A New Species of the Genus Rhabdophis Fitzinger,1843 (Squamata:Colubridae)in Southwestern Sichuan,China
2020-06-28YigePIAOZeningCHENYanqingWUShengchaoSHIHirohikoTAKEUCHITeppeiJONOMasayaFUKUDAAkiraMORIYezhongTANGQinCHENandLiDING
Yige PIAO ,Zening CHEN ,Yanqing WU ,Shengchao SHI ,Hirohiko TAKEUCHI ,Teppei JONO ,Masaya FUKUDA,Akira MORI,Yezhong TANG,Qin CHEN* and Li DING*
1 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Key Laboratory of Bio-Resource and Eco-Environment of Ministry of Education,College of Life Sciences,Sichuan University,Chengdu 610065,Sichuan,China
4 Nanjing Institute of Environmental Sciences,Ministry of Ecology and Environment of China,Nanjing 210042,Jiangsu,China
5 College of Bioresource Sciences,Nihon University,Fujisawa,Kanagawa 2520880,Japan
6 Tropical Biosphere Research Center,University of the Ryukyus,Nishihara,Okinawa 9030213,Japan
7 Department of Zoology,Graduate School of Science,Kyoto University,Sakyo,Kyoto 6068502,Japan
Abstract The genus Rhabdophis is a group of widely distributed snakes with more than 20 species.Recent field surveys uncovered a species in southwestern China,which has long been considered as R.pentasupralabialis.Combined molecular and morphological analyses revealed it as a new species Rhabdophis chiwen sp.nov. Based on 12 specimens,this new species is distinguished by the following characters:1) dorsal body saddlebrown,dorsal scales typically with black margins forming spots and stripes,the margin of the outer row forming two faint dorsolateral black cross-bars alongside body;2) ventral scales 151-159,the outer margin of ventral scales and several lateral rows of dorsal scales forming ventrolateral longitudinal brownish-red coloration,with faint black spots in the middle of ventral scales;3) a black oblique stripe present below eyes,often with a black spot between the 2nd and 3rd supralabial and a black stripe on the 5th supralabial;4)eyes dark khaki,pupils black;5) infralabials usually 7,the first four in contact with anterior chin-shields;6) temporal scales 1+1;7) dorsal scales in 15 rows,feebly keeled except the outer 1-2 rows;8) anal scale divided;subcaudals 45-59;9) preocular 1 and postoculars 3 (occasionally 2);10) body medium-sized (snout-vent length:adult males 404-431 mm,adult females 409-476 mm);11) tail moderate (tail length/total length in adult males 0.205-0.238,in adult females 0.172-0.193).With the discovery of this new species,the total number of species in genus Rhabdophis is 28 with 12th species known to occur in China.
Keywords Rhabdophis chiwen sp.nov.,morphology,Natricinae,phylogenetics,taxonomy
1.Introduction
The genusRhabdophisis a group of widely distributed snakes with a unique defensive system that relies on nuchal glands(Moriet al.,2012).In 1960,Malnate defined this genus based on morphological characters,i.e.hemipenes with sulcus spermaticus divided;last two maxillary teeth strongly enlarged,recurved and usually preceded by a diastema;internasals broad anteriorly,nostrils lateral;apical pits present or absent;vertebral glands present in several species (Malnate,1960).
Currently,27 species are recognized and are distributed in eastern and southern Asia (Zhuet al.,2014;Takeuchiet al.,2018).Two new species were discovered in the past five years,Rhabdophis akraios(Doriaet al.,2013) andRhabdophis guangdongensis(Zhuet al.,2014).
Eleven species ofRhabdophisare now known to occur in China (Zhuet al.,2014),which areR.adleriZHAO,1997,R.guangdongensisZhu,Wang,Takeuchi and Zhao,2014,R.himalayanusGünther,1864,R.lateralisBerthold,1859,R.leonardiWall,1923,R.nigrocinctusBlyth,1855,R.nuchalisBoulenger,1891,R.pentasupralabialisJiang and Zhao,1983,R.subminiatusSchlegel,1837,R.formosanusMaki,1931 andR.swinhonisGünther,1868.During several field work in the southwestern part of Sichuan Province,China,we collected a number of samples which were originally referred to asR.pentasupralabialis.Nevertheless,those snakes can be distinguished fromR.pentasupralabialisbased on morphological characters.Moreover,the phylogenetic analysis based on the mitochondrial cytochromeb(cyt b) and nuclear oocyte maturation factor (cmos) gene sequences revealed that those samples are different fromR.pentasupralabialisand other congeners in China.Therefore,we propose that those specimens be a new species in the genusRhabdophis.
