The effect of sintering and cooling process on geometry distortion and mechanical properties transition of PTFE/Al reactive materials
2020-06-28HaifuWangBaoqunGengHuanguoGuoYuanfengZhengQingboYuChaoGe
Hai-fu Wang, Bao-qun Geng, Huan-guo Guo, Yuan-feng Zheng, Qing-bo Yu, Chao Ge
State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, China
Keywords:Reactive material Sintering Cooling Geometry distortion Mechanical properties
ABSTRACT In this research, the effect of the sintering and cooling process on geometry distortion and mechanical properties of PTFE/Al reactive material is investigated. Six particularly selected sintering temperatures,three different cooling modes(annealing cooling,normalizing cooling and rapid cooling),three different initial cooling temperatures,as well as six different final cooling temperatures were designed to compare the effects of sintering temperature, cooling rate, initial cooling temperature and final cooling temperature on the properties of reactive materials. Geometry distortion was quantitatively analyzed by a statistic on the dimensional changes of the specimens and microscopic morphology. A mechanical response properties transition from brittle to ductile was found and analyzed. By combining the thermodynamic properties of PTFE and unsteady heat conduction theory, mechanisms of cooling induced morphology change, temperature induced distortion and strength decrease were obtained. The results showed that the cooling rate has the most significant effect on the morphology transformation, while initial cooling temperature has more significant effect on the dimensional distortion than final cooling temperature.As to the mechanical properties transition from brittle to plastic,a more prominent effect of initial cooling temperature than cooling rate and final temperature was revealed.
1. Introduction
Reactive material has received extensive attention in military fields as potential structural material due to the unique mechanical and chemical properties [1-8]. Much different from traditional metals,reactive material not only achieves to penetrate the target,but also releases sufficient energy caused by non-self-sustaining exothermic reactions under dynamic loading impact. In order to improve the damage effect of reactive materials, researches focus on the formulations, mechanical properties and energy release characteristics [9-27].
As for the purpose of achieving high-efficiency damage enhancement when the reactive materials are impacted or impact at the target, mechanical properties, especially the strength, play vital role.Mainly by focusing on the formula design,high strength inert metal component (tungsten) was added to improve the strength performance. Yet excessive addition of inert particles affects the energy release rate and conversely limits the damage efficiency.
Mainly fabricated by aluminum and polytetrafluoroethylene(PTFE)via a press/sintering process,the majorities of the materials are sintered for a certain period of time by heating at a temperature ranging from 360°C to 370°C [1], then cooled with the vacuum oven to room temperature or held at a crystallization temperature for a period of time and then cooled with the annealing cooling.Crystallization refers to the transition of polymer matrix from molten state to crystalline state and is significantly affected the cooling process which in essence is a thermal transition process[28-30]The crystallization of the reactive material is related to the strength of the material. For quasi-static compression of reactive materials, related research is more reflected in materials reaction characteristics [30]. Experiments involving the sintering process are carried out to analyze the mechanics of reactive materials,and the effect of crystallinity on the mechanical properties are demonstrated[28,29,31].
For the typical fabrication process of reactive materials, a longterm sintering cycle is crucial to the crystalline state of the material.This sintering cycle contains a heating stage, sintering stage and cooling stage. During these three stages, detailed controlling parameters could be proposed, such as heating rate, sintering temperature, cooling rate and mode, initial and final cooling temperature, which have significant effect on the crystalline state of the material. All these on the property changes and the corresponding mechanisms are not yet well understood.
This paper presents a study on the effects of sintering and cooling process on the geometry distortion, microscopic morphology change and mechanical property transition. Scanning electron microscope (SEM) is used to study the surface material microscopic morphology, and quasi-static test is used for investigating the compressive response of reactive materials in different sintering and cooling states. Analysis and discussions are carried out to analyze the mechanism of compression characteristics,combing with the residual stresses and relative crystallinity forming in the non-steady-state heat conduction. Research results can provide a useful guide to the mechanical property design and the engineering applications, especially for the sintering and cooling process of the PTFE/Al reactive materials.
2. Experiment
2.1. Sample preparation
In this research, PTFE/Al (73.5 wt%/26.5 wt%) reactive material,of which the mass ratio of each constituent was determined through zero-oxygen-balance between PTFE and Al,was prepared.The fabrication process consists of three stages: mixing and isostatic pressing stage, heating and sintering stage and cooling stage, and is presented below.
