类金刚石薄膜固体超滑的研究现状和挑战
2020-07-01王康陈新春马天宝
王康,陈新春,马天宝
类金刚石薄膜固体超滑的研究现状和挑战
王康,陈新春,马天宝
(清华大学 摩擦学国家重点实验室,北京 100084)
类金刚石(Diamond-like carbon,DLC)薄膜,具有高硬度、高化学惰性及低摩擦磨损等特性,特别是在一定条件下的超滑特性(摩擦系数低于0.01),为真正的近零摩擦和磨损的实现提供了可能性,因此在固体润滑领域展现出巨大的应用前景。从元素掺杂种类和键合结构特点,概述了DLC薄膜的种类多样性,归纳了不同DLC薄膜的力学及摩擦学特性。通过对比分析不同DLC薄膜在不同环境条件下的摩擦学行为,阐述了DLC薄膜超滑实现的环境敏感性,其中薄膜和环境中氢原子的作用十分关键,同时提出Si等元素掺杂改善超滑环境敏感性的可行方案。重点介绍了3种DLC超滑机理——界面钝化理论、界面石墨化理论以及转移膜形成理论,这三者均具有一定的局限性,如何更深入且全面认识DLC超滑仍是一个科学难题。最后强调了先进界面检测和表征技术对探秘DLC超滑态界面组成的重要性,并对今后亟需开展的深入研究方向进行了展望。
类金刚石薄膜;超滑;种类多样性;环境敏感性;超滑机理;界面表征技术
类金刚石(Diamond-like carbon,简称DLC,也可称为非晶碳)薄膜自1971年由Aissenberg等人通过离子束沉积(Ion beam deposition)方法制备出后[1],以其优异的力学和摩擦学性能引起广泛研究热潮[2-8]。DLC主要由金刚石结构的sp3杂化碳原子和石墨结构的sp2杂化碳原子相互混杂形成三维网状结构[6,9],通常掺杂不同元素(H、Si、W等)以实现综合力学及摩擦学等性能[10-12],在具备较高硬度的同时,又兼顾优异的减摩抗磨特性。超润滑(摩擦系数低于0.01,简称超滑)作为DLC最为显著的摩擦学特性[13-14],虽然自2000年就已被实验证实[15-16],但研究者对其超滑机制的认识至今仍不完善。造成这种情况的主要因素有:1)DLC碳膜的种类众多,不同sp2、sp3比例及掺杂元素的不同都会导致碳膜力学及摩擦性能产生较大差异;2)DLC的摩擦性能不仅受到载荷等实验参数的影响,同时对环境氛围十分敏感;3)DLC超滑缺乏普适、系统的理论体系,不同理论之间的联系尚不清晰;4)DLC超滑态界面厚度通常在纳米尺度,其化学和微观结构特征表征难度大。因此近年来,DLC的超滑机制研究备受世界各国研究者的重视且亟需系统性的理解和完善。对DLC超滑的深入认识有助于人们理解其摩擦过程中能量耗散机制,继而为碳膜制备技术及进一步应用提供坚实的理论依据。本文以上述四个超滑影响因素为导向,详细介绍并阐述了DLC超滑机制的研究进展及现存挑战,最后展望进一步的研究方向。
1 DLC薄膜种类的多样性
DLC薄膜是由同时含有sp3杂化键和sp2杂化键的碳原子构成的一种亚稳态非晶物质[5,9,14]。由于金刚石结构中碳原子以sp3杂化键合,而石墨结构中碳原子以sp2杂化键合,因此一般而言,类金刚石薄膜(DLC)的性质介于金刚石和石墨之间,即有较高硬度的同时又兼顾较低的摩擦系数。研究发现,通过掺杂H原子改变sp3和sp2杂化键的比例,可以显著影响DLC薄膜的力学和摩擦性质,因此可将DLC薄膜分为含氢DLC(a-C:H)和不含氢DLC(ta-C和a-C)两类,其中含氢DLC薄膜又可根据含氢量细分为四类[17-18]:类聚合物a-C:H(PLCH)、类金刚石a-C:H(DLCH)、四面体a-C:H(ta-C:H)和类石墨a-C:H(GLCH)。除此之外,还可通过掺杂各类金属或非金属元素(如Al、W、Si、O等)来进一步改善DLC在各种实际工况下的性能[11,12,19-21]。由此可见,DLC薄膜其实是一个集合术语,包含了各种性能各异的非晶复合碳膜[6,18],如表1所示。据报道,DLC的超滑特性被广泛发现于各类含氢DLC薄膜中,包括:a-C:H、GLCH、TLCH、a-C:H:F、a-C:H:Si、(Si/Al)a-C:H等[14-16,19,20,22-24]。
表1 DLC薄膜的种类及特征
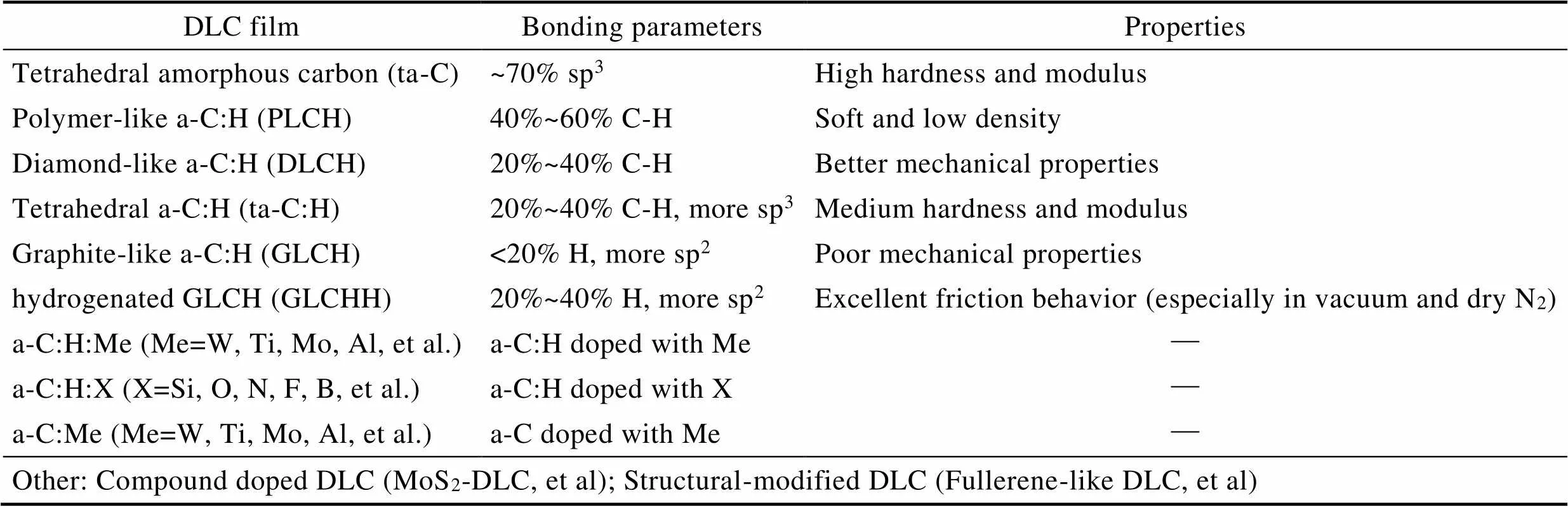
Tab.1 Varieties and characteristics of DLC films
2 DLC超滑的环境敏感性
