三维氧化铁/石墨烯的构建及其对CL-20的热分解性能的影响
2020-06-30张婷李翠翠王伟郭兆琦庞爱民马海霞
张婷 ,李翠翠 ,王伟 ,郭兆琦 ,庞爱民 , ,马海霞 ,
1西北大学化工学院,西安 710069
2湖北航天化学技术研究所,航天化学动力技术重点实验室,湖北 襄阳441003
1 Introduction
Nano metal oxides are of great importance in catalysts, sensor,capacitor and water treatment because of their unique properties,such as surface effect, small size effect and quantum size effect1-3.For explosives or propellants, nano metal oxides are important additives as combustion catalysts, which are mainly utilized in regulating the thermal decomposition behavior and burning rate as well as enhanced combustion performance4-6, which contribute to evidently modify the thermal conductivity, the heat of reaction and energy barrier of pyrolysis of energetic ingredients7. However, nano-materials are easy to aggregate,resulting in the decreased specific surface area and reduced active sites.
Graphene has large surface area, high conductivity and excellent mechanical properties8. Benefiting from its excellent properties, graphene can be used as an ideal carrier for constructing new supported materials. Coupling metal oxides with graphene forming a binary composite is an effective strategy due to the synergistic effects. And composite materials often exhibit excellent properties, which cannot be achieved with a single component. Research finds that metal oxide/graphene composite, as a new type of combustion catalyst, shows an improved catalytic activity on the pyrolysis of energetic components by the multi-component synergistic effect9.Besides, graphene is also an excellent additive for constructing the insensitive energetic materials, which can be used to improve the safety performance of energetic component10,11.
Hexanitrohexaazaisowurtzitane (CL-20) is mainly used as energetic component in solid propellants12. However, its high sensitivity limits its application13. Researches mainly focus on the catalytic activity of metal oxides and metal oxides/graphene on the thermal decomposition of some energetic materials(EMs),such as ammonium perchlorate (AP),cyclotrimethylenetrinitramine (RDX), and cyclotetramethylenete-tranitramine (HMX) with less concern on the effect on the sensitivity to EMs14-17. Herein, exploring new materials with increased catalytic activity and decreased sensitivity are highly desirable. Iron oxide is a common metal oxide in nature and the most commonly used combustion catalyst in solid propellants. In our previous work, different morphological iron oxides had been synthesized and used to study the catalytic action on the thermal decomposition of energetic components. Results found that the specific surface area of iron oxide has a vital role in thermal decomposition of nitrocellulose18. Inspired by this study, iron oxide/graphene binary composites with three-dimensional macroporous structure and high specific surface area through the coupling between iron oxide and graphene were constructed by interface self-assembly. On the one hand, these composite materials are expected to promote the thermal decomposition of CL-20. On the other hand, it is expected to improve the safety performance of CL-20. In this study, the thermal behaviour of CL-20 under the catalytic action of Fe2O3/G was investigated systematically.And the safety performance of CL-20 was further evaluated by impact sensitivity test.
2 Experimental and computational section
2.1 Reagents and materials
All of the reagents were analytical reagent (AR) from Sinopharm Chemical Reagent Co., Ltd. and no further purified.CL-20 is explosive hazardous material and provided by Xi’an modern chemistry research institute. Metal shovel is strictly prohibited. Fire and electrostatic discharge should be avoided.
2.2 Preparation of rFe2O3 and rFe2O3/G
The synthetic procedures of rFe2O3and rFe2O3/G are as follows: 0.249 g Fe(NO)3·9H2O was dissolved into 10 mL H2O with stirring for 30 min. After that, 2.5 mol·L−1NaOH aqueous solution was dropped into the above solution with constantly stirring. Then the prepared mixture was transferred into 25 mL teflon-lined stainless steel autoclave and maintained at 150 °C for 12 h. At last, the yellow products (FeO(OH)) were obtained and washed with deionized water and absolute ethanol for several times and freezy-dried at −50 °C for 12 h. Subsequently,the samples were calcined in a muffle furnace at 300 °C for 1 h.The product was labeled as rFe2O3. And then the as-prepared rFe2O3was re-dispersed to 100 mL GO (1 mg·mL−1) aqueous solution in a round bottom flask. The mixture was ultrasonicated for 2 h to obtain a homogeneous solution. 0.8 g NaBH4was added into the solution slowly, and then heated at 80 °C for 2 h with stirring constantly. Finally, the obtained product was washed with deionized water and absolute ethanol and freezydried at −50 °C for 12 h, which was denoted as rFe2O3/G.