2.Materials and Methods
2.1.Specimens samplingSpecimenswere collected from the Xingou Village (XG,10 specimens) in Tianquan County and Jiguan Mountain (JG,2 specimens) in Chongzhou City,Sichuan Province,China (Table 1).Fresh liver or muscle tissues were taken and immediately preserved in 95% ethanol and stored at -20 ℃.The specimens were dehydrated in absolute ethanol and then later transferred to and stored in 70% ethanol.In addition,another 10 samples ofR.leonardi(4 specimens),R.nuchalis(4 specimens) andR.pentasupralabialis(2 specimens)that represent 3 sympatric species with relatively close niche in Sichuan Province,were used for molecular analyses.
2.2.Molecular phylogenetic analysisGenomic DNA was extracted from the collected tissue samples in Chengdu Institute of Biology (CIB).PCR amplifications were performed in 25 μl reactions (12.5 μl I-5™ 2×High-Fidelity Master Mix,10 μl ddH2O,1 μl F-primers,1 μl R-primers,0.5 μl DNA template)using the following cycling conditions:initial denaturation for 2 min at 95°C,followed by 35 cycles:denaturation at 94°C for 40 s,annealing at different temperatures (48.5 ℃ forcyt bor 56 ℃ forcmos) for 25 s,elongation at 72 °C for 15 s,and then finalized with elongation step of 2 min at 72 °C,with the PTC-100 thermal cycler (BioRad,USA).Purified PCR products were sequenced using the same PCR primers.Sequencing was completed by Beijing Qingke New Industry Biotechnology Co.,Ltd.Sequences for comparison of available species were downloaded from GenBank.Amphiesma stolatumandNatrix natrixwere used as outgroups.
Thecyt bandcmossequences were combined into one dataset to build gene trees.All sequences were aligned with other retrieved sequences in the same gene loci by using software MEGA 7 (Kumaret al.,2016).Raw trace files were edited in Geneious 7 (Biomatters Limited,New Zealand).Partition finder 2.1.1 under BIC identified the optimal models of sequence evolution for each partition (Lanfearet al.,2012).Phylogenetic relationships derived from the combined gene fragments were performed based on Bayesian Inference (BI) by using MrBayes 3.2 (Ronquistet al.,2012).We ran our analysis for 20 million generations with the chains,sampling every 1000 generations.The average standard deviation of split frequencies (ASDSF <0.01).The first 1000 trees (of 20,000) were discarded as the burnin.A 50 majority-rule with compatible groups consensus was taken from the remaining trees and posterior probabilities(pp) of 0.95 or above were considered significant.We also performed maximum-likelihood (ML) analysis by using the program RaxML v8 (Stamatakis,2014) and IQ tree (Nguyenet al.,2015).Reliability of the ML tree was assessed by calculating bootstrap probability (BP) with 1000 replications.Additionally,the uncorrectedcyt bandcmosp-distance matrix was compared using MEGA 7.0 (Kumaret al.,2016).

Table 1 Morphological characters of Rhabdophis chiwen sp.nov.
2.3.Morphological analysisTwelve adult snakes (7 males and 5 females) were examined.Information on some morphological characters of known species were obtained from literature(Boie,1827;Boulenger,1896,1900,1906;Bourret,1935;Cantor,1839;Das,2010;David and Vogel,2010;de Lang and Vogel,2006;Doriaet al.,2013;Dumérilet al.,1854;Günther,1858,1864;Leviton,1970;Smith,1943;Stuebing and Lian,2002;Tweedie,1953;Zhao,1997;Zhaoet al.,1998;Zhao and Adler,1993;Zhao and Jiang,1981) and are shown in Table 2.Color description was done according to wiki color-coding.