(1) Mixing and isostatic pressing
With the assistant of a vacuum chamber, powders of PTFE(DuPont, MJ 1500J) and Al (FLPA 250) were uniformly mixed via a dry mixing process.Average diameter of the PTFE and Al particles is approximately 100 μm,while the density values of pure crystalline PTFE and amorphous PTFE are 0.46 g/cm3and 2.3 g/cm3respectively. Studies have shown that PTFE/Al reactive material would undergo tip cleaving reaction under quasi-static compression due to the adding of fine Al particles[16],thus Al particle of an average diameter of 114 μm was selected to ensure a stable mechanical response during quasi-static tests.The powder mixtures were then encapsulated in a rigid φ9.5 mm cylindrical mandrel with a moving piston, to produce 9.5 mm high and 10.0 mm in diameter samples of 1.55-1.59 g. Pressure applied to the reactive material mixtures during the followed cold isostatic pressing was 150 MPa with a dwell time of approximately 1 min, at ambient temperature.Pressed samples were then relaxed at ambient pressure and temperature for 24 h to remove any trapped air or residual stresses.
(2) Heating and sintering
After mixing and pressing, the reactive material samples were placed into an argon atmosphere protected vacuum oven to undergo a heating and sintering cycle.Heating temperature first rose from 20.0°C up to 200.0°C in 30 min. On the basis of the initial heating, the temperature was further raised to six different particularly selected sintering temperatures: 250.0°C, 275.0°C,300.0°C,325.0°C,350.0°C and 375.0°C,at the rate of 1.0°C/min,to characterize the effect of sintering temperatures on material properties,as shown in Fig.1(a).Five samples under each sintering temperatures were prepared for subsequent tests.
(3) Cooling
After held at the sintering temperature for 1 h to ensure sufficient heat exchange between the samples and atmosphere, three cooling processes with distinct cooling rates were designed:annealing cooling, normalizing cooling and rapid cooling, which denote cooled the samples in the vacuum oven, ambient temperature and water, respectively. Relationships between cooling rates and time are depicted in Fig.1(b).
In order to compare the effect of initial cooling temperature on material properties,an annealing cooling to rapid cooling process is designed.Samples sintered to 375.0°C and held at this temperature for 1 h at the state of fully molten, were first annealing cooled to different temperatures(357.0°C,327.0°C and 297.0°C),then rapid cooled to room temperature, as shown in Fig.1(c).
Different final cooling temperatures were also applied to characterize the difference of material properties. After sintered to 375.0°C, samples were cooled to six different final temperatures(95.3°C, 80.2°C, 60.6°C, 40.0°C, 20.5°C and 0.3°C) via the rapid cooling process, as depicted in Fig. 1(d). Rapid cooling process assisted by 200 mL water is shown in Fig. 2.
2.2. Quasi-static compression test
The fabricated reactive materials are processed into a cylindrical specimens with a final size of φ9.5 mm×9.5 mm by a milling machine. Quasi-static uniaxial compression tests were conducted according to ISO 604-2002 for plastics and performed with a computer controlled universal testing machine (MTS) at ambient temperature. The loading speed of the crosshead is determined based on the height of the sample corresponding to a nominal strain rate of 10-3s-1. Schematic of the quasi-static compression tests and typical experimental photograph are shown in Fig. 3.
The true stress and strain can be calculated following Eq. (1):

Where σTand εTare the true stress and strain obtained in the quasistatic compression,F and X are the pressure and displacement data recorded by the MTS during compression, D and H are the initial diameter and height of the reactive material specimen.
3. Experimental results
3.1. Geometry distortion effects
PTFE/Al specimens sintered to 375.0°C and cooled to ambient temperature by rapid cooling and annealing cooling process(Fig.1(b)) respectively are shown in Fig. 4.
Obvious geometry distortion and radial size shrink of the two specimens indicate the significant effect of cooling stage. In Fig. 4(a), specimens rapidly cooled to room temperatures lead to non-uniform shrinkage and sever distortion. The shrank surface could be represented by a hyperbolic-like line. By comparison, an annealing cooling process results in uniform shrinkage and straight cylindrical surfaces, which could be represented by a straight line.
