Effects of Acute Temperature Stress on mRNA Expression of Transferrin in the Yellow Pond Turtle Mauremys mutica
2020-06-28YufengWEIYangchunGAODainanCAOYanGEHaitaoSHIandShipingGONG
Yufeng WEI ,Yangchun GAO ,Dainan CAO ,Yan GE ,Haitao SHI and Shiping GONG*
1 Ministry of Education Key Laboratory for Ecology of Tropical Islands,College of Life Sciences,Hainan Normal University,Haikou 571158,Hainan,China
2 Guangdong Key Laboratory of Animal Conservation and Resource Utilization,Guangdong Public Laboratory of Wild Animal Conservation and Utilization,Guangdong Institute of Applied Biological Resources,Guangdong Academy of Science,Guangzhou 510260,Guangdong,China
Abstract The yellow pond turtle Mauremys mutica is widely cultured using both greenhouse-reared and outdoor pond-reared models.Individuals from the two models often show different tolerances to dramatic temperature changes caused by extreme weather events.However,the mechanism underlying the difference is unclear.In this study,we found that for greenhouse-reared turtles (GRTs),the expression levels of an immune-related gene for transferrin were significantly different (P < 0.05) between the control group and the acute cold stress (ACS) group for most time points (3 h,6 h and 48 h),while at two time points (6 h and 12 h) there was a significant difference (P <0.05) between the control group and the acute heat stress(AHS) group.However,for the outdoor pond-reared turtles(OPTs),we found the opposite pattern:the ACS group showed no significant difference (P > 0.05) from the control group for all time points (3 h,6 h,12 h,24 h and 48 h),whereas two time points (12 h and 24 h) were significantly different (P < 0.05) for the AHS group.Our results indicate that ACS may influence the immunity of GRTs and have no influence on OPTs,whereas AHS may largely affect the immunity of OPTs and have little influence on GRTs.The findings provide insights into the mechanism underlying the different morbidity and mortality rates of turtles from different culture models after extreme weather events.
Keywords acute temperature stress,immunity,culture model,transferrin,Mauremys mutica
1.Introduction
Freshwater aquaculture plays a dominant role in contributing to food resources and improving human living standards by providing high-quality proteins (Wanget al.,2015a),thus highlighting the importance of developing optimal freshwater aquaculture practices.Evidence shows that sustainable development of freshwater aquaculture largely relies on healthy culture models.Greenhouse-rearing and outdoor pond-rearing are two commonly used systems for many aquaculture species,especially for turtles (Zhouet al.,2014;Longet al.,2017).The former is characterized by short cultural periods under high temperature conditions,high yields,but poor quality (e.g.low percentages of fresh flavor amino acids),whereas the latter has the opposite characteristics:long cultural periods at low temperature conditions,low yields,but good quality (Qian and Zhu 2001;Zhouet al.,2014;Longet al.,2017).Many observations have also shown that greenhouse-reared individuals are vulnerable to drastic environmental change,such as acute cold stress (ACS) caused by extreme weather events,and frequently show cold-stress symptoms and even death,whereas outdoor pond-reared individuals often display weak responses to ACS and strong resistance to pathogens (Wanget al.,2007;Yang and Cao,2013).However,the underlying mechanism is still unknown.
Pre-acclimation theory has shown that long-term adaptive training of animals can improve immune function and mitigate the damage caused by acute environmental stresses(Hangalapuraet al.,2003;Suet al.,2019).For example,cold stimulation of broilersArbor Acresat 3°C for 34 days can lead to cold acclimation,which improves immune function and resistance to ACS,and alleviates the damage caused by ACS (Suet al.,2019).Therefore,we propose a hypothesis based on pre-acclimation theory,which predicts that greenhousereared individuals have the potential to tolerate acute heat stress (AHS) but easily sustain damage due to ACS.Longterm high temperature environments may facilitate the physical adaptation of greenhouse-reared individuals to high temperature conditions or AHS,while the same cultural conditions may cause high sensitivity to ACS in greenhousereared individuals.Conversely,outdoor pond-reared individuals may show high tolerance to ACS but obvious stress responses to AHS due to pre-acclimation to long-term low temperature environments.However,the hypothesis has so far lacked adequate evidence.