2.3 Preparation of pFe2O3 and pFe2O3/G
0.249 g Fe(NO)3·9H2O was dissolved into 10 mL·H2O with stirring for 30 min. Next, 2.5 mol·L−1urea aqueous solution (5 mL) was dropped in the above solution with constant stirring.The mixture was transferred into 25 mL teflon-lined stainless steel autoclave and maintained at 150 °C for 12 h. The products were labeled as pFe2O3. The prepared procedure of pFe2O3/G is the same as rFe2O3/G.
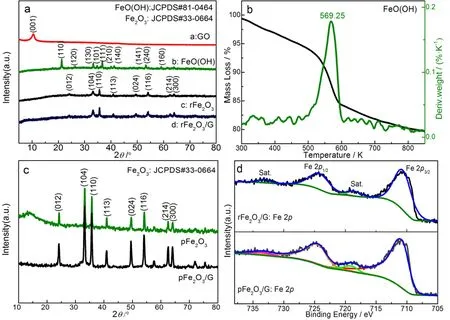
Fig. 1 (a) XRD patterns of rFe2O3 and rFe2O3/G; (b) TG-DTG curve of FeO(OH); (c) XRD patterns of pFe2O3 and pFe2O3/G; and(d) XPS spectra of Fe 2p of pFe2O3/G and rFe2O3/G.
2.4 Characterization
The structures of as-prepared materials were characterized by X-ray diffractometer (XRD) with CuKαradiation (Rigaku,Japan). The morphologies were detected by scanning electron microscope (SEM, Zeiss SIGMA, Germany) and transmission electron microscope (TEM, Zeiss Libra 200FE, Germany).Element composition and valence state were investigated by X-ray photoelectron spectroscopy (XPS, Thermo Scientific, USA).The specific surface area was measured using a N2adsorptiondesorption equipment (TriStar II plus 2.02, USA). The thermal behaviors of the samples were determined using differential scanning calorimetry (DSC, Q2000, TA, USA.) and simultaneous thermal analysis (SDT, Q600, TA, USA) in N2atmosphere.
3 Results and discussion
3.1 Characterization of Fe2O3 and Fe2O3/G
As shown in Fig. 1a, XRD analysis indicates that the precursor is goethite, all diffraction peaks can be assigned to the (110),(120), (130), (101), (024), (111), (210), (140), (141), (240) and(106) lattice planes of FeO(OH) (JCPDS No. 81-0464). Because goethite could form hematite by dehydration, a rod-like Fe2O3can be obtained by the calcination of FeO(OH) at 573.15 K for 1 h (The calcination temperature of FeO(OH) is determined by TG-DTG analysis as shown in Fig. 1b). The diffraction patterns of rFe2O3and rFe2O3/G in Fig. 1a are almost identical. All the diffraction peaks could be indexed as the (012), (104), (110),(113), (024), (116), (122), (214) and (300) lattice planes of α-Fe2O3(JCPDS No. 33-0664)19. The XRD patterns of pFe2O3and pFe2O3/G are shown in Fig. 1c. When sodium hydroxide was replaced by urea, the obtained red products also can be indexed as α-Fe2O3(JCPDS No. 33-0664). After coupling with graphene,the characteristic peaks of rFe2O3/G and pFe2O3/G do not change, indicating the phase composition and crystallinity remain constant. Furthermore, XPS was used to identify the chemical state of Fe in Fe2O3/G composite. The corresponding Fe2pspectra of rFe2O3/G and pFe2O3/G are shown in Fig. 1d.For pFe2O3/G, the peaks located at 710.66 eV and 724.13 eV correspond to the Fe 2p3/2 and Fe 2p1/2, respectively. Two accompanying satellite peaks located at 718.53 eV and 733.05 eV further reveal the existence of Fe3+3,20. And the deconvolution Fe 2ppeaks in pFe2O3/G assigned to Fe 2p1/2and Fe 2p3/2are located 710.84 eV and 724.41 eV, respectively. Two satellite peaks similarly identify the chemical state of Fe is Fe3+.

Fig. 2 SEM and TEM images of (a, b) FeO(OH) and (c, d) rFe2O3; (e, f) SEM image of GO and rFe2O3/G; and(g, h) particle size distribution of rFe2O3

Fig. 3 (a, b) SEM image of pFe2O3 and pFe2O3/G; and (c) the statistical distribution of particle size of pFe2O3.