自Enke等[25]首次报道了DLC薄膜具有超低摩擦系数之后,DLC的摩擦学性能便受到了各国研究者们的广泛关注。如前所述,DLC薄膜实际上是一个集成术语,包含的碳膜种类众多且性能各异,不同的碳膜在不同的实验条件下展现出截然不同的摩擦学性能。研究发现,DLC超滑对试验环境条件(真空、惰性气体、活性气体、湿度、温度等)十分敏感,对于同种碳膜,通过变换实验条件,其前后的摩擦系数变化可相差两个数量级[13,26-32]。
2.1 真空及惰性气体环境
研究表明,多种a-C:H碳膜可在超高真空环境(Ultra-high vacuum,简称UHV)以及惰性气体环 境中实现超滑[15,20,22,23,30]。最早由Donnet和Fontaine等[2,15,30,33]采用PECVD制备出了不同含氢量的a-C:H薄膜,并在UHV环境下对其摩擦性能进行了测试(对偶面为钢销,平均接触应力0.5 GPa,真空度小于10−7Pa),结果如图1所示。较低含氢量的a-C:H薄膜(34% H,对应图1中的AC8试样)在UHV环境中,先经历一个较短时间的跑合,摩擦系数低至0.01以下,随后突然剧烈上升,摩擦系数达到1左右,即超滑失效;而含氢量较高的a-C:H薄膜(40% H,对应图1中的AC5试样)在达到超滑状态后具有较长的寿命。对实验后样品表面磨痕的观测发现,低含氢量碳膜(AC8试样)出现明显划痕,且有黑色磨屑,说明摩擦过程中产生了强烈的界面粘附,而高含氢量碳膜(AC5试样)的超滑态磨痕非常浅且光亮。据此研究者们提出,当a-C:H表面接触滑移时,裸露出的碳悬键会被氢原子饱和,薄膜表面存在一层超薄类聚合物的碳氢化合物,因为碳氢链间的范德华力作用结合能只有~0.08 eV[6],所以形成易剪切的界面层,表现出超低的摩擦系数,而含氢量不足会导致一部分表面碳悬键无法被充分钝化,从而摩擦过程中产生剧烈的摩擦化学反应,使摩擦力显著上升[2]。为了进一步验证H的作用,Donnet等[15]在不同氢气压强环境中对两种样品进行了摩擦测试,结果表明样品中的H原子以及环境中的H原子/分子都可以有效延长超滑态的寿命。Fontaine等通过实验确定了在UHV环境中a-C:H超滑的临界含氢H量,其依赖于不同的碳膜沉积技术[30,34,35]。
Erdemir等[16,36]在干燥氮气环境中对不同碳源(C/H比不同)制备的a-C:H薄膜进行了测试,结果与其在UHV中类似,即含氢比例越高的a-C:H薄膜,摩擦系数越小,磨损率越低,且寿命越长。同时也发现,当对偶面为更光滑的蓝宝石球时,摩擦系数可进一步降低至0.001。惰性气体环境下a-C:H的超滑与其在UHV中非常相似,两种环境都能提供一个惰性环境来实现a-C:H薄膜的超滑特性,同时它们又存在着不同,主要体现在UHV环境中a-C:H的超滑寿命明显比氮气环境中的短。首先,两种环境都不能代表绝对惰性,两种条件虽然会显著降低活性分子的分压,但仍然会保留环境中的少量活性分子,如氧气和水蒸气等,会导致a-C:H表面产生不同强度的气体吸附作用,继而影响摩擦行为[26]。其次,两种环境下气体的扩散能力(分子平均自由程)有明显差异,且 真空下的对流传热非常微弱,摩擦界面温度相对较高,这些原因的综合影响导致a-C:H薄膜在UHV环境中的超滑寿命相对于氮气中较短。此外Ji和Wang等[37-38]发现,同为惰性环境,a-C:H在氮气中的摩擦系数要明显低于氩气中的,这种摩擦性质的差异可用气体-表面相互作用机理来解释。
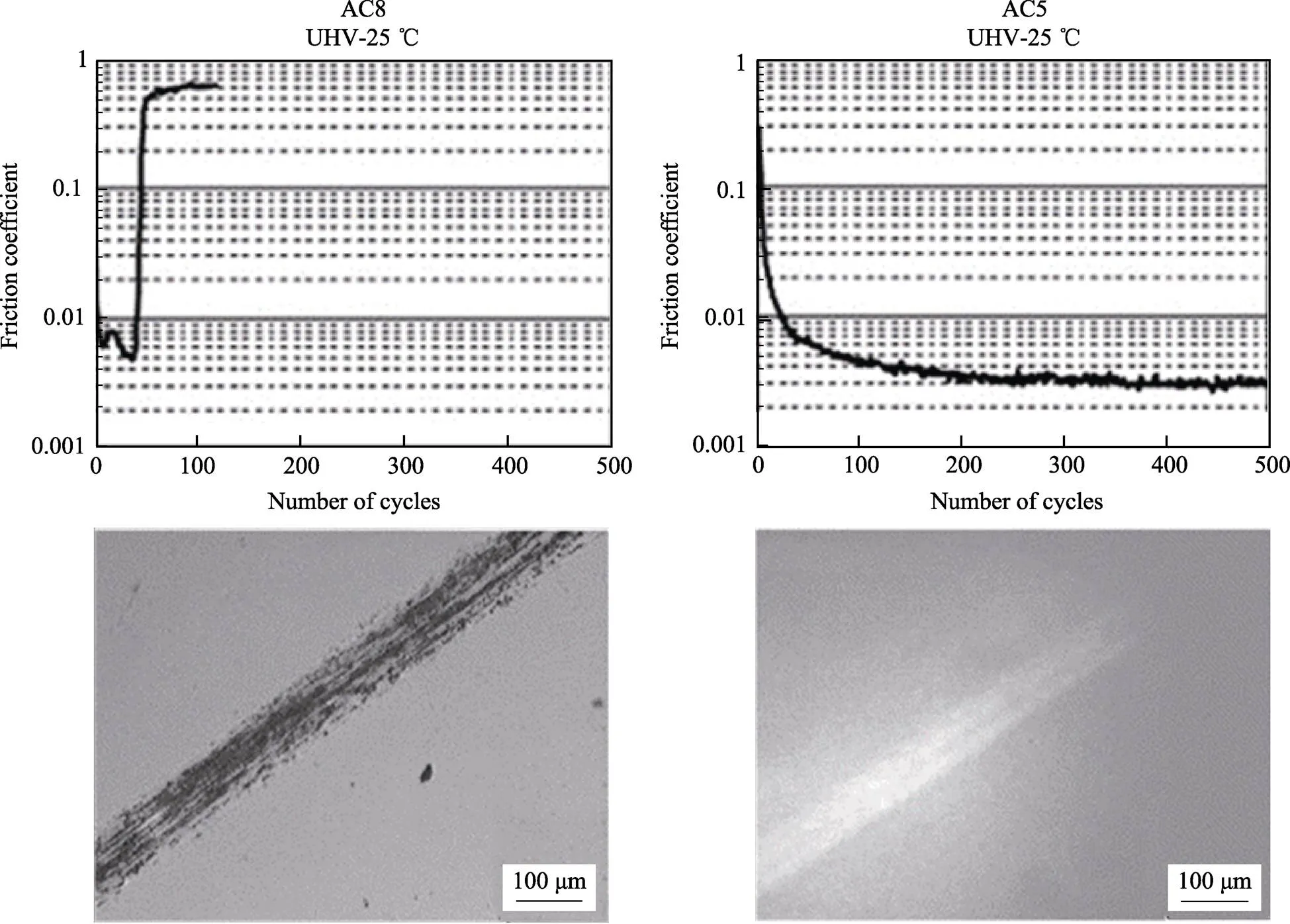
图1 a-C:H薄膜(AC8: 34% H,AC5: 40% H)在超高真空UHV环境下的摩擦曲线和磨痕光镜图[33]
2.2 活性气体环境
对于大部分实际工况,其应用环境往往是大气,且包含了各种活性气体(如氧气、水蒸气等),而大量研究表明,活性气体环境(主要指不同湿度下的 惰性气体、氧气和空气)不利于a-C:H薄膜实现超 滑[6,21,26,27,39-42]。Kim等[27]将水蒸气、氧气和氮气分别通入摩擦试验的真空腔内,研究了不同气压下三种气体对a-C:H(40% H)摩擦行为的影响,如图2a所示。结果发现:H2O和O2的通入会增加碳膜表面粘附性,导致摩擦系数迅速增大,加快a-C:H薄膜的失效,其中H2O分子的影响最显著,仅达到~1.3×103Pa压强便会使a-C:H的摩擦系数从真空下的~0.004迅速增大至~0.07,而通入N2的影响最小。从转移膜形成的角度出发,对偶副材料(如金属、陶瓷等)表面在活性气体环境下都会参加界面摩擦化学反应,形成高度氧化的转移膜,其不仅含有过氧基团,还包括许多金属氧化物和碳化物等,导致较大的界面粘附;同时,DLC表面在活性气体中摩擦导致原位氧化,继而抑制了易剪切层的形成[21]。