Terminology for morphological measurements and descriptions is as follows:body and tail length were measured with a tape ruler to the nearest 1 mm;total length (TL),from the tip of snout to the tip of tail;snout-vent length (SVL),from the tip of snout to posterior margin of cloaca;tail length(TaL),from posterior margin of cloaca to the tip of tail.Other measurements were conducted with a digital caliper to the nearest 0.1 mm:head length (HL),from the snout tip to the posterior margin of the mandible;head width (HW) was measured at the widest part of the head on posterior side;the eye horizontal diameter (ED),the greatest diameter of the orbit.Biometric measures were performed exclusively on the right eye.Ratio of tail length to total length (TaL/TL) was recorded.The dorsal scale rows (DSR) were counted at one head length behind head,at mid-body,and at one head length before vent;the number of ventral scales (VEN) was counted according to the method proposed by Dowling (Dowling,1951),half ventral was counted as one.For subcaudals (SC),first scale under the tail meeting its opposite was regarded as the first subcaudal scale,and the unpaired terminal scute was not included in the number of subcaudals.Paired scales on head were counted on both sides of the head and presented in left/right order.Supralabials (SL) were considered being those shields that were behind the rostral and bordering the mouth gap;infralabials(IL) were considered being those shields that were behind the mental,completely below a supralabial and bordering the mouth gap.Number of supraocular (SPO),preoculars (PRO)and postoculars (PTO) were counted as shields above,at the anterior and posterior margin of the orbit.Loreal scales (LR)were counted as scales between the nasal scale and preocular.Number of temporal scales (TEM) were counted as scales behind the postoculars and between the parietal scales and supralabials.The sex was determined by inspection of the existence of hemipenes.

Table 2 Significant characters of R.chiwen sp.nov.and the other known 27 species of the genus Rhabdophis.
This new species is most similar to and has long been considered asR.pentasupralabialis.To illuminate this problem,22 specimens (11 males and 11 females) collected from the type locality (Jiulong County,JL) ofR.pentasupralabialiswere examined and measured.Morphological characters of specimens were analyzed using Principal Component Analysis (PCA)(Wüsteret al.,1992).Male specimens were from Jiulong County,Jiguan Mountain and Xingou Village,using the characters VEN,SC,left IL,right IL,left PRO,left PTO and right PTO.Female specimens were from Jiulong County and Xingou Village,using the characters VEN,SC,left IL,right IL,left PTO and right PTO.All statistical analysis were conducted with SPSS 25.0 (IBM Inc.,Armonk,U.S.A.).
3.Results
3.1.Molecular phylogenetic analysisWe obtained alignments of the mitochondrial genecyt b(1074 bp) and nuclear genecmos(535 bp).Sequence data were uploaded to GenBank,available accession numbers showed in supplementary Table S1.The best evolution models of each partition combination are shown in Table 3.The result show thatcyt bandcmosgenes consistent phylogenetic trees were achieved by using Bayesian Inference(BI) and Maximum likelihood RaxML (Figure 1).
The topological structures of combinedcyt bandcmossequences phylogenetic tree are identical with the earlier study(Takeuchiet al.,2018).Our results also strongly supported the monophyly ofRhabdophis.The individuals from the Jiguan Mountain and Xingou Village were placed in the genusRhabdophiswith a high support (Figure 1).The Results also showed that they share a common ancestor withR.nuchalis,R.leonardi,andR.pentasupralabialis.
The specimen (HKV36838) from Hongya County,Sichuan,China was also nested within the clade with individuals from Jiguan Mountain and Xingou Village.
The uncorrectedp-distances between species are shown in Table 4.The pairwise distances between species in the genus were wide,ranging from 0.004 to 0.198.The largest disparityoccurred betweenR.subminiatusfrom Thailand andR.chrysargosfrom Malaysia (0.198).In comparison,the specimen ofRhabdophisfrom Jiguan Mountain (CIB116092) differed fromR.pentasupralabialisfrom Jiulong County,Sichuan,China (GP1065,type locality) by 0.081,and was different fromR.guangdongensis(SYSr000018) by 0.066 substitutions per site to the other species.Moreover,the specimen (HKV36838) from Hongya County,Sichuan,China was identified asR.nuchalisbefore (Alfaro and Arnold,2001),but its pairwise distance withR.nuchalis(HT0854 and CIBDL1807208) were rather large,by 0.063 and 0.058 respectively.

Table 3 The best evolution models of each partition combination.
3.2.Taxonomic conclusionsTheindividuals from the Jiguan Mountain and Xingou Village were placed as monophyletic clade with strong supports (100% in PP and BP).In addition,thep-distance between the individuals of the clade and other species indicated very large values (more than 0.058).Combined with further evidence from morphology mentioned below,the individuals from southwestern Sichuan Province represent an undescribed species of the genusRhabdophis.Here,we described it as a new species.