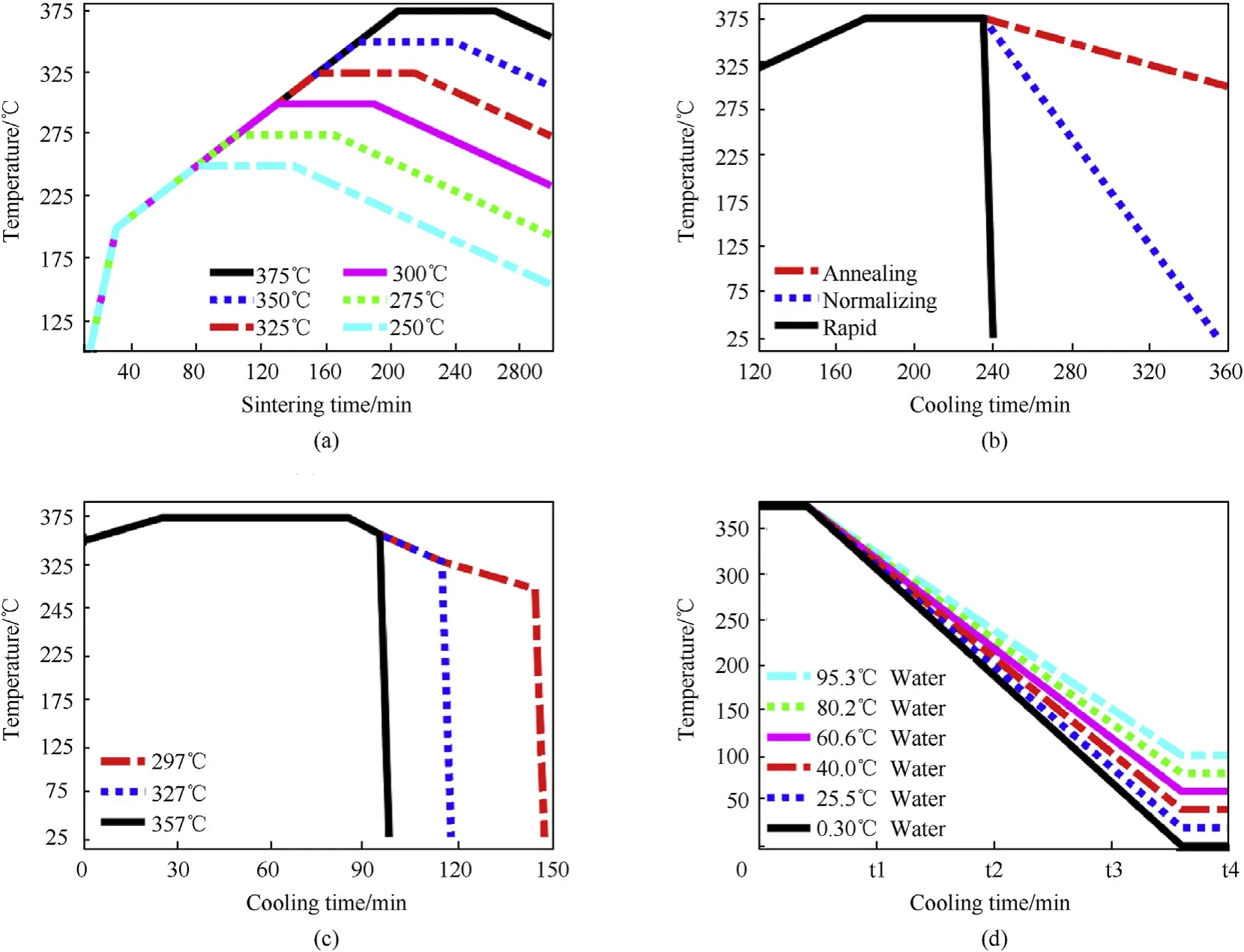
Fig.1. The time history of different sintering and cooling process.(a)sintering stages;(b)annealing,normalizing and rapid cooling process;(c)annealing cooling to rapid cooling;(d) rapid cooling with different final cooling temperatures.

Fig. 2. Experiment setup for rapid cooling in water with different temperature: (from left to right) 95.3°C, 80.2°C, 60.6°C, 40.0°C, 20.5°C and 0.3°C.