Erdemir等[5]在同一腔体内不断改变环境条件(潮湿空气、干燥N2和潮湿N2)来考察含氢DLC薄膜的摩擦性质受环境的影响,如图2b所示。结果发现不断地改变环境,a-C:H薄膜的摩擦行为是可逆的,即摩擦副可迅速排出之前高湿度环境下生成的磨屑,并再次在摩擦界面形成致密的转移膜。
与此同时,环境湿度对无氢DLC(a-C和ta-C)的影响与对a-C:H截然相反,前者由于没有掺杂H原子,真空条件下界面剪切暴露出的大量悬键(σ键)使得界面间产生极强的粘附作用,因此摩擦系数较大[43];而少许H2O的存在可以水解生成—H和—OH基团,并钝化界面作用较强的C—C键,从而降低界面间的粘附,并实现超低摩擦和磨损[44]。
研究者通常利用金属/非金属元素掺杂来改善a-C:H在潮湿大气环境下的摩擦适应性[10,12,19,45]。Chen等[45]合成了不同氢含量的a-C:H:Si薄膜(Si原子数分数为8.9%~9.9%),并在潮湿空气环境(22% RH)下进行了摩擦实验(载荷2 N,速度15 cm/s),结果如图3所示。研究表明,掺杂Si元素后,a-C:H在潮湿空气环境中的摩擦性能明显得到改善,未掺杂的a-C:H薄膜在潮湿空气中的摩擦系数一般大于0.1,而掺杂Si后(原子数分数~9%),其在潮湿空气中经历短时间的跑合后可实现超低的摩擦系数(小于0.03),而当a-C:H:Si薄膜中的氢的原子数分数控制在20%~35%时,其在潮湿空气中甚至可以实现超滑,如图3b所示。研究发现,Si掺杂能够改善a-C:H在湿度环境中的摩擦性能,主要是其滑移界面在相互摩擦剪切过程中,能发生摩擦化学反应,生成亲水的硅氧基团(Si—OH),并吸附着边界水膜,即形成极易剪切的有序化纳米结构滑移界面[20,45]。Koshigan 等[12]采用PECVD方法制备了a-C:H:Si:O薄膜,发现Si和O的掺杂可以明显改善a-C:H薄膜在氢气及氧气环境下的摩擦性能,但改善效果与环境条件密切 相关(H2或O2的压强大于103Pa时,摩擦系数明显下降)。

图3 a-C:H:Si薄膜(Si原子数分数为8.9%~9.9%,H原子数分数为17%~36%)与SUJ2钢球对磨副在大气环境下的摩擦行为(湿度(22±2)% RH)[45]
3 DLC超滑理论
基于实验和分子动力学模拟(MD-simulation)的综合结果,研究者们提出了DLC超滑的微观及原子尺度机理,主要有:界面原子钝化理论、界面相变石墨化理论和转移膜形成理论。
3.1 界面钝化理论
对于a-C:H在真空和惰性气体环境中的超滑,Erdemir等[22]提出氢钝化模型来解释,如图4所示。1)a-C:H薄膜本身通过碳原子与氢原子的杂化存在大量的化学惰性C—H sp3结构(比C—C更强的C—H键能),在摩擦过程中,表面的C—H键可以有效钝化摩擦表面,避免对偶之间的粘附作用并减小摩擦(抑制碳碳之间形成共价键和π—π*键作用); 2)a-C:H薄膜内部同样存在一些未成键的氢原子/氢分子,当不可避免的界面粘附或磨损发生时,游离氢能够迅速填补裸露的碳悬键,在表面重新富集化学惰性的C—H键,从而持续有效钝化滑移界面并维持低摩擦力;3)氢钝化界面形成后,C—H键中H原子的电子密度转移到原子核的另一端,这样使得对偶面的氢质子互相接近时,形成C—H/H—C排斥作用,因此进一步减小表面间的相互吸引,使得摩擦系数更低。
Hayashi和Li等[46-47]分别采用紧束缚量子化学动力学(TB-QCMD)和反应力场分子动力学(RMD)研究了氢原子对自配副a-C界面的钝化行为,无氢DLC的滑移界面始终保持着较为剧烈的键合过程,因此摩擦力较大,而氢修饰的a-C:H表面可以有效增大接触距离,避免界面粘附作用。通过模拟细节发现,某些局部应力较大的地方,C—H可能会裂解(C—H键被拉伸且处于不稳定状态),但裂解的氢原子会彼此结合并生成氢气分子,其在滑移界面游荡直至某处的C—H再次裂解后,氢分子可以二次裂解并通过原子转移再次钝化碳悬键。同时Li等发现这种界面钝化受载荷的影响十分显著,随着载荷的逐渐升高,钝化效果逐渐减弱,界面的原子重构行为更加剧烈,因此相变对摩擦的贡献占到主导地位。