Individuals vulnerable to acute stresses are easily infected by various pathogenic bacteria or viruses,suggesting their immunity is greatly influenced by ambient stresses (Bowdenet al.,2007;Zhanget al.,2015;Chenet al.,2019a).Generally,acute stresses decrease the immunity of aquaculture species to pathogens,leading to illness and even death.Acute stress can lead to sharp increases in both glucocorticoids and norepinephrine,which can suppress the synthesis and release of immune globulins (Moriciet al.,1997;Ray and Maiti 2001).In addition,molecular evidence has shown that the expression pattern of immunity-related genes can be significantly influenced by ACS.For example,Chinese soft-shelled turtlesPelodiscus sinensiswere subjected to ACS (15 °C) to explore the influence of abrupt temperature changes on immune function.After 24 h of the treatment,the mRNA expression levels of pro-inflammatory cytokines (IL-1β,TNFα,IL-6,IL-12β,IL-8 and IFNγ) were all enhanced in both spleen and intestine(Zhanget al.,2015).Moreover,AHS can also significantly affect the immunity of aquaculture species by influencing the mRNA expression of immunity-related genes.For example,when sea cucumbersApostichopus japonicuswere subjected to AHS (26 °C),after 6 h,48 h and 192 h of exposure,RNAseq based transcriptional analysis revealed large numbers of immune-related differential expressed genes,which were classified into six immune-associated groups,including transferrin superfamily members (Xuet al.,2018).These findings demonstrated that both ACS and AHS can have significant influences on the immunity of aquaculture species by affecting the mRNA expression levels of immunity-related genes,suggesting the expression levels of key immunity-related genes can mirror the levels of stress and provide insights into variations in immunity under different temperature stresses.
The gene for transferrin (TF) is one of the key immunityrelated genes in many aquaculture species,such as the yellow pond turtleMauremys mutica(Gaoet al.,2011;Liuet al.,2018),the channel catfishIctalurus punctatus(Liuet al.,2010),and the sea bassDicentrarchus labrax(Neveset al.,2009).As an innate immune protein,TF can contribute to host nutritional immunity and help organisms to evade bacterial infection by preventing iron from binding with TbpA,a TF surface receptor on bacteria,which allows pathogens to scavenge iron from their hosts (Barber and Elde,2014).The rapid up-regulation of TF mRNA levels after bacterial infection in many aquaculture species further highlights the key role of TF in resistance to pathogens.For example,the mRNA expression of TF was significantly up-regulated after channel catfishI.punctatuswere challenged with the bacteriaEdwardsiella ictaluri(Liuet al.,2010).Unlike other immune genes,such as pro-inflammatory cytokines and matrix metalloproteinase 3 (Zhaoet al.,2014),which have their highest expression in the spleen,the TF mRNA was predominantly expressed in the liver for seven freshwater turtle species (Gaoet al.,2011;Liuet al.,2018).Another study showed that TF expression was only detected in the liver of walking catfishClarias macrocephalus(Sriboonsanet al.,2008).This evidence suggests that TF is mainly produced in the liver for many aquaculture species (Neveset al.,2009).
The yellow pond turtleM.muticais one of the most commonly cultured aquaculture species in southern China due to its high value in traditional Chinese medicine,as a food and the species’ popularity as a pet (Zhaoet al.,2015;Wanget al.,2019).However,high morbidity and mortality rates in both outdoor pond-reared and greenhouse-rearedM.muticaare frequently reported after extreme weather events that are associated with rapid temperature elevations or drastic temperature declines (Weiet al.,2007;Chenet al.,2019b).In the present study,we examined the expression patterns of TF genes in the livers of both greenhouse-reared turtles (GRTs) and outdoor pond-reared turtles(OPTs) to reveal the effect of acute temperature stress on the immunity ofM.muticareared using different culture models.The findings of this study facilitate understanding of the mechanisms underlying the varying morbidity and mortality rates in different aquaculture models after extreme weather events.
2.Material and Methods
2.1.Experimental animalsSamples of 2-year-oldM.muticafrom different culture models (greenhouse-reared and outdoor pond-reared) were obtained from a turtle farm (Guangdong Fujinyuan Ecological Agriculture Co.Ltd,Guangdong,China),where some populations ofM.muticawere reared in outdoor ponds with an average annual temperature of 21.6°C and others were cultured under greenhouse conditions with a water temperature of 30 ± 0.5 °C.Prior to the experiments in July 2019,allM.muticaindividuals from both rearing conditions were acclimated in plastic tanks (380 mm × 277 mm × 144 mm,Shishan Lian Plastic Hardware Factory,Foshan,China) for one week at a water temperature of 28 ± 0.5°C in the laboratory.During the acclimation period,the turtles were fed with commercial food specifically formulated for all types of turtle(Shenzhen Inch-Gold Fish Food Co.,Ltd,Guangdong,China)on a daily basis.All experimental procedures were performed in accordance with the regulations of the Animal Care and Welfare Committee,Guangdong Institute of Applied Biological Resources (Guangzhou,China;Authorization Number:GIABR20190501,Date of approval:1 May 2019).