Scanning electron microscope (Hitachi S-4800) was applied to compare the structures of the specimens shown in Fig. 4 in microscopic scale. After cutting along the central axis, two randomly selected regions on the partition surfaces were chosen to observe the morphology of the PTFE matrix and PTFE/Al interfacial adhesion (Fig. 5). For annealing cooled specimens (Fig. 5(a)),interfaces between PTFE and Al particles show ideal combination.Large amount of PTFE fibers could be found for the bonding of the two constituents (Fig. 5(b)). As well as this, PTFE fibers densely cover the embedded metal particle, providing a further improved combination between matrix and particles (Fig. 5(c)). By comparison, on specimen by rapid cooling (Fig. 5(d)), less filamentary bonding and clear gaps between matrix and particles could be observed(Fig.5(e)).Different from specimen of annealing cooling,only few PTFE filaments scatter on the surface of metal particles(Fig. 5(f)). Thus generally, rapid cooling of the specimen results in inferior particle/matrix bonding and fiber formation compared with slow cooling. These all would have significant influence on mechanical response of materials.
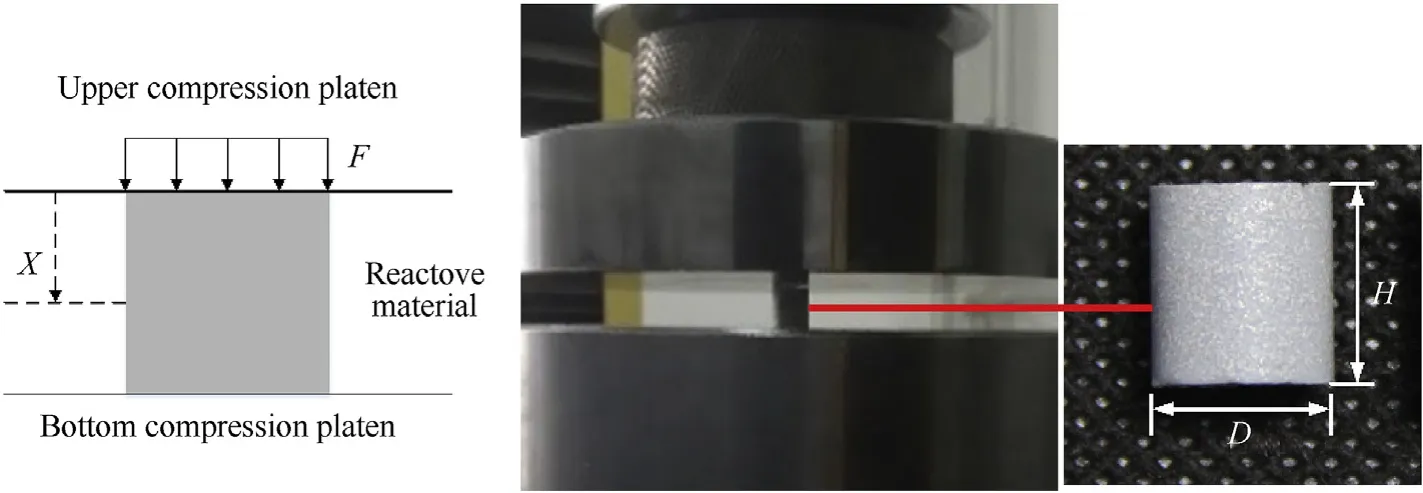
Fig. 3. Schematic of quasi-static compression tests and specimen.
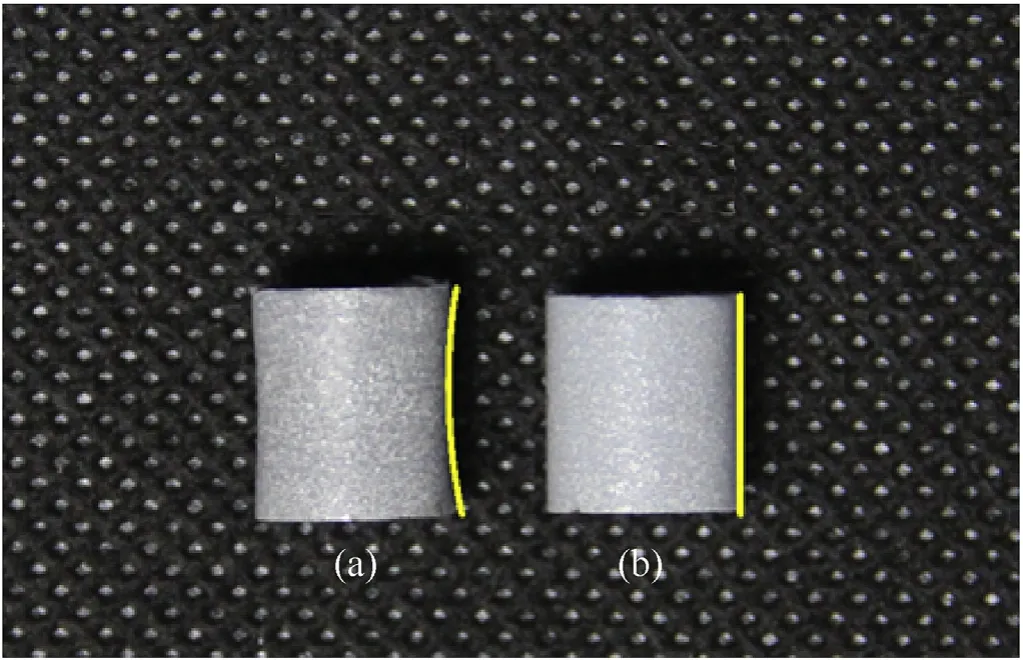
Fig.4. Typical photograph of the PTFE/Al specimens prepared by sintering to 375.0°C and different cooling processes to room temperature: (a) rapid cooling and (b)annealing cooling. The yellow lines on the right side indicate the geometry of each specimen.