Taxonomy
Rhabdophis chiwensp.nov.Chen,Ding,Chen and Piao,2019(Figures 2,3,4,6);
Rhabdophis nuchalis pentasupralabialis:Jiang and Zhao,1983 pp.59-62;
Rhabdophis pentasupralabialis:Zhao,1998,pp.271-274;Zhao,2006,pp.268-269;Takeuchiet al.,2018,Figure 2 and 3,p.10226 (part).
Diagnosis.1) nuchal groove present,with enlarged and paired scales on each side;2) Dorsal body saddlebrown,DSR in 15 rows throughout,feebly keeled,the outer 1-2 rows smooth;3)dorsal scales typically with black margins forming some spots and stripes,the margin of the outer row forming two faint dorsolateral black cross-bars alongside body;4) a black oblique stripe below the eye,often with a black spot between the 2nd and 3rd SL and a black stripe (or separated as black spots) on the 5th SL and the 1st TEM;5) Eyes dark khaki,pupils black;6) SL 5,the 3rd and 4th touching the eye:7) usually 7 ILs (occasionally 6 or 8),the first four contact with anterior chin-shields;8) 1 LR,and TEM 1+1;9) VEN 151-159,the outer margin of ventral scales and several lateral rows of dorsal scales forming ventrolateral longitudinal brownish-red coloration,with faint black spots in the middle of each ventral scale and between scales which line a black stripe;10) anal divided;SC 45-59;11) PRO 1 (rarely 2) and PTO usually 3 (occasionally 2);12) medium-sized body (SVL of adult males:404-431 mm and adult females:409-476 mm);13)tail moderate and longer in males than in females (adult males:104-131 mm,adult females:91-107 mm).
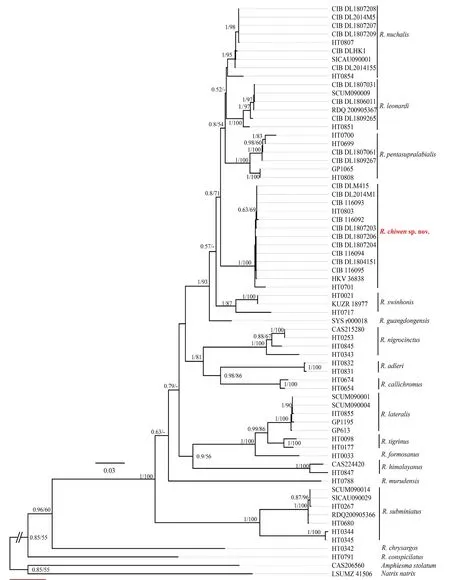
Figure 1 Phylogenetic tree derived from the combined gene fragment (cmos+cyt b).Bayesian posterior probabilities (PP) and bootstrap probabilities (BP) from maximum likelihood are shown at the nodes.

Etymology.The species name of the new species “chiwen” is in reference to the ninth son of Loong in ancient Chinese myth who likes eating fire,and indicates the firefly-eating habit of this new species (Yoshidaet al.,2020).Its common name is suggested as “Chiwen Keelback” in English and “螭吻颈槽蛇”in Chinese.
Holotype.CIB116092,an adult male,collected from Jiguan Mountain,Chongzhou City,Sichuan Province (30°45'57.64" N,103°14'3.52" E,and 1846 m a.s.l.) collected on 1st July 2019 by Li Ding (Figure 2 and 3).
Paratypes.CIB116093,an adult male,collected from the same locality asholotypeon 1st July 2019 by Li Ding.CIB116094-95,two specimens,an adult male and an adult female,collected from Xingou Village,Tianquan County,Sichuan Province(29°55'42.39" N,102°23'9.19" E,and 1461 m a.s.l.) collected on 22nd July 2018 by Li Ding and Zening Chen.
3.3.Description of the holotypeAdult male with TL 540 mm (SVL 422 mm and TaL 118 mm).TaL/TL 21.9%.Body elongated and cylindrical.Nuchal groove present;seven scales on each side of groove more or less distinctly enlarged and paired.DSR in 15 rows throughout,feebly keeled,the outer 2 rows smooth.VEN 152;anal divided;SC 56,paired,final spine present at tip of tail.