2.2.ACS and AHSAfter the acclimation,a total of 33 greenhouse-reared turtles (GRTs) were randomly selected and used for the ACS and AHS experiments.Body weights ranged from 130.5 g to 246.7 g with an average (mean ± SD) of 187.2 ±27.7 g.15 GRTs were randomly selected and transferred into a plastic tank (440 mm × 290 mm × 80 mm,Shishan Lian Plastic Hardware Factory,Foshan,China) with a water temperature of 32°C (AHS group),and other 15 individuals were also randomly selected and transferred into a plastic tank with a water temperature of 24 °C (ACS group).The remaining three individuals were not treated with ACS or AHS and were used as a control group (28°C).For the outdoor pond-raised turtles(OPTs),a total of 33 individuals with body weights ranging from 45.5 g to 95.9 g with an average (mean ± SD) of 68.7 ±12.1 g were also randomly selected and also divided into three groups (AHS group,ACS group and control group) as described above.
Therefore,there were six groups:AHS group for GRTs;ACS group for GRTs;control group for GRTs;AHS group for OPTs;ACS group for OPTs;and control group for OPTs.For the four treatment groups (AHS group for GRTs,ACS group for GRTs,AHS group for OPTs and ACS group for OPTs),three turtles were sampled at each time point (3 h,6 h,12 h,24 h and 48 h after treatments).Liver tissues were collected from each turtle and immediately frozen in liquid nitrogen for at least two hours,then stored at -80°C until RNA extraction was conducted.For the two control groups (control group for GRTs and control group for OPTs),three turtles were sampled for each control group and subsequent collection and storage of liver samples was performed as described above.
2.3.Total RNA extraction and first-strand cDNA synthesisTotal RNA was extracted from the liver tissues using Trizol reagent (Catalog # B511311-0100,Sangon,China) according to the manufacturer's instructions.Subsequently,genomic DNA contaminants were removed by digesting the total RNA samples using RNase-free DNase I (Catalog # A610099-0250,Sangon,China).The integrity of the total RNA was examined by inspecting the 28S and 18S ribosomal bands using 1% agarose gel electrophoresis.The quality and quantity were assessed using a Nanodrop One spectrophotometer (Nanodrop Technologies,Wilmington,DE,USA).The first-strand cDNA was synthesized from 1.0 μg total RNA in 10 μl reaction system using the M-MuLV First-Strand cDNA Synthesis Kit (Catalog # B532435-0100,Sangon,China) with the Oligo dT primer.The synthesized cDNA was stored at -20°C until it was used for subsequent realtime PCR assay.
2.4.Real-time PCRReal-time quantita tive PCR was performed on the MX3000PTMReal-Time PCR system (Agilent,Santa Clara,CA,USA) to investigate the expression of TF mRNA using TF-specific primers (TF-F:AGTTCTATCTGGGCTACC,TF-R:ACTGCACTCCATTCATCA) (Gaoet al.,2011).Each PCR reaction was carried out in a total volume of 10 μl containing 5.0 μl of SGExcel UltraSYBR mixture (Catalog # B532957-0005,Sangon,China),0.2 μM of each primer,0.4 μl cDNA template and 4.2 μl RNase-Free ddH2O.The PCR program was composed of a denaturation step of 5 min at 95 °C,followed by 40 cycles of 95 °C for 10 sec,60 °C for 20 sec and 72 °C for 10 sec.Three technological replicates were employed.To rule out potential contamination,negative controls in each run were set up with ddH2O and RNA as templates,respectively.Melting curve analysis of the PCR products was performed at the end of each PCR reaction to confirm the specificity of the primers.β-actin (β-actin-F:GATGTGGATCAGCAAGCA;β-actin-R:GGGCAAAGTTTACAAGTAA) (Zhaoet al.,2012) was used as an internal control to normalize the relative expression of TF mRNA with the 2-ΔΔCtmethod (Pfaffl 2001).For the convenience of comparisons between groups,the expression level of the control samples was set to a value of 1.The expression levels of stressed samples thus represent fold changes relative to each control sample,and values of the 2-ΔΔCtlarger than 1 or smaller than 1 indicate up-or down-regulation of expression levels of TF mRNA respectively.