As shown in Fig. 2a,b, a yellow FeO(OH) with a rod-like morphology can be obtained. The inserted high resolution transmission electron microscope (HRTEM) image shows a distinct lattice fringe with a spacing of 0.41 nm, corresponding to the (110) plane of FeO(OH). It is found that the micromorphology of Fe2O3remains constant after high temperature calcination from Fig. 2c,d. The lattice distance of Fe2O3 is 0.27 nm corresponding to (104) plane as shown in the inserted image of Fig. 2d. Fig. 2e reveals a wrinkled and multilayer structured graphene oxide prepared by improved hummer method. It can be seen that Fe2O3 nanoparticles (NPs) attach on the graphene sheets and form three-dimension porous skeleton structure from Fig. 2f. Graphene could prevent Fe2O3from agglomerating and in turn Fe2O3can restrict the stack of graphene sheets via the interfacial interaction. The particle size distribution patterns of rFe2O3/rGO are shown in Fig. 2g,h. The average width of Fe2O3is about (38.66 ± 1.84) nm and the average length is about(285.47 ± 2.17) nm. When sodium hydroxide was replaced by urea, the granular Fe2O3can be formed and a serious agglomeration phenomenon emerges as shown in Fig. 3a. The average particle size is about (40.69 ± 0.59) nm (Fig. 3c) from the statistical distribution diagram of the particle size of pFe2O3.Fig. 3b shows that the surface of graphene sheet is overlaid by pFe2O3 NPs. As shown in Fig. 4, the hysteresis loops appear at relative highP/P0region, which shows a typical “type IV”isotherm from the adsorption-desorption isotherms of rFe2O3/G21.In comparison with pure rFe2O3, its BET surface area increases from 34.8 to 80.4 m2·g−1. And the N2adsorption-desorption isotherms of pFe2O3/G and pFe2O3 also can be identified as ‘type IV’. According to the Brunauer-Emmett-Teller (BET) equation,pFe2O3/G exhibits a larger surface area (78.1 m2·g−1) than that of pFe2O3(33.6 m2·g−1).
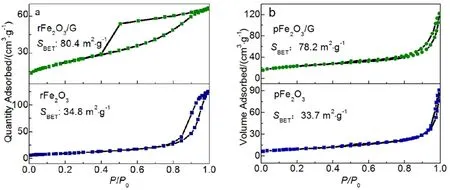
Fig. 4 Nitrogen adsorption-desorption isotherms of (a) rFe2O3 and rFe2O3/G and (b) pFe2O3 and pFe2O3/G.
3.2 Catalytic performance
DSC is used for evaluating the thermal decomposition characteristics and thermal behaviors of energetic materials22.In this work, we firstly studied the catalytic activity of asprepared Fe2O3and Fe2O3/G on the thermal decomposition of CL-20 by DSC at the heating rate (β) of 10 K·min−1. The samples were placed in aluminium pans and sample sizes of about 0.2 mg were employed. Fig. 5a demonstrates pure CL-20 has an exothermic behaviour with a peak temperature ofTp= 525.28 K.And the decomposition temperature of CL-20 decrease to 516.94, 517.59, 519.00 and 519.44 K under the catalytic action of rFe2O3/G, pFe2O3/G, rFe2O3and pFe2O3, respectively. All prepared samples show a degree of catalytic effect for thermal decomposition of CL-20. rFe2O3/G, pFe2O3/G, rFe2O3and pFe2O3decrease the thermal decomposition peak temperature of CL-20 by 8.34, 7.69, 6.28 and 5.84 K, respectively. The corresponding TG curves reveal that the weight loss rate reaches the maximum at 515.04, 515.75, 516.87 and 517.82 K, which are all lower than that of pure CL-20 (Tp= 522.88 K; Fig. 5b).Besides, one can see that rFe2O3/G/CL-20 shows a more enhanced catalytic activity on the thermal decomposition of CL-20 compared with rFe2O3/CL-20. The same trend occurs in pFe2O3/G and pFe2O3, which may be due to the synergistic effect between Fe2O3and graphene.

Fig. 5 (a) DSC and (b) TG-DTG curves of CL-20 mixed with different catalysts.