Geometrical distortion of specimens following different cooling process is quantitatively compared by dimensionless analysis of characteristic dimensions, as depicted in Fig. 6. Specimens from rapid cooling demonstrate hyperbolic distortion, that is, the side face of the material changed from cylindrical surface to single-leaf hyperboloid, and end faces shrink from flats to a double-leaf hyperboloid. On the basis of standard cylindrical shape obtained by annealing and normalizing cooling process, quantitative description of the distortion size is expressed in Eq. (2):
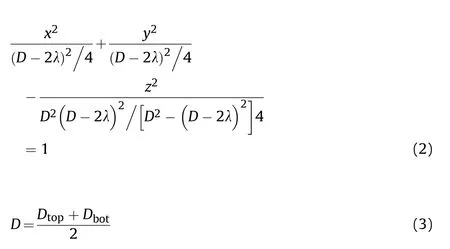
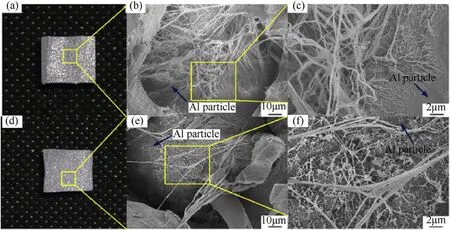
Fig. 5. Microstructures of reactive materials by (a-c) annealing cooling and (d-f) rapid cooling processes.
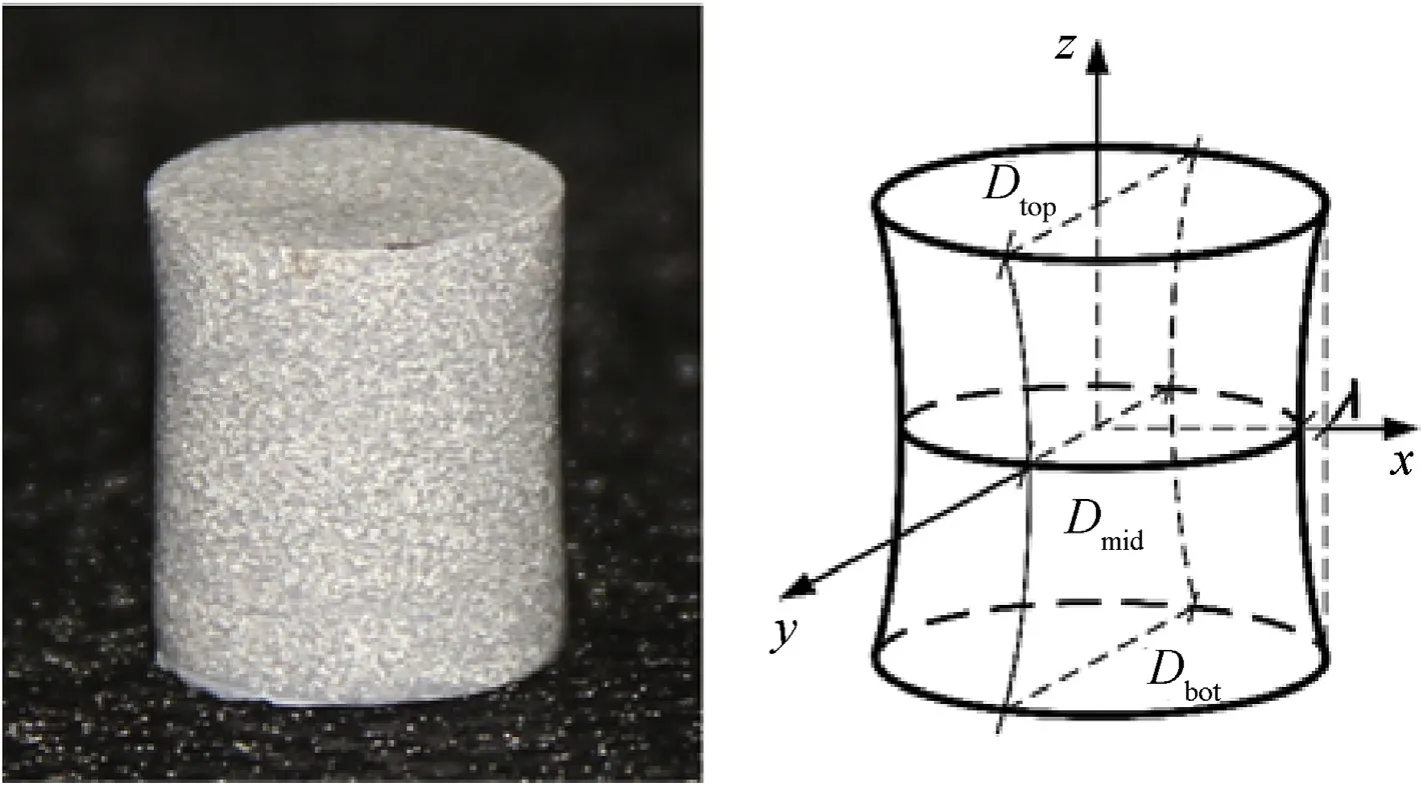
Fig. 6. Typical distorted specimen and schematic of characteristic dimensions.

Where Dtopand Dbotare the diameters of the top and bottom faces,and Dmidis the waist circle diameter of the cylindrical sample surface, while λ represent the radial size shrink.
The radial size shrink induced by different cooling processes is shown in Fig.7,calculated by Eqs.(3)and(4),which corresponds to the cooling rates, initial cooling temperatures and final cooling temperatures.
With a rising cooling rate, the average size shrink increases significantly (Fig. 7 (a)). Radial distortion size of annealing cooled material specimens show the least scattering, which could be neglected. As the cooling rate increasing, the shrink size variance increases from 1.5×10-4to 8.9×10-3, which indicates the unstable shrink size increase. The average shrink size increases two orders of magnitude from 10-3mm to 10-1mm with an average value fluctuating within the order of 10-1mm. The maximum radial size shrink appears during rapid cooling process.