2.5.StatisticsAll data are presented as mean ± standard deviation (SD).To comprehensively understand the influences of ACS and AHS on TF expression of GRTs and OPTs,we performed four comparisons (ACS and AHS in GRTs,ACS and AHS in OPTs,GRTs and OPTs after AHS,GRTs and OPTs after ACS).For the first two comparisons (ACS and AHS in GRTs,ACS and AHS in OPTs),variations in the relative expression of TF gene between unstressed (control) and treated groups and between two different treated groups at the same time points were analyzed using a non-parametric Mann-WhitneyUtest (Dytham,2011).For the remaining comparisons(GRTs and OPTs after AHS,GRTs and OPTs after ACS),the analysis between GRTs and OPTs after the same treatment(AHS or ACS) at the same time points was carried out using the Mann-WhitneyUtest (Dytham,2011).Statistical analysis was performed in SPSS v.25.0.The level of significance was defined atP< 0.05.
3.Results
3.1.Expression patterns in GRTsFor the ACS treatment(24°C),a consistent pattern was observed.The average levels (1.26± 0.17 -4.52 ± 2.26) of TF mRNA at 3 h -48 h were generally higher in comparison with those (1.00 ± 0.49) in the control group (Figure 1).The variation showed an oscillating pattern,increasing initially,then decreasing,followed by another increase along the time axis.The relative expression of TF mRNA was significantly (P< 0.05) higher at 3 h,6 h and 48 h in comparison with the control group.Although the average levels at 12 h and 24 h were also higher than those in the control group,there was no statistical difference between 12 h and the control (P=0.13) and between 24 h and the control (P=0.28).
For the AHS treatment (32 °C),the average levels of TF mRNA showed a different pattern compared to the ACS group (Figure 1).The average levels (1.31 ± 0.09 -1.61 ± 0.17) at 3 h,12 h and 24 h were higher than those (1.00 ± 0.49) in the control group,while the average levels (0.58 ± 0.15 -0.99 ± 0.38)at 6 h and 48 h were lower than those in the control group.However,the variation showed the same trend as the ACS group along the time axis:the levels of TF mRNA in the AHS group also firstly increased,subsequently decreased,and then increased again.Significant differences (P< 0.05) were detected between the control group and two stressed time points (6 h and 12 h),while the remaining three time points (3 h,24 h and 48 h) showed no significant difference (P=0.18,0.13 and 0.83,respectively) from the control group.
TF mRNA showed lower expression in the AHS group than in the ACS group for all time points except for 24 h (Figure 1).Specifically,significant differences (P< 0.05) at 3 h,6 h and 48 h were observed between the two groups,while there was no statistical difference between the two groups at 12 h (P=0.51) and 24 h (P=0.51).While showing the same trend along the time axis,the ACS group also showed a delayed response to external stresses in comparison to the AHS group.At 3 h,both groups simultaneously reached the first wave peak.The first wave hollow and the second wave peak in the AHS group were found at 6 h and 12 h,respectively,whereas the first wave hollow and the second wave peak in the ACS group were found at 24 h and 48 h at the earliest,respectively.
3.2.Expression patterns in OPTsFor the ACS treatment(24°C),compared to the control group (1.00 ± 0.53),all stressed time points showed higher expressions (1.01 ± 0.52 -1.20 ± 0.19)of TF mRNA except for 12 h (0.90 ± 0.53) on average (Figure 2).The average levels showed an oscillating pattern,first increasing at 3 h and 6 h,decreasing at 12 h and increasing again at 24 h along the time axis.The highest and lowest levels,on average,were detected at 6 h and 12 h,respectively.However,they showed no significant difference (P> 0.05) in comparison with the control group,and there was also no statistical difference (P> 0.05) between the control group and other stressed time points.