Pastewka和Chen等[48-49]通过对体相a-C:H薄膜(H均匀分布在a-C中)摩擦行为的分子动力学模拟发现,除了氢钝化外,接触表面的原子级粗糙度随着滑移过程逐渐减小,滑移高度势垒逐渐降低,摩擦力进一步减小;同时体相H含量的增多,有利于提高a-C:H的钝化能力(可承受更高的法向载荷)。Cui等[50]通过实验发现,a-C:H的界面钝化对速度和真空气压条件也十分敏感(改变了界面钝化气体分子的吸附行为)。此外,除了氢的钝化,氟原子掺杂的氟化DLC薄膜在惰性环境中可实现更有效的钝化效果[51](C—F键的结合能为5.6 eV,相对于C—H键的3.5 eV更加稳定)。
3.2 界面剪切局域化和石墨化理论
除了基于氢钝化的钝化理论,界面原子重构导致的类石墨结构相变(界面相变石墨化)也是一种DLC超滑理论。研究人员在实验中发现DLC薄膜超滑通常伴随着摩擦界面的结构相变(sp3→sp2)[20,23,52,53]。Pastewka等[48]利用MD模拟了a-C:H的界面剪切行为,结果发现摩擦力的下降虽然伴随着界面碳原子的sp2化,但其不足以成为摩擦力下降的主要原因。Ma等[54-55]通过研究a-C/a-C摩擦界面的结构相变过程,发现剪切局域化会导致剧烈的界面结构相变及原子排列有序化(界面高达90% sp2),如图5a所示。这种石墨化过程使得摩擦力迅速减小,而接触压强(载荷)是影响石墨化最核心的因素,当载荷足够大使得体系密度升高时,界面剪切局域化才会有效发生。同时Wang等人[56]在纳米尺度直接观测了a-C界面在剪切诱导下形成的相变石墨纳米晶。近几年来,研究者们发现DLC在边界润滑条件下,通过摩擦化学的诱导也可生成界面类石墨烯层。Bouchet等[57]通过实验和光谱表征发现,油酸润滑下的ta-C表面通过摩擦化学反应生成氧化膜石墨烯,使得摩擦系数达到0.005,随后Kuwahara等[58]通过量子动力学模拟(QMD)发现ta-C界面在少量甘油分子作用下会发生原子结构重排,生成超薄类石墨烯的界面纳米结构(包含了5-,6-和7-碳元环),如图5b所示。界面剪切使得甘油分子发生机械-化学分解,生成的氢和氧原子仅能钝化部分碳悬键,从而诱导界面氧化石墨烯层的形成,继而实现超滑态。
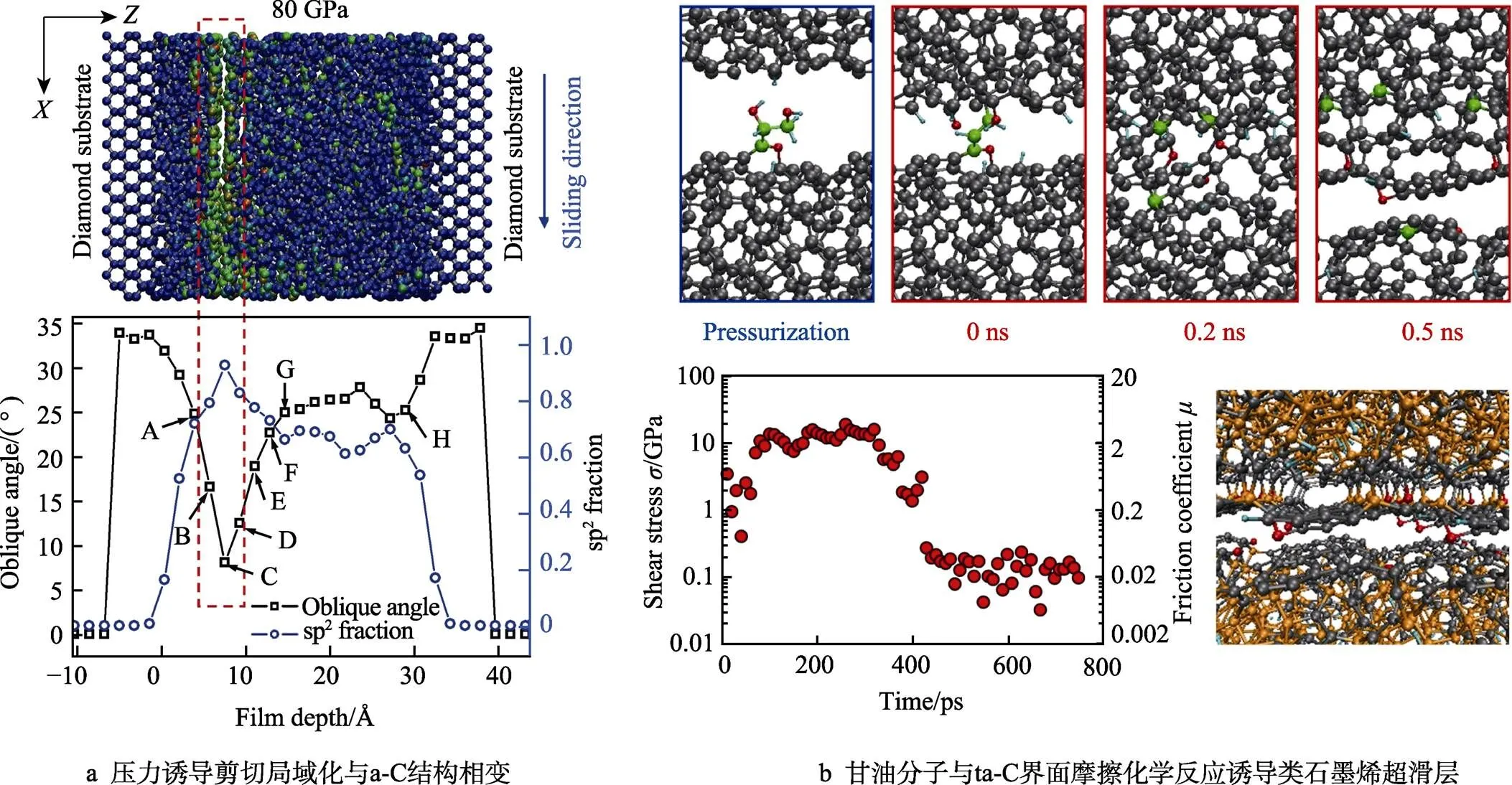
图5 压力[54]及摩擦化学反应[58]诱导界面原子结构相变
3.3 转移膜形成理论
DLC超滑总是伴随着对偶面上转移膜的形成(尤其是非自配副DLC界面),当转移膜形成后,滑移界面即从对偶面与DLC薄膜之前转移到转移膜层间,因此建立高质量的转移膜是实现超滑的核心。Chen和Koshigan等[12,45]发现,使用Si和O元素对a-C:H掺杂来改善其环境敏感性的关键在于形成类聚合物特性的易剪切转移膜,Chen通过纳米压痕测得转移膜的硬度及杨氏模量分别在2.0 GPa和40 GPa左右,明显低于体相的16.2 GPa和155.7 GPa,说明了转移膜本身低硬度和易剪切的特点[45]。Koshigan将已生成转移膜的钢球与硅片对磨,发现其摩擦系数明显低于钢/硅片,进一步说明了转移膜自身的润滑特性[12]。Liu等[59-60]研究了速度和载荷对Al2O3球/a-C:H对磨在真空中摩擦学行为的影响规律,结果发现,速度越高,对偶面上越难形成致密的碳转移膜,从而极大缩短了a-C:H碳膜的超滑寿命,如图6所示。而载荷对转移膜形成的影响和实验环境有关,如研究发现,大气环境中高载荷有利于形成转移膜,而在真空中却是低载荷,然而过高载荷不利于提高超滑持续寿命[60]。此外,Diao等[61]通过在DLC表面覆盖纳米厚的石墨烯纳米晶层,加快对偶面上石墨化转移膜的形成,继而缩短磨合阶段,并减小界面摩擦。
4 超滑态界面检测和表征手段
DLC超滑态的界面层厚度通常在纳米尺度,而对其界面化学及结构性质的直接检测依赖于先进的表征手段。Chen等[20]通过扫描透射电子显微镜(STEM)结合电子能量损失谱(EELS)表征解析了钢球表面的纳米级a-C:H超滑转移膜(厚~27 nm),如图7所示,提出了异质界面纳米化转移膜的分层结构(纳米颗粒钝化表层+富碳低密度中间层+C-Fe-O过渡层)对超滑界面的协同润滑效应,此外还揭示了氧气氛下导致超滑界面失稳的表面去氢化和高粘附性表层[21]。