Head oval and distinctly wider than neck,HL 13.67 mm,2.53% of TL;HW 9.10 mm,HL/HW 1.50.Eyes are relatively large,with rounded pupil,EW 2.95 mm,EW/HL 21.58%.Rostral semi-circular when viewed from the front,wider than long,barely visible from above.Nostrils large and open laterally,nasal completely divided,in contact with rostral.Internasals slightly shorter than prefrontals.Prefrontals wide,bending to the loreal region.Frontal slightly pentagonal,longer than wide,equal to the distance from the rostral to frontal.LR 1,longitudinally longer.SPO 1;PRO 1;PTO left 2 and right 3;subocular absent.SL 5,the 3rd and 4th touching the eye,with a black oblique stripe below the eye,a black spot between the 2nd and 3rd SL and a black stripe on the 5th SL and the 1st TEM.Mental triangular,width approximately twice of length.IL left 7 and right 6,the first one in contact with each other behind the mental and the first four contact with anterior chinshields,the 4th to 6th infralabials contact with posterior chinshields.At the left side,the 7th infralabial slightly longer than the 6th infralabial,but much narrower,width about 1/3 of its height.TEM 2 each side,anterior temporal single and posterior temporal single.

Figure 2 Dorsal (A) and ventral (B) view of the holotype of Rhabdophis chiwen sp.nov. (CIB116092).Photo by Shengchao Shi.
Hemipenis bilobed,extends to the 11th subcaudal,forked at the level of the 9th subcaudal.When wholly everted,the hemipenes have small and hard spines resembling hooks covering the entire organ with the highest density of spines on the tip.The length of each lobe is about 1/4 of the hemipenis.Basal big spine absent,skin shallow cupped.The centripetal sulcus bifurcates at the fork and extends to the tip of per lobe.Lips evident and smooth (Figure 4).
Coloration of specimens in life.Dorsal body saddlebrown with rusty red.Dorsal scales are typically with black margins forming some scattered spots and stripes (for holotype,those dispersed black spots forming a black stripe around the neck,see Figure 3 A,C,E),the margin of the outer row forming two faint dorsolateral black cross-bars alongside body (Figure 2 A;Figure 5 A,C).Coloration of ventral is seashell and the outer margin of ventral scales and several lateral rows of dorsal scales forming ventrolateral longitudinal brownish-red coloration,with faint black spots in the middle of each ventral scale and between scales at the anterior part of body which line a black stripe and merge into black patches covering the whole scale with dispersed brownish-red spots at the posterior part of body(Figure 2 B).Hatchlings similar to adults except for darker dorsal coloration,a yellow stripe present on nape,separated by the nuchal groove (Figure 5 D);ventral scales typically shallow black.

Figure 3 The holotype of Rhabdophis chiwen sp.nov.(CIB116092) in life.A-E:Different views of head.C and E:showing the same 5 supralabials as R.pentasupralabialis.D:ventral view of head,displaying the representative 7 infralabials.F:Lateral view of trunk,demonstrating several smooth outer rows.Photo by Shengchao Shi.
Coloration of specimens in preservative.Coloration pattern in preservative similar to alive,but the faint black spots in the middle of each ventral scale may become much dispersed,forming large black patches alongside the ventral surface after one year in preservative.
3.4 VariationOther specimens generally resemble the holotype except the following characters (Table 1):IL rarely 8.PRO rarely 2 and PTO occasional 2.SVL ranges from 409-476 mm in females and 404-431 mm in males.Female specimens tend to have a shorter tail and fewer SC than male specimens(for TaL 91-107 mm versus 104-131 mm,for SC 45-52 versus 55-59).The ventrolateral longitudinal brownish-red coloration may be lighter (Figure 5 A,C) and faint black spots on the ventral scales could merge into large patches and cover the whole scale at the posterior part of body (Figure 5 B).The dorsal scales of some specimens (such as CIB116093) are feebly keeled even on the second outer row,with only the outer row smooth.

Figure 4 The hemipenis of the holotype of Rhabdophis chiwen sp.nov.(CIB116092).Photo by Shengchao Shi.

Figure 5 Photos of Rhabdophis chiwen sp.nov.. A and B:Ventral view of an adult female displaying the coloration variations,photo by Yige Piao.C:An adult female in life.D:A yearling in life.For C and D,photo by Masaya Fukuda.
3.5.ComparisonsComparative data ofR.chiwenwith 27 known species of the genusRhabdophiswere obtained from literature (see Materials and methods) and are shown in Table 2.