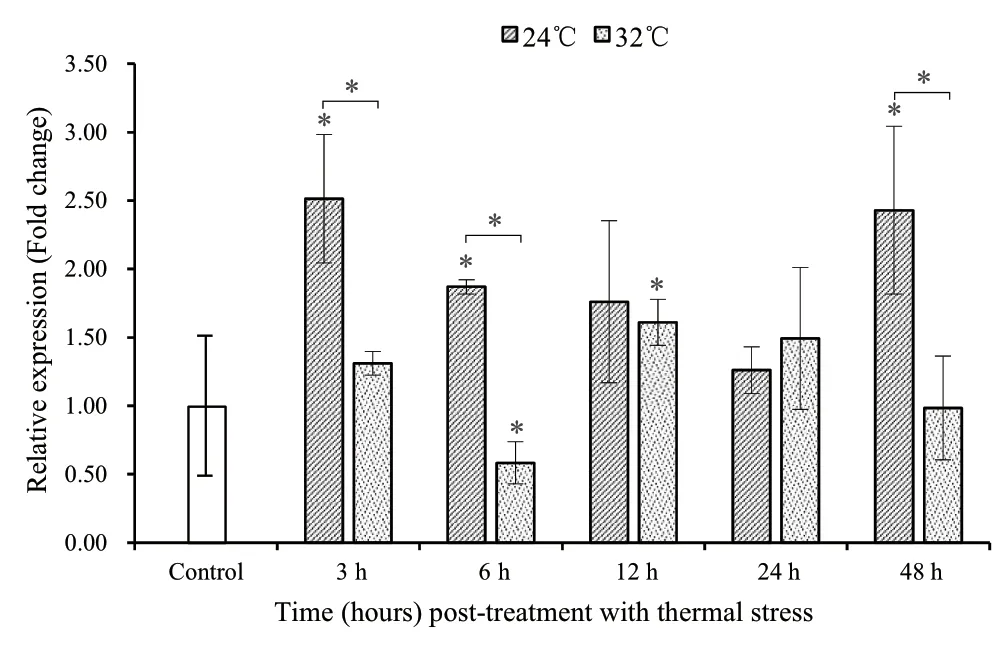
Figure 1 Expression of TF mRNA in stressed turtles with acute cold stress (24°C) and acute heat stress (32°C) at different time points for greenhouse-reared turtles.The Mauremys mutica β-actin gene was used as an internal control to calibrate the cDNA template for all samples.Data are expressed as mean ± SD for triplicate samples.Significant differences between the control and each treatment and between the two treatments at each time point at P < 0.05 are denoted with “*”.
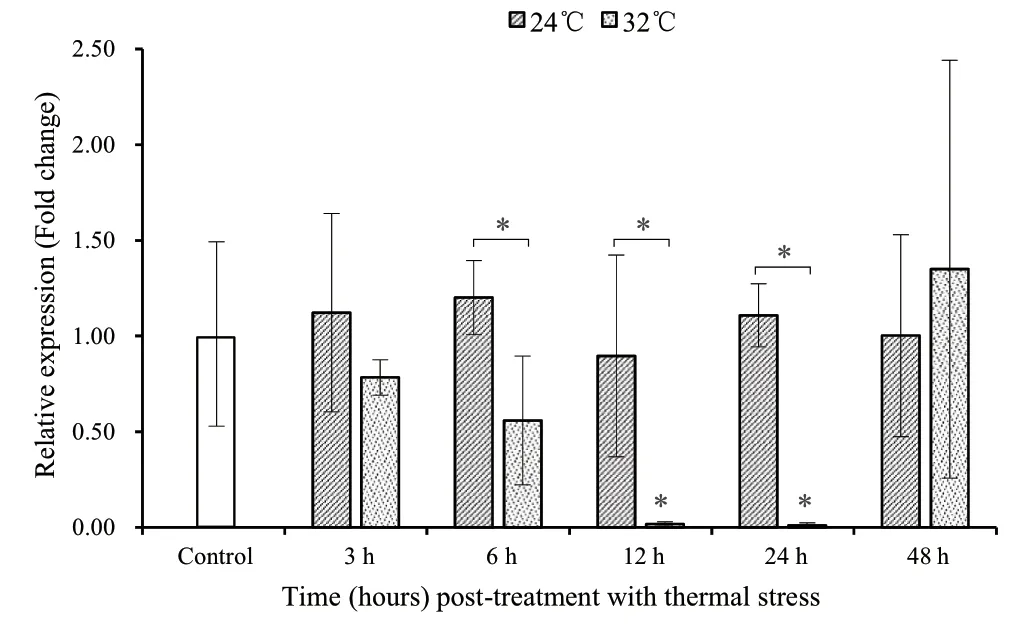
Figure 2 Expression of TF mRNA in stressed turtles with acute cold stress (24°C) and acute heat stress (32°C) at different time points for outdoor pond-reared turtles.The Mauremys mutica β-actin gene was used as an internal control to calibrate the cDNA template for all samples.Data are expressed as mean ± SD for triplicate samples.Significant differences between the control and each treatment and between the two treatments at each time point at P < 0.05 are denoted with “*”.