Furthermore, we studied the kinetic parameters [apparent activation energy (Ea, kJ·mol−1), pre-exponential constant(lg(A/s−1))] and the most probable model function of the exothermic decomposition process of prepared samples by DSC at four different heating rates (5, 7.5, 10 and 12.5 K·min−1). The values ofEOcorresponding toα(reaction fraction)were obtaineby Flynn-Wall-Ozawa method (Eq. 1)23and an integral iso-conversional non-linear (NL-INT) method (Eq. 2)24was used to check the validity ofEO. As shown in Fig. 6, the values ofEOare almost equal toEN, which further demonstrates thatEOis credible25,26.
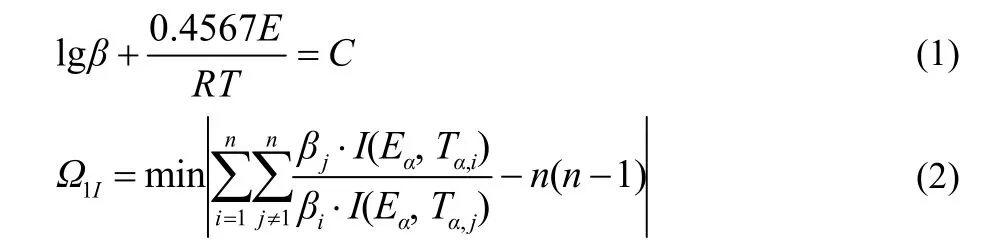
Substituting forty-one types of kinetic model functions and original data (the temperatures corresponding to the selection range ofαand the corresponding heating rate) into five integral ethods (general integral, universal integral, MacCallum-Tanner,Šatava-Šesták, Agrawal)27, a newEa can be obtainedviaa logical choice method and is approximately equal with the calculated activation energy obtained from the peak temperature(Tp) by Kissinger method and Ozawa method. And the No. 11 function (Avrami-Erofeev equation:f(a) = 3(1 −a)[−ln(1 −a)]2/3) can be selected as the most probable model function for the exothermic decomposition reaction of rFe2O3/G/CL-20 by logical choice method. Substitutingf(α) = 3(1 −ɑ)[−ln(1 −ɑ)]2/3,A(1013.76s−1) andEa(157.67 kJ·mol−1) into the (Eq. 3), the most probable kinetic equation (Eq. 4) for rFe2O3/G/CL-20 can be obtained. Besides, we found that the kinetic equations of other prepared samples (CL-20, rFe2O3/CL-20, pFe2O3/G/CL-20 and pFe2O3/CL-20) all follow the No. 11 mechanism function. The detailed data for five prepared samples are listed in Tables 1 and 2.


Fig. 6 E vs α curves of prepared samples (EO obtained by Ozawa method and EN obtained by NL-INT method).
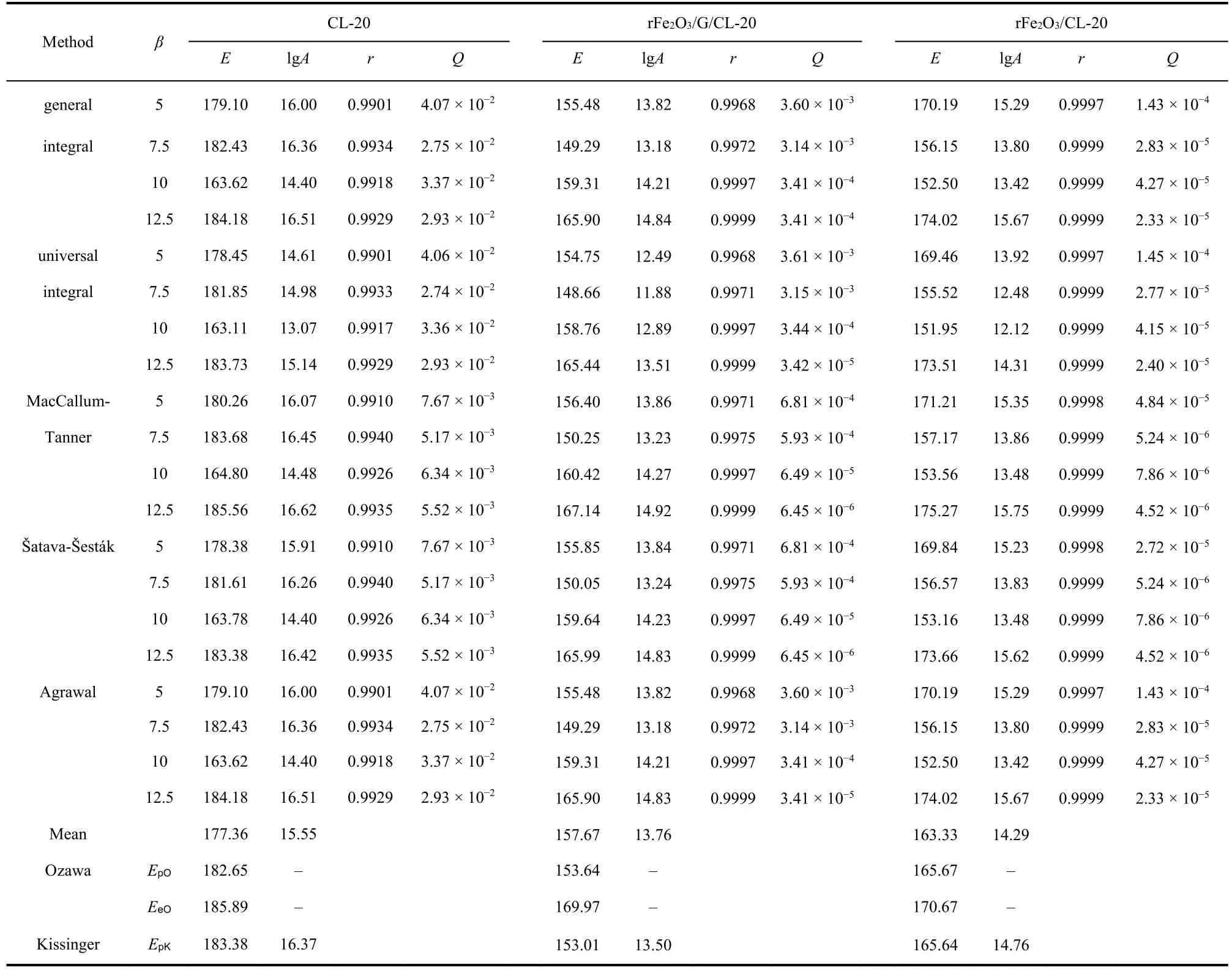
Table 1 The calculated thermal decomposition kinetics parameters of CL-20, rFe2O3/G/CL-20 and rFe2O3/CL-20.