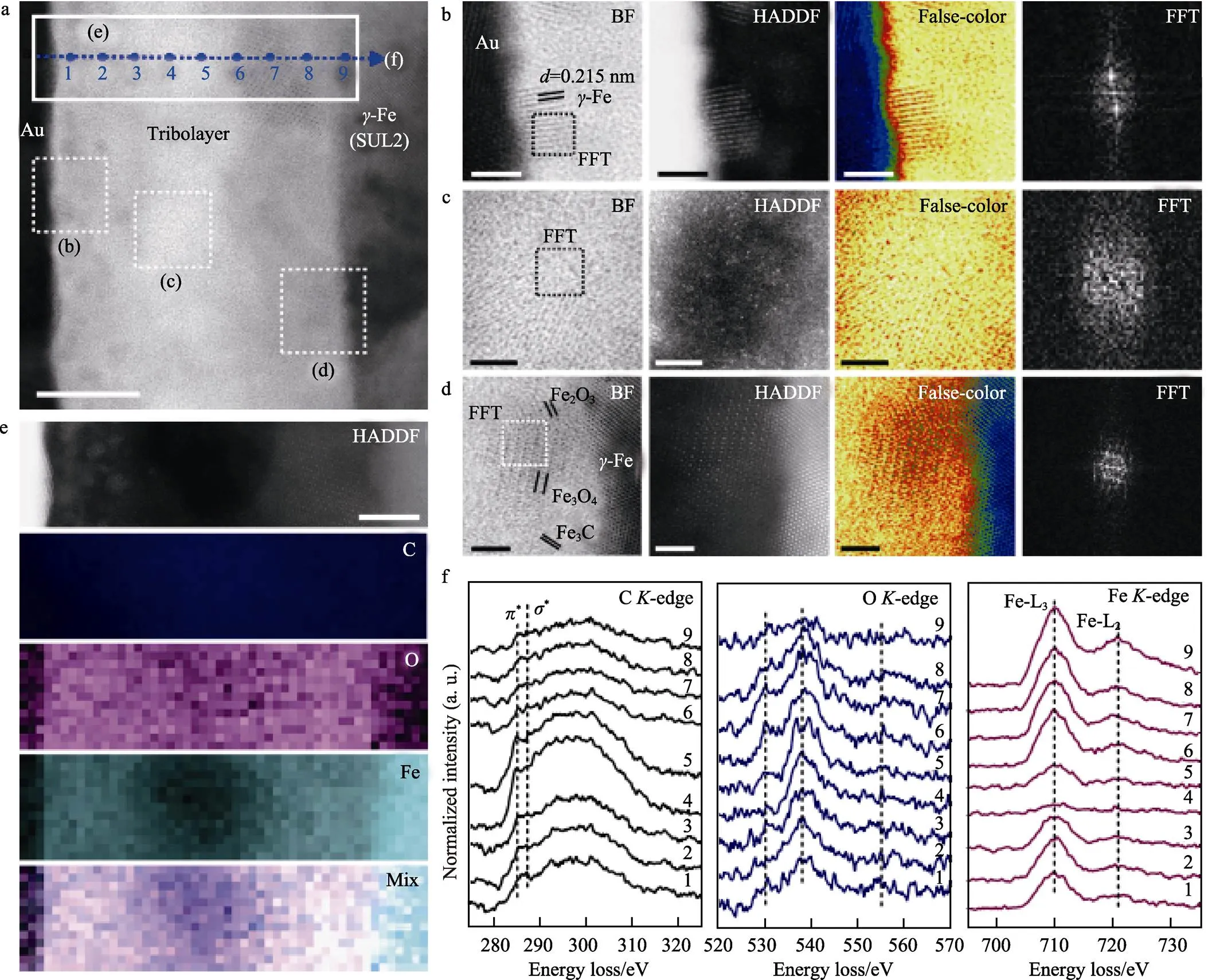
图7 钢球表面27 nm厚碳基转移膜的BF-STEM及EELS表征(对磨副:a-C:H)[20]
Wang等[23]利用透射电子显微镜(TEM)结合拉曼光谱,对类石墨a-C:H薄膜(GLC)和类富勒烯a-C:H薄膜(FLC)的超滑态磨屑进行了分析表征,如图8所示,发现磨痕表面存在极薄的有序石墨化相变层(3~5层),强调了剪切诱导的结构相变对超滑态的重要作用。Manimunda等利用原位拉曼摩擦试验机,对a-C:H薄膜的摩擦系数、转移膜厚度和转移膜碳键杂化结构演变进行了原位观测和表征,如图9所示[62]。同时在纳米尺度,原位TEM(in-situ TEM)为揭示DLC纳米级接触界面的结构演变及粘附状态特征提供了新的研究方案[63-65]。此外,飞行时间-二次离子质谱(TOF-SIMS)和近边X-ray吸收精细结构(NEXAFS)由于其对物质表面化学状态的高检测灵敏度及原子级深度分辨率,也成为DLC超滑态原子级界面化学性质分析的重要表征手段[44,66,67]。
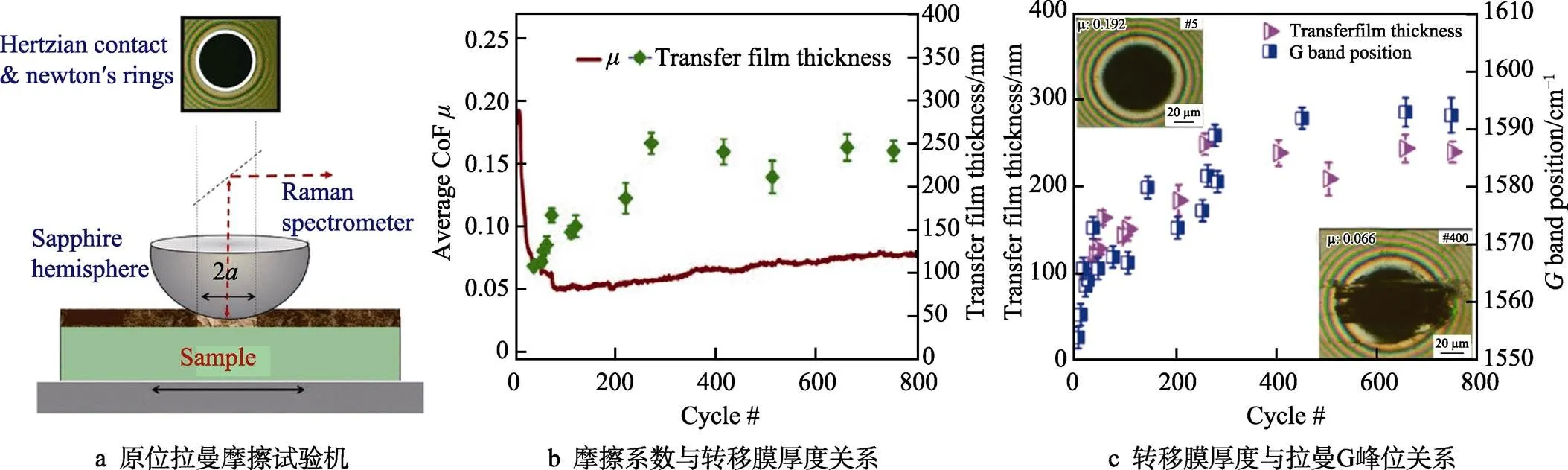
图9 利用原位拉曼摩擦试验机对摩擦系数、转移膜厚度及碳键杂化结构进行同步观测[62]
5 总结与展望
从四个关键方面综述了DLC薄膜固体超滑研究的主要进展,包括DLC薄膜的种类多样性、摩擦行为的环境敏感性、超滑机理的复杂性和先进的超滑态界面检测和表征手段。通过不同的制备技术得到不同化学性质及结构特点的DLC薄膜,在不同的实验环境条件下展现出不同的摩擦学行为。通常富氢DLC薄膜在真空和惰性环境下可实现超滑态,而无氢DLC薄膜可在活性气体环境中(特别是湿度环境)实现超低摩擦,这主要归结于滑移界面中活性键的钝化,避免了界面强粘附作用,继而实现超低摩擦和磨损。通过元素掺杂(如Si、N等),可有效改善DLC薄膜的环境敏感性,如在Si掺杂改性DLC薄膜中(a-C:H:Si),由于Si—C键能(3.21 eV)小于C—C键能(3.70 eV),因此掺Si后可使得之前拘束的碳网状结构变得松弛,大幅降低薄膜内应力,更重要的是提高了a-C:H薄膜的环境敏感性,即在湿度环境下也可实现超滑态,其超滑机制主要归结于界面纳米结构类硅胶易剪切转移膜的形成。除了界面钝化及转移膜机理,通过剪切局域化诱导滑移界面的石墨化相变来减小摩擦磨损并实现超滑态也被广泛提出和验证,特别是在无氢DLC和类石墨a-C:H(GLCH)体系。此外,更深层次探索DLC超滑的原子尺度机制,还需依赖于先进的表征技术和手段。
为了进一步深入探索神秘的DLC超滑机制,并促进其在各种实际工况下的应用(如宽温度、极端环境工况等),可从以下几个方面入手,加强深入研究:
1)建立完善、体系的DLC超滑理论,系统研究超滑态界面化学性质变化(如钝化)与微观结构演变(如石墨化)过程的联系性以及两者对最终超滑态实现的协同作用机制。
2)结合计算机模拟等方法,建立超滑态转移膜形成的动力学模型,深入研究载荷、速度和气氛环境等因素对转移膜形成质量的影响机制,有效并定量评估转移膜形成质量与超滑态之间的联系性。
3)开发多元掺杂改性DLC的制备技术,改善DLC涂层的环境敏感性,有效提高膜基结合力,促进其在一些跨环境条件实际工况中的应用。
[1] 牟魁峰. 低摩擦系数DLC膜的制备及摩擦性能的研究[D]. 哈尔滨: 哈尔滨工业大学, 2006. MOU Kui-feng. Fabrication and tribological property of low friction coefficient DLC films[D]. Harbin: Harbin Institute of Technology, 2006.
[2] FONTAINE J, BELIN M, MOGNE T L, et al. How to restore superlow friction of DLC: the healing effect of hydrogen gas[J]. Tribology international, 2004, 37(11-12): 869-877.
[3] HAUERT R. An overview on the tribological behavior of diamond-like carbon in technical and medical applications [J]. Tribology international, 2004, 37(11-12): 991-1003.