For the AHS treatment (32 °C),a different expression pattern was observed compared to all other groups (Figures 1 and 2).All the AHS time points showed lower expression levels (0.01± 0.01 -0.78 ± 0.09) except for 48 h (1.35 ± 1.09),on average,in comparison with the control group.Moreover,the expression levels of TF mRNA gradually decreased from 3 h to 24 h,and recovered to the control level at 48 h.Specifically,both 12 h and 24 h exposures showed the lowest expressions (0.02 ± 0.01 and 0.01 ± 0.01,respectively) with significant differences (P< 0.05) in comparison with the control group.No statistical difference (P> 0.05) was found between the remaining three stressed time points (3 h,6 h and 48 h) and the control group.
TF mRNA showed lower expression in the AHS group than that in the ACS group for all time points except for 48 h (Figure 2).Specifically,the AHS group showed significantly (P< 0.05)lower expression than the ACS group at 6 h,12 h and 24 h,while there was no statistical difference (P> 0.05) between the two stressed groups for the remaining two time points (3 h and 48 h).
3.3.Expression patterns under ACSWhen β-actin was used as an internal control in this study,the expression levels of TF were 1.09 ± 0.57 for OPTs and 1.02 ± 0.27 for GRTs in the control group,and they showed no statistical difference (P>0.05).To understand the immune responses of turtles from different culture models to ACS (24 °C),we compared TF mRNA expressions between GRTs and OPTs (Figure 3).A consistent pattern was observed:the average levels of expression(1.26 ± 0.17 -2.51 ± 0.47) in GRTs were generally higher than those (0.89 ± 0.53 -1.22 ± 0.52) in OPTs for all time points (3 h,6 h,12 h,24 h and 48 h) after ACS.Specifically,compared to OPTs,GRTs showed significantly (P< 0.05) higher expression of TF mRNA at 3 h,6 h and 48 h.Although higher average levels of TF mRNA in GRTs were detected at 12 h and 24 h,there was no statistical difference (P> 0.05) between the two aquaculture models at both time points.
3.4.Expression patterns under AHSTo understand the immune responses of turtles from different culture models to AHS (32°C),we compared TF mRNA expressions between GRTs and OPTs (Figure 4).However,we found an inconsistent pattern:the expression (0.58 ± 0.54 -1.61 ± 0.17) in GRTs was generally higher than that (0.01 ± 0.01 -0.78 ± 0.09) in OPTs at 3 h,6 h,12 h and 24 h after AHS,whereas the opposite pattern was observed at 48 h.Moreover,GRTs showed an oscillating trend with levels increasing first,then decreasing,and increasing again along the time axis,whereas expression in OPTs continued to decline over the first 4 time points (3 h,6 h,12 h and 24 h) and sharply recovered to the control level at 48 h.Specifically,GRTs showed significantly higher expressions (P< 0.05) of TF mRNA at 3 h,12 h and 24 h in comparison with OPTs.Although different expressions of TF mRNA between the two aquaculture models were detected at 6 h and 48 h,there was no statistical difference (P> 0.05).
4.Discussion
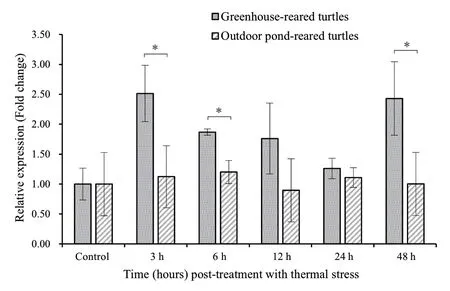
Figure 3 Comparison of TF expression between greenhouse-reared turtles and outdoor pond-reared turtles after acute cold stress (24°C) at different time points.The Mauremys mutica β-actin gene was used as an internal control to calibrate the cDNA template for all samples.Data are expressed as mean ± SD for triplicate samples.Significant differences between the control and each treatment and between the two treatments at each time point at P < 0.05 are denoted with “*”.
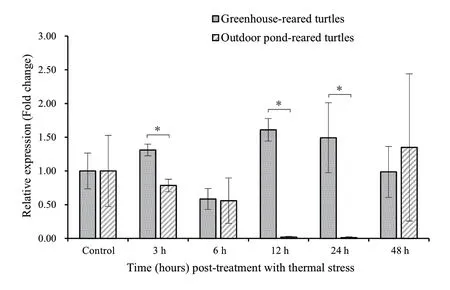
Figure 4 Comparison of TF expression between greenhouse-reared turtles and outdoor pond-reared turtles after acute heat stress (32°C) at different time points.The Mauremys mutica β-actin gene was used as an internal control to calibrate the cDNA template for all samples.Data are expressed as mean ± SD for triplicate samples.Significant differences between the control and each treatment and between the two treatments at each time point at P < 0.05 are denoted with “*”.