By the contrast analysis, bothEandAof rFe2O3/G/CL-20 are the lowest, indicating rFe2O3/G can facilitate the thermal decomposition of CL-20 more easily. It may be the synergistic action between rFe2O3and graphene which endows the composite a bigger surface area and more pore structure than other samples, and makes it easily adsorb small molecules and ions produced by the decomposition of CL-20.
Furthermore, we also studied whether rFe2O3/G has high catalytic activity on HMX and RDX. Fig. 7 shows the exothermic decomposition temperatures of pure HMX and RDX are 556.31 and 515.65 K at the heating rate of 10 K·min−1,respectively. After mixed with prepared catalyst, a significant change has been found in the decomposition process of HMX and RDX. There is a distinct endothermic melting peak for pure HMX, which entirely disappears after the addition of rFe2O3/G or rFe2O3(marked with red box). In fact, the degradation of HMX occurs in the melting state. In other word, the liquid phase of HMX is composed of HMX molecules and some decomposition products. When the catalyst was added, the decomposition rate of HMX in the liquid phase was accelerated28,29.The initial decomposition temperature of rFe2O3/G/HMX is lower than that of rFe2O3/HMX and the exothermic decomposition peak temperatures decrease to 551.72 K and 553.18 K from 556.31 K, respectively. In Fig. 7b, though there is no change in the position of the endothermic temperature of RDX, the exothermic peak has an evident change under the addition of rFe2O3or rFe2O3/G. The DSC curve of rFe2O3/RDX show a sharp peak, and a broaden heat flow curve was obtained by the addition of rFe2O3/G with a peak temperature of 507.23 K. It follows that rFe2O3/G can catalyze the exothermic decomposition of CL-20 as well as HMX and RDX.

Table 2 The calculated thermal decomposition kinetics parameters of pFe2O3/G/CL-20 and pFe2O3/CL-20.

Fig. 7 DSC curves of (a) rFe2O3/G and (b) rFe2O3 mixed with HMX and RDX.