[4] JOHNSON J A, WOODFORD J B, CHEN X, et al. Insights into “near-frictionless carbon films”[J]. Journal of applied physics, 2004, 95(12): 7765-7771.
[5] ERDEMIR A, DONNET C. Tribology of diamond-like carbon films: recent progress and future prospects[J]. Journal of physics D: applied physics, 2006, 39(18): R311-R327.
[6] 薛群基, 王立平. 类金刚石碳基薄膜材料[M]. 北京: 科学出版社, 2012. XUE Qun-ji, WANG Li-ping. Diamond-like carbon-based film materials[M]. Beijing: Science Publishing Company, 2012.
[7] ERDEMIR A, RAMIREZ G, ERYILMAZ O L, et al. Carbon-based tribofilms from lubricating oils[J]. Nature, 2016, 536(7614): 67-71.
[8] BERMAN D, DESHMUKH S A, SANKARANARAYANAN S K, et al. Macroscale superlubricity enabled by graphene nanoscroll formation[J]. Science, 2015, 348(6239): 1118-1122.
[9] 崔丽, 孙丽丽, 郭鹏, 等. 自组织分层金属掺杂类金刚石薄膜的研究进展[J]. 表面技术, 2019, 48(11): 23-35. CUI Li, SUN Li-li, GUO Peng, et al. Research progress in metal-doped diamond-like carbon films with self-organized multilayer structure[J]. Surface technology, 2019, 48(11): 23-35.
[10] CHEN X, KATO T. Growth mechanism and composition of ultrasmooth a-C:H:Si films grown from energetic ions for superlubricity[J]. Journal of applied physics, 2014, 115(4): 044908.
[11] WANG A Y, LEE K R, AHN J P, et al. Structure and mechanical properties of W incorporated diamond-like carbon films prepared by a hybrid ion beam deposition technique[J]. Carbon, 2006, 44(9): 1826-1832.
[12] KOSHIGAN K D, MANGOLINI F, MCCLIMON J B, et al. Understanding the hydrogen and oxygen gas pressure dependence of the tribological properties of silicon oxide- doped hydrogenated amorphous carbon coatings[J]. Carbon, 2015, 93: 851-860.
[13] ERDEMIR A, ERYILMAZ O. Achieving superlubricity in DLC films by controlling bulk, surface, and tribochemistry[J]. Friction, 2014, 2(2): 140-155.