Understanding the mechanisms underlying the acute stress responses of aquaculture species from different culture models is particularly important for the sustainable development of aquaculture industries in the context of global climate change and intense anthropogenic activity.We characterized and compared the expression patterns for one key immunity-related gene (TF) after acute temperature stresses inM.muticasamples from both greenhouse-reared and outdoor pond-reared models.TF plays important roles in both iron transport and immune responses to bacterial infection (Liuet al.,2010;Barber and Elde 2014).An anti-microbial study showed that the recombined TF protein can significantly inhibit the growth of three bacteria:Staphylococcus aureus,Escherichia coliandSerratia marcescens(Gaoet al.,2012).The protein level of TF was similar to the mRNA level of TF in different tissues ofM.mutica(Gaoet al.,2012).Both up-regulation and down-regulation of TF mRNA at different time points inM.muticaafter bacterial infection highlighted the important roles of TF in immune responses to bacterial challenges,and also indicated that TF can act either as a positive or a negative acute phase protein (Gaoet al.,2011).In the present study,we detected significant up-regulation of TF mRNA at most time points for both GRTs under ACS and OPTs under AHS,suggesting that TF is a positive acute phase protein inM.muticain thus conditions,to increase iron storage and make it unavailable for bacterial growth and reproduction (Neveset al.,2009;Liuet al.,2010).In contrast,a significant down-regulation of TF mRNA was observed in OPTs under AHS,suggesting a role as a negative acute phase protein in these conditions.This finding was similar to other studies on aquaculture species,such as the sea bassDicentrarchus labrax,where studies showed that TF was both a positive acute phase protein and a negative acute phase protein in different tissues or at different time points,after being challenged (Bayne and Gerwick 2001;Neveset al.,2009).When OPTs were treated with AHS,TF may have acted as a negative acute phase protein for the first 24 h.There may be two reasons for this observation:(1) TF levels in outdoor-rearedM.muticamay be already high enough to quickly remove circulating iron,facilitating down-regulation of TF mRNA in the first 24 h of AHS;and (2) bacteria have different strategies to get iron from the host by directly binding to haemin (do Valeet al.,2002) or producing siderophores (Fouzet al.,1997),which enable bacteria to get iron from TF;thus down-regulation of TF mRNA during the course of AHS would reduce the rates of iron uptake in the OPTs.
For the GRTs,TF mRNA expressions showed significant differences (P< 0.05) between the control group and the ACS group for most time points (3 h,6 h and 48 h),and the remaining time points (12 h and 24 h) showed no significant difference (P> 0.05).In contrast,only two time points (6 h and 12 h) showed a significant difference (P< 0.05) between the control group and the AHS group,and most time points(3 h,24 h and 48 h) showed no statistical difference (P> 0.05).Similar findings have also been obtained in other aquaculture species such as scallopChlamys farreri(Chenet al.,2019a) and Chinese soft-shelled turtleP.sinensis(Liuet al.,2019),indicating that immunity is significantly influenced by large variations in immune-related gene expression at most time points.The pattern indicated that AHS may have little or no influence on the immunity of GRTs,whereas ACS may significantly influence the immunity of GRTs.This is supported by the comparison of TF expressions between GRTs and OPTs under the same ACS:the former showed significantly higher expression (P< 0.05) than the latter at most time points (3 h,6 h and 48 h).Generally,impaired immunity due to temperature stress may increase the risk of disease and even death for many aquaculture species,such as rainbow troutOncorhynchusmykiss(Wanget al.,2015b) and senegalese soleSolea senegalensis(Conde-Sieiraet al.,2018).Thus our results provide molecular evidence for the hypothesis that greenhouse-reared individuals are sensitive to ACS and may easily suffer damage caused by extreme cold weather events,while greenhouse-reared individuals do not show obvious stress responses to high temperature conditions and exhibit high tolerance to AHS.In general,this is because the greenhouse-reared individuals are cultured under high temperature conditions for long periods of time,which may hinder tolerance to low temperature conditions or stresses but facilitate acclimatization and/or local adaptation to high temperature environments or stresses based on pre-acclimation (Hangalapuraet al.,2003;Suet al.,2019).As expected,the observations provide an explanation for the high morbidity and mortality rate of greenhouse-rearedM.muticaand other aquaculture species after extreme cold weather events and/or anthropogenic ACS (Wanget al.,2007;Yang and Cao 2013).