Currently,11 species are known to occur in China,andRhabdophis chiwencan be distinguished from these species by following characters (a detailed comparison betweenR.chiwenandR.pentasupralabialisis separately listed in a latter paragraph).R.chiwendiffers fromR.adleriandR.himalayanusby DSR 15 rows throughout vs.DSR 19-19-17 inR.adleriandR.himalayanus.R.chiwendiffers fromR.formosanusandR.lateralisby DSR 15 rows throughout vs.DSR 19-19-17(15) inR.formosanusandR.lateralis.The new species differs fromR.guangdongensisby 5 SLs,VEN 151-159,SC 45-59,dorsal body saddlebrown vs.6 SLs,VEN 126,SC 39,brownish-grey coloration inR.guangdongensis.The new species is different fromR.leonardiby DSR 15 rows throughout vs.DSR 18(17)-17-15 inR.leonardi.The new species is different fromR.nigrocinctusandR.subminiatusby DSR 15 rows throughout vs.DSR 19-19-17 inR.nigrocinctusandR.subminiatus.The new species differs fromR.nuchalisby 5 SLs,7(6,8) ILs,TEM 1+1,dorsal body saddlebrown vs.6 SLs,8(7) ILs,TEM 1+2,olive green coloration with black and magneta spots inR.nuchalis(Figure 6).R.chiwendiffers fromR.swinhonisby 5 SLs,7(6) ILs,dorsal body saddlebrown vs.6 SLs,8(7) ILs,medium brown coloration with several rows of black spots inR.swinhonis.
Rhabdophis chiwen,R.pentasupralabialis,R.guangdongensis,R.swinhonis,R.angeliandR.nuchalishave the minimal number of dorsal scale rows in this genus,equal to 15 rows.Nevertheless,R.chiwencan be readily distinguished from the other five species and all other species ofRhabdophisby SLs,DSR,VEN,SC,PRO,PTO or different coloration.BecauseR.guangdongensis,R.swinhonisandR.nuchalisare distributed in China,their comparisons withR.chiwenare listed in the previous paragraph.The new species differs fromR.angeliby VEN 151-159,SC 45-59,dorsal body saddlebrown vs.VEN 117-126,SC 39-46,brownish coloration with a dorsolateral series of small reddish spots inR.angeli.
For the remaining species within the genusRhabdophisexceptR.pentasupralabialis,R.chiwensp.nov.can be readily distinguished by its dorsal scales in 15 rows throughout vs.DSR 17-17-15 inR.auriculata;DSR 19 at midbody inR.barbouri,R.callichromus,R.chrysargos,R.conspicillatus,R.lineatusandR.spilogaster;DSR 21 at midbody inR.callistusandR.chrysargoides;DSR 19-19-15(17) inR.murudensis;DSR 19-19-17(15) inR.tigrinus.
Specimens collected from Xingou Village and Jiguan Mountain differ from specimens ofR.pentasupralabialisby:1)larger size (mean TL 536.83 mm) vs.mean TL 483.64 mm inR.pentasupralabialis(Table S2);2) saddlebrown coloration of dorsal body vs.dark green or olive green coloration of dorsal body inR.pentasupralabialis;3) the margin of the outer row forming two faint dorsolateral black cross-bars alongside body vs.absent inR.pentasupralabialis;4) the outer margins of ventral scales and several lateral rows of dorsal scales forming ventrolateral longitudinal brownish-red coloration,with faint black spots in the middle of each ventral scale and between scales which line a black stripe vs.absent inR.pentasupralabialis(for the comparison of ventral coloration,see Figure S1).
For male specimens,the first two principal components accounted for 55.900% of cumulative coefficients.Principal component 1 (PC1) accounted for 31.464% and principal component 2 (PC2) for 24.436%.For female specimens,the first two principal components accounted for 64.567%.PC1 accounted for 39.106% and PC2 for 25.461%.Table 5 displays which characters are important in PC1 and PC2 for male and female specimens.

Figure 6 The lateral or ventral view of R.pentasupralabialis (A),holotype (CIB116092) of R.chiwen sp.nov.(B) and R.nuchalis (C),displaying the 6 ILs of R.pentasupralabialis,7 ILs of R.chiwen sp.nov.and 8 ILs of R.nuchalis.

Table 5 Component matrix of Principal Component Analysis for male and female specimens.
Figure 7 shows the plots of the first two principal components for males (A) and females (B).The PCA for male specimens did not reveal much differentiation among the localities,while the female specimens could be easily distinguished from each other.
