demands less FFA molecules, compared to glucose molecules, for a similar amount of ATP production. However, against the background of the observation that the saturated acyl chain percentages in erythrocyte membrane phospholipids of control individuals, people with type 2 diabetes without retinopathy, and people with type 2 diabetes with retinopathy increased within a life-time, the conclusion is that circulating saturated FFAs play a main role in the pathophysiology of type 2 diabetes.
A detailed working hypothesis proposes that a key feature in the etiology of type 2 diabetes, which appears in the prediabetic phase, is an essentially raise, compared to healthy controls, in the flux of intermembrane-space protons, which re-enter the matrixviaUCP1, and thereby causes hyperthermia in and around the mitochondria.The reviewed data highlight the need for studies to find the cause of the essential raise in this proton flux through UCP1, because it will improve the possibilities for diagnosis of type 2 diabetes, treatment, and care.
ACKNOWLEDGEMENTS
I need hardly say that this review article has benefited greatly from the expertise of Joep Maeijer from the Audiovisual service and Bert Berenschot from the medical library.
杂志排行
World Journal of Diabetes的其它文章
- Role of sodium-glucose co-transporter-2 inhibitors in the management of heart failure in patients with diabetes mellitus
- Severity of the metabolic syndrome as a predictor of prediabetes and type 2 diabetes in first degree relatives of type 2 diabetic patients: A 15-year prospective cohort study
- Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters
- Maternal low protein diet induces persistent expression changes in metabolic genes in male rats
- Shared (epi)genomic background connecting neurodegenerative diseases and type 2 diabetes