For the OPTs,we observed an opposite gene expression pattern:TF mRNA levels showed no significant difference (P> 0.05) between the control group and the ACS group,whereas the levels of TF mRNA in the AHS group continuously decreased at the first 24 h,with significant differences (P< 0.05)at 12 h and 24 h in comparison with the control group.Similar to the GRTs,the results indicated that ACS may not affect the immunity of OPTs,whereas AHS may have significant influence on their immunity.This is supported by the comparison of TF expression between GRTs and OPTs under the same AHS:the latter showed significantly lower expressions of the TF gene (P< 0.05) than the former at most time points (3 h,12 h and 24 h).Hence our results provide molecular evidence for the assumption that outdoor pond-reared individuals have potential adaptation to low temperature conditions and reflect their high tolerance to ACS,but a strong sensitivity to high temperature environment,meaning they may be vulnerable to extreme hot weather events.The explanation is analogous to that for the greenhouse-reared individuals:the long cultivation period of outdoor pond-raised individuals under low temperature conditions may impede rapid adaptation to AHS but promote acclimatization or adaptation to ACS based on pre-acclimation (Hangalapuraet al.,2003;Suet al.,2019).Similar results were found in a previous study on broilers,which indicated that low temperature cultivation ofArbor Acresbroilers for 42 days enhanced immunity and improved adaptation to ACS (Suet al.,2019).Our results also provide insights into the high morbidity and mortality rates ofM.muticaand other aquaculture species after extreme weather events and/or anthropogenic temperature stresses (Wanget al.,2007;Yang and Cao,2013).
In conclusion,our results showed that for the GRTs,the expression levels of TF mRNA were significantly different (P< 0.05) between the control group and the ACS group for most time points (3 h,6 h and 48 h),while at only two time points (6 h and 12 h) was there a significant difference (P< 0.05) between the control group and the AHS group.However,for the OPTs,we found the opposite pattern:no significant difference (P>0.05) was found between the control group and the ACS group for all time points (3 h,6 h,12 h,24 h and 48 h),whereas the AHS group showed significant differences (P< 0.05) for two time points (12 h and 24 h) in comparison with the control group.The variations in TF mRNA reflected the immunity variations induced by acute temperature stresses.Remarkably,our study provided molecular evidence for the hypothesis that greenhouse-reared individuals have the potential to tolerate AHS but are easily damaged by ACS,and outdoor pondreared individuals may show a high tolerance to ACS but have an obvious stress response to AHS.Given that the expression pattern of immune-related genes may vary depending on gene categories,these results and conclusions based on only one gene cannot provide a complete insight into the effects of acute temperature stresses on immunity in both greenhouse-reared and outdoor pond-reared individuals.Hence,it is necessary to use high throughput sequencing-based methods (e.g.RNAseq) to obtain a more complete understanding.The molecular mechanism of our hypothesis,based on pre-acclimation,may involve epigenetic modifications such as DNA methylation (Ruet al.,2017).It would be helpful to use epigenetic modification methods and theory to explore which epigenetic loci are involved and how they regulate the expression of key immunerelated genes.
AcknowledgementsWe would like to thank Mr.Zhijian XU for his help during materials collection and turtle feeding experiments.This research was funded by the thousand PhD program of Guangdong Academy of Sciences(No.2018GDASCX-0932;No.2020GDASYL-20200103099),the Training Fund of Guangdong Institute of Applied Biological Resources For PhDs,Masters and Postdoctoral Researchers(No.GIABR-pyjj201603),the GDAS Special Project of Science and Technology Development (No.2018GDASCX-0107),the Scientific and Technological Program of Guangdong Province(No.2017A020219004),the National Natural Science Foundation of China (No.31772486).
杂志排行
Asian Herpetological Research的其它文章
- Description of a New Species of Amolops Cope,1865 (Amphibia:Ranidae) from Nepal and Nomenclatural Validation of Amolops nepalicus Yang,1991
- A New Species of the Genus Rhabdophis Fitzinger,1843 (Squamata:Colubridae)in Southwestern Sichuan,China
- Ring Clinal Variation in Morphology of the Green Odorous Frog (Odorrana margaretae)
- Behavior and Approximate Entropy of Right-eye Lateralization During Predation in the Music Frog
- Distribution Changes of Chinese Skink (Eumeces chinensis) in China:the Impacts of Global Climate Change
- Hotspot of Adult Cuban Treefrog and Cane Toad Multi-organ Abnormality in Suburban South-west Florida