[14] CHEN X, LI J. Superlubricity of carbon nanostructures[J]. Carbon, 2020, 158: 1-23.
[15] DONNET C, FONTAINE J, GRILL A, et al. The role of hydrogen on the friction mechanism of diamond-like carbon films[J]. Tribology letters, 2001, 9(3-4): 137-142.
[16] ERDEMIR A, ERYILMAZ O L, FENSKE G. Synthesis of diamondlike carbon films with superlow friction and wear properties[J]. Journal of vacuum science & technology A: vacuum, surfaces, and films, 2000, 18(4): 1987-1992.
[17] CASIRAGHI C, PIAZZA F, FERRARI A C, et al. Bonding in hydrogenated diamond-like carbon by raman spectroscopy[J]. Diamond and related materials, 2005, 14(3-7): 1098-1102.
[18] CASIRAGHI C, FERRARI A C, ROBERTSON J. Raman spectroscopy of hydrogenated amorphous carbons[J]. Physical review B, 2005, 72(8): 085401.
[19] LIU X, YANG J, HAO J, et al. A near-frictionless and extremely elastic hydrogenated amorphous carbon film with self-assembled dual nanostructure[J]. Advanced materials, 2012, 24(34): 4614-4617.
[20] CHEN X, ZHANG C, KATO T, et al. Evolution of tribo- induced interfacial nanostructures governing superlubricity in a-C:H and a-C:H:Si films[J]. Nature communications, 2017, 8(1): 1675.
[21] CHEN X, YIN X, QI W, et al. Atomic-scale insights into the interfacial instability of superlubricity in hydrogenated amorphous carbon films[J]. Science advances, 2020, 6: eaay1272.
[22] ERDEMIR A. Genesis of superlow friction and wear in diamondlike carbon films[J]. Tribology International, 2004, 37(11-12): 1005-1012.
[23] WANG Y, GAO K, ZHANG B, et al. Structure effects of sp2-rich carbon films under super-low friction contact[J]. Carbon, 2018, 137: 49-56.
[24] FONTAINE J, LOUBET J, MOGNE T L, et al. Superlow friction of diamond-like carbon films: a relation to viscoplastic properties[J]. Tribology letters, 2004, 17(4): 709-714.
[25] ENKE K, DIMIGEN H, HÜBSCH H. Frictional properties of diamondlike carbon layers[J]. Applied physics letters, 1980, 36(4): 291-292.
[26] HEIMBERG J A, WAHL K J, SINGER I L, et al. Superlow friction behavior of diamond-like carbon coatings: time and speed effects[J]. Applied physics letters, 2001, 78(17): 2449-2451.
[27] KIM H I, LINCE J R, ERYILMAZ O L, et al. Environmental effects on the friction of hydrogenated DLC films[J]. Tribology letters, 2006, 21(1): 51-56.
[28] LIU H, TANAKA A, UMEDA K. The tribological characteristics of diamond-like carbon films at elevated temperatures[J]. Thin solid films, 1999, 346(1-2): 162-168.
[29] LI H, XU T, WANG C, et al. Friction behaviors of hydrogenated diamond-like carbon film in different environment sliding against steel ball[J]. Applied surface science, 2005, 249(1-4): 257-265.
[30] FONTAINE J, MOGNE T L, LOUBET J L, et al. Achieving superlow friction with hydrogenated amorphous carbon: some key requirements[J]. Thin solid films, 2005, 482(1-2): 99-108.
[31] DONNET C, MOGNE T L, PONSONNET L, et al. The respective role of oxygen and water vapor on the tribology of hydrogenated diamond-like carbon coatings[J]. Tribology letters, 1998, 4(3-4): 259-265.
[32] ERDEMIR A, ERYILMAZ O, KIM S. Effect of tribochemistry on lubricity of DLC films in hydrogen[J]. Surface and coatings technology, 2014, 257: 241-246.
[33] FONTAINE J, DONNET C, GRILL A, et al. Tribochemistry between hydrogen and diamond-like carbon films[J]. Surface and coatings technology, 2001, 146: 286-291.
[34] SANCHEZ-LOPEZ J C, DONNET C, FONTAINE J, et al. Diamond-like carbon prepared by high density plasma[J]. Diamond and related materials, 2000, 9(3-6): 638-642.
[35] DONNET C, FONTAINE J, MOGNE T L, et al. Diamond- like carbon-based functionally gradient coatings for space tribology[J]. Surface and coatings technology, 1999, 120: 548-554.
[36] ERDEMIR A, ERYILMAZ O, NILUFER I, et al. Effect of source gas chemistry on tribological performance of diamond-like carbon films[J]. Diamond and related materials, 2000, 9(3-6): 632-637.
[37] JI L, LI H, ZHAO F, et al. Effects of environmental molecular characteristics and gas-surface interaction on friction behaviour of diamond-like carbon films[J]. Journal of physics D: applied physics, 2009, 42(13): 135301.
[38] WANG C, LI B, LING X, et al. Superlubricity of hydrogenated carbon films in a nitrogen gas environment: adsorption and electronic interactions at the sliding interface [J]. RSC advances, 2017, 7(5): 3025-3034.
[39] ERYILMAZ O L, ERDEMIR A. Surface analytical investigation of nearly-frictionless carbon films after tests in dry and humid nitrogen[J]. Surface and coatings technology, 2007, 201(16-17): 7401-7407.
[40] MARINO M J, HSIAO E, CHEN Y, et al. Understanding run-in behavior of diamond-like carbon friction and preventing diamond-like carbon wear in humid air[J]. Langmuir, 2011, 27(20): 12702-12708.
[41] KONCA E, CHENG Y T, WEINER A M, et al. Vacuum tribological behavior of the non-hydrogenated diamond- like carbon coatings against aluminum: effect of running- in in ambient air[J]. Wear, 2005, 259(1-6): 795-799.
[42] ZENG Q, ERDEMIR A, ERYLIMAZ O. Ultralow friction of ZrO2ball sliding against DLC films under various environments[J]. Applied sciences, 2017, 7(9): 938.
[43] ANDERSSON J, ERCK R, ERDEMIR A. Frictional behavior of diamondlike carbon films in vacuum and under varying water vapor pressure[J]. Surface and coatings technology, 2003, 163: 535-540.
[44] KONICEK A R, GRIERSON D S, SUMANT A V, et al. Influence of surface passivation on the friction and wear behavior of ultrananocrystalline diamond and tetrahedral amorphous carbon thin films[J]. Physical review B, 2012, 85(15): 155448.
[45] CHEN X, KATO T, NOSAKA M. Origin of superlubricity in a-C:H:Si films: a relation to film bonding structure and environmental molecular characteristic[J]. ACS applied materials & interfaces, 2014, 6(16): 13389-13405.
[46] HAYASHI K, TEZUKA K, OZAWA N, et al. Tribochemical reaction dynamics simulation of hydrogen on a diamond-like carbon surface based on tight-binding quantum chemical molecular dynamics[J]. The journal of physical chemistry C, 2011, 115(46): 22981-22986.
[47] LI X, WANG A, LEE K R. Atomistic understanding on friction behavior of amorphous carbon films induced by surface hydrogenated modification[J]. Tribology international, 2019, 136: 446-454.