3.6.Distribution,habitat and behaviorRhabdophis chiwenis currently known to be distributed in several parts of Sichuan Province,including Xingou Village of Tianquan County,Jiguan Mountain of Chongzhou City and Hongya County.During several field surveys in Xingou Village,individuals of this species were commonly encountered,including hatchings,juveniles and adults.At more than six different field sites with the GPS records,we speculate thatRhabdophis chiwenlives at the altitude range of 1100-2200m,typically near farmland and the source of water (Figure 8).With the stomach content by forced regurgitation and observation of feeding behaviors in the laboratory,it has been confirmed that this species primarily prey on earthworms and fireflies (Yoshidaet al.,2020).The defensive behavior of this species is the typical body lift described by Mori (Moriet al.,2016).
4.Discussion
The genusRhabdophisis widely distributed among Eurasia.Species of this genus are known to occupy a wide range of microhabitat and different altitudes (Zhao,2006).Sichuan Province lies in the southwestern part of China,which stretches across Palearctic Realm and Oriental Realm,and can mainly be divided into two parts:the western part is the West Sichuan High Plateau and the eastern part is the Sichuan Basin (Zhao,2002).The complex natural environment provides varied habitats for a large quantity of species (Wanget al.,2010).Currently,5 species ofRhabdophisare reported to inhabit Sichuan Province,i.e.R.pentasupralabialis,R.nuchalis,R.leonardi,
R.subminiatusandR.lateralis.There are doubtful records ofR.nigrocinctus,because this species typically inhabits low altitude habitat with high humidity rather than dry-hot valleys as the recorded locality in southwestern Sichuan (Li Ding,personal communication).R.chiwenwas also found in Hongya County during several field surveys,where a specimen (HKV36838) was considered asR.nuchalisin a previous study (Alfaro and Arnold,2001).With our long-term field records since 2012,it is shown that the distribution range ofR.chiwenis partly overlapped with that ofR.pentasupralabialisandR.nuchalis.Together withR.leonardi,these four species all primarily feed on earthworms and form a earthworm-eating clade while most species in this genus prey on frogs and fish (Zhao,2006;Yoshidaet al.,2020).Intriguingly,all these earthworm-eating species can be found in Sichuan,and it remains to be investigated whether and how they show some niche differentiations.
A previous study has pointed out substantial genetic divergence withinR.nigrocinctus,R.swinhonis,R.nuchalis,and especiallyR.subminiatus,and suggested the possibility for several undescribed species (Takeuchiet al.,2018).In the study of this genus,our field records clearly support this possibility (Li Ding,personal communication).It is sure that the western and southern parts of Sichuan Province still provide shelters for certain cryptic species ofRhabdophis.

Figure 7 The plots of the first two principal components for males (A) and females (B) of specimens.

Figure 8 Habitat of Rhabdophis chiwen in Xingou Village,Tianquan County,Ya’an City,Sichuan Province.Photo by Yige Piao on June 2nd,2019.
AcknowledgementsThis study was supported by the Biodiversity Survey,Observation and Assessment Programme(2019-2023) of Ministry of Ecology and Environment of China to Li DING and Yanqing WU;grants of the National Natural Science Foundation of China (No.31301882,No.31970423) to Qin Chen,and Science and Technology Foundation of Sichuan(No.2018SZ0335) to Qin CHEN.;grants from Japan-China Joint Research Project (2014-2016) between the Japan Society for the Promotion of Science (JSPS) and National Natural Science Foundation of China (NSFC,31411140033) to Yezhong TANG and Akira MORI,and grants from JSPS KAKENHI Grant Numbers JP26440213,JP17H03719,and JP18KK0205 to Akira MORI.In addition,we are much indebted to the Museums of Herpetology in Chengdu Institute of Biology and their staff for their help and permission to examine preserved specimens under their care.
杂志排行
Asian Herpetological Research的其它文章
- The Effects of Prey Items Diversity and Digestible Materials in Stomach on Digestive Tract Length in Hylarana guentheri
- Hotspot of Adult Cuban Treefrog and Cane Toad Multi-organ Abnormality in Suburban South-west Florida
- Distribution Changes of Chinese Skink (Eumeces chinensis) in China:the Impacts of Global Climate Change
- Effects of Acute Temperature Stress on mRNA Expression of Transferrin in the Yellow Pond Turtle Mauremys mutica
- Behavior and Approximate Entropy of Right-eye Lateralization During Predation in the Music Frog
- Ring Clinal Variation in Morphology of the Green Odorous Frog (Odorrana margaretae)