The gases products were detected by the real time FTIR during the whole pyrolysis process of CL-20 mixed with rFe2O3/G and rFe2O3, which reveals that a complex mixture forms in Ar atmosphere. And the apparent variations in the IR absorption peaks of the gaseous decomposition products of three samples are shown in Fig. 8 at five typical temperatures (initial temperatureTi, extrapolated onset temperatureTo, peak temperatureTp; the extrapolated end temperatureTeand the final temperatureTf). The main gas phase products can be identified by their characteristic IR absorbances: H2O (3600-3740 cm−1),CO2(2300-2380 cm−1), CO (2150-2194 cm−1), NO2(1593-1635 cm−1), N2O (2200-2300 cm−1) and HCHO (2700-2900 cm−1)23,30,31. At the initial decomposition temperature of CL-20,the H2O and CO2 are formed, along with some amount of NO2.As the temperature increases, the other gases could be detected.One can see that more gases release at the initial temperature of rFe2O3/G/CL-20, including NO2, N2O, H2O and CO2. Notably,for rFe2O3/G/CL-20, the whole decomposition process occurs at a lower temperature compared with CL-20 or rFe2O3/CL-20,indicating that rFe2O3/G can accelerate the bond cleavage of CL-20 faster than rFe2O3.

Fig. 8 FTIR spectra of the evolved gases at different temperature for (a) CL-20; (b) rFe2O3/CL-20 and (c) rFe2O3/G/CL-20.
Moreover, the self-accelerating decomposition temperature(TSADTorTe0, K), thermal ignition temperature (Tbe0orTTIT, K),critical thermal explosion temperature (Tb,K), the corresponding entropy of activation (ΔS≠, J·K−1·mol−1), enthalpy of activation(ΔH≠, kJ·mol−1) and free energy of activation (ΔG≠, kJ·mol−1) of the decomposition reaction of prepared samples could be obtained by following Equations (5-9), respectively. According these results, one can evaluate its thermal safety of prepared samples26,27,32.
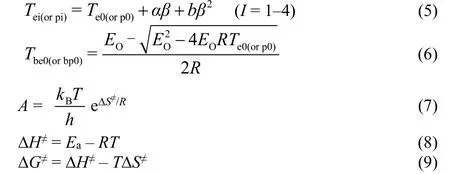
whereaandbare coefficients.T=Tp0,Ea=EKandA=AK.kBis the Boltzmann constant (1.381 × 10−23J·K−1) andhis the Planck constant (6.626 × 10−34J·s).
As shown in Table 3, the values of thermal safety parameters show a decreasing trend with the additions of rFe2O3and rFe2O3/G, respectively. However, the effect of rFe2O3/G on the explosion temperature of CL-20 is lower than that of rFe2O3.Whether this phenomenon is the effect of graphene, we test the impact sensitivity (IS) of prepared samples. A fall hammer apparatus is used for the determination of impact sensitivity using 2 kg drop weight with maximum height of 120 cm. The explosion of CL-20 emerges at a height of 75 cm with an IS value of 14.7 J. The IS values of rFe2O3/CL-20 is 100 cm corresponding to an impact energy of 19.6 J. However,rFe2O3/G/CL-20 shows reduced impact sensitivity (110 cm,21.56 J) than rFe2O3/CL-20. Combined with the calculated explosion temperature, we deduce that the mechanical property and heat-conduction of graphene may responsible for the reducing of internal hot spots10. In other words, the interaction between rFe2O3and graphene is helpful for accelerating the thermal decomposition of CL-20, other than initiate explosion,indicating that graphene-based catalyst has a better thermal safety for storage or transport.

Table 3 Thermal safety parameters of three prepared samples.
4 Conclusions
In summary, Fe2O3 NPs with two morphologies of rod-like and granular were synthesized, and then Fe2O3NPs coupled with graphene sheets were achieved through an interface assembly process. Compared with rFe2O3, pFe2O3and pFe2O3/G,rFe2O3/G showed the best catalytic activation for the pyrolysis of CL-20 with the lowest thermal decomposition temperature(516.94 K), apparent activation energy (157.67 kJ·mol−1) and pre-exponential constant (1013.76s−1). The most probable kinetic model function of rFe2O3/G/CL-20 is classified as Avrami-Erofeev equation:f(ɑ) = 3(1−ɑ)[−ln(1 −ɑ)]2/3. As a conducting framework for sustaining rFe2O3 NPs, graphene nanosheets endow its abundant pore structure and large surface area, which is helpful to accelerate the thermal decomposition of CL-20.Besides, the decreased impact sensitivity of rFe2O3/G/CL-20 compared with CL-20 may due to the excellent mechanical property and conduction of graphene, which can reduce the internal hot spots. This work may greatly stimulate the practical application of graphene/metal oxides in propellants as catalytic material.