[48] PASTEWKA L, MOSER S, MOSELER M. Atomistic insights into the running-in, lubrication, and failure of hydrogenated diamond-like carbon coatings[J]. Tribology letters, 2010, 39(1): 49-61.
[49] CHEN Y, MA T, CHEN Z, et al. Combined effects of structural transformation and hydrogen passivation on the frictional behaviors of hydrogenated amorphous carbon films[J]. The journal of physical chemistry C, 2015, 119(28): 16148-16155.
[50] CUI L, LU Z, WANG L. Probing the low-friction mechanism of diamond-like carbon by varying of sliding velocity and vacuum pressure[J]. Carbon, 2014, 66: 259-266.
[51] BAI S, MURABAYASHI H, KOBAYASHI Y, et al. Tight- binding quantum chemical molecular dynamics simulations of the low friction mechanism of fluorine-terminated diamond-like carbon films[J]. RSC advances, 2014, 4(64): 33739.
[52] ZENG Q, ERYILMAZ O, ERDEMIR A. Superlubricity of the DLC films-related friction system at elevated temperature[J]. RSC advances, 2015, 5(113): 93147-93154.
[53] SONG H, JI L, LI H, et al. Perspectives of friction mechanism of a-C:H film in vacuum concerning the onion- like carbon transformation at the sliding interface[J]. RSC advances, 2015, 5(12): 8904-8911.
[54] MA T, WANG L, HU Y, et al. A shear localization mechanism for lubricity of amorphous carbon materials[J]. Scientific reports, 2014, 4: 3662.
[55] MA T, HU Y, WANG H. Molecular dynamics simulation of shear-induced graphitization of amorphous carbon films[J]. Carbon, 2009, 47(8): 1953-1957.
[56] WANG D, CHANG S, HUANG Y, et al. Nanoscopic observations of stress-induced formation of graphitic nanocrystallites at amorphous carbon surfaces[J]. Carbon, 2014, 74: 302-311.
[57] BOUCHET M I D B, MARTIN J M, AVILA J, et al. Diamond-like carbon coating under oleic acid lubrication: Evidence for graphene oxide formation in superlow friction[J]. Scientific reports, 2017, 7: 46394.
[58] KUWAHARA T, ROMERO P A, MAKOWSKI S, et al. Mechano-chemical decomposition of organic friction modifiers with multiple reactive centres induces superlubricity of ta-C[J]. Nature communications, 2019, 10(1): 151.
[59] LIU Y, YU B, CAO Z, et al. Probing superlubricity stability of hydrogenated diamond-like carbon film by varying sliding velocity[J]. Applied surface science, 2018, 439: 976-982.
[60] LIU Y, CHEN L, ZHANG B, et al. Key role of transfer layer in load dependence of friction on hydrogenated diamond-like carbon films in humid air and vacuum[J]. Materials (basel), 2019, 12(9): 1550.
[61] CHEN C, XUE P, FAN X, et al. Friction-induced rapid restructuring of graphene nanocrystallite cap layer at sliding surfaces: short run-in period[J]. Carbon, 2018, 130: 215-221.
[62] MANIMUNDA P, AL-AZIZI A, KIM S H, et al. Shear-induced structural changes and origin of ultralow friction of hydrogenated diamond-like carbon (DLC) in dry environment[J]. ACS applied materials & interfaces, 2017, 9(19): 16704-16714.
[63] MERKLE A P, ERDEMIR A, ERYILMAZ O L, et al. In situ TEM studies of tribo-induced bonding modifications in near-frictionless carbon films[J]. Carbon, 2010, 48(3): 587-591.
[64] M'NDANGE-PFUPFU A, ERYILMAZ O, ERDEMIR A, et al. Quantification of sliding-induced phase transformation in N3FC diamond-like carbon films[J]. Diamond and related materials, 2011, 20(8): 1143-1148.
[65] BERNAL R A, CHEN P, SCHALL J D, et al. Influence of chemical bonding on the variability of diamond-like carbon nanoscale adhesion[J]. Carbon, 2018, 128: 267-276.
[66] ERYILMAZ O L, ERDEMIR A. TOF-SIMS and XPS characterization of diamond-like carbon films after tests in inert and oxidizing environments[J]. Wear, 2008, 265(1-2): 244-254.
[67] ERYILMAZ O L, ERDEMIR A. Investigation of initial and steady-state sliding behavior of a nearly frictionless carbon film by imaging 2- and 3-D TOF-SIMS[J]. Tribology letters, 2007, 28(3): 241-249.
Research Status and Challenges of Solid Superlubricity of Diamond-like Carbon Film
,,
(State Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China)
Diamond-like carbon (DLC) films have shown significant application prospects in the field of solid lubrication due to their high hardness, good chemical inertness, low friction and wear properties and especially the superlubricty (friction coefficient lower than 0.01) performance under some certain conditions, which can provide new opportunities for the realization of near-zero friction and wear. Various DLC films (DLCs) were summarized according to the differences in doped elements and bonding structures, and the corresponding mechanical and tribological properties were also overviewed. By comparing the tribological properties of DLCs under different environmental conditions, the sensitivity of DLCs’ friction to environmental atmosphere was mainly illustrated, where the hydrogen atoms in films and atmosphere played important roles. At the same time, a feasible scheme to improve the environmental sensitivity of superlubricity by doping Si element or other elements was proposed. Three kinds of DLCs’ superlubricity mechanisms were mainly introduced: surface passivation theory, graphitization theory and transfer film formation theory. All three mechanisms mentioned above had limitations in some extent, and it was still a scientific difficulty to systematically understand and explore the superlubricity mechanisms of DLCs. Finally, the importance of advanced characterization techniques for the detection of chemistry and microstructure of superlubric interface was emphasized, and further research directions were also proposed.
diamond-like carbon film; superlubricity; structural diversity; environmental sensitivity; superlubricity mechanisms; characterization techniques
2020-04-22;
2020-05-15
WANG Kang(1995—),Male, Ph. D. candidate, Research focus: solid superlubricity of diamond-like carbon.
马天宝(1980—),男,博士,副教授,主要研究方向为固体超滑的机理和实现。
Corresponding author:MA Tian-bao (1980—), Male, Doctor, Associate professor, Research focus: realization and mechanism researches of solid superlubricity.
王康, 陈新春, 马天宝. 类金刚石薄膜固体超滑的研究现状和挑战[J]. 表面技术, 2020, 49(6): 10-21.
TH117;O313.5
A
1001-3660(2020)06-0010-12
10.16490/j.cnki.issn.1001-3660.2020.06.002
2020-04-22;
2020-05-15
国家自然科学基金项目(51935006,51975314);国家科技重大专项(2017-VII-0013-0110)
Fund:Supported by the National Natural Science Foundation of China (51935006, 51975314) and National Science and the Technology Major Project (2017-VII-0013-0110)
王康(1995—),男,博士研究生,主要研究方向为类金刚石薄膜固体超滑。
WANG Kang, CHEN Xin-chun, MA Tian-bao. Research status and challenges of solid superlubricity of diamond-like carbon film[J]. Surface technology, 2020, 49(6): 10-21.