Fig.7(b)shows that the shrink size presents an increasing trend with increasing initial cooling temperatures. The mean values of the specimen size shrink cooled to ambient temperature are reduced from 0.20 mm to 0.15 mm and 0.03 mm, and the size shrink maximum and standard deviation are also decreasing,which is consistent with the shrink size by rapidly cooled from initial cooling temperature of 375.0°C. When the initial cooling temperature is lower than 327°C, the size shrink is significantly reduced,and the size shrink is close to those by annealing cooling.
Fig. 7(c) shows that, as the final temperature increasing from 40.0°C to 80.6°C and 95.3°C, the shrinks of the reactive material cooled to ambient temperature reduce from 0.16 mm to 0.13 mm and 0.08 mm,the size shrink and variance of the size shrink are also reduced correspondingly, which is consistent with the shrink size by rapidly cooled to 20.0°C.
Calculation on the size shrink also indicates that the reactive material is more sensitive to the initial cooling temperature than the final cooling temperature. Reducing the initial cooling temperature by 60°C,the shrink size is 0.17 mm.Correspondingly,the dimensional shrinkage degree reduces to 0.08 mm, when the final cooling temperature reduces by 60°C. Thus a more significant effect on the dimensional distortion by initial cooling temperature is demonstrated than final cooling temperature. Furthermore, the change of cooling rate and cooling temperature is consistent with the trend of statistical parameters of reactive materials, which further proves that rapid cooling has a significant effect on the morphology transformation of reactive materials.
3.2. Mechanical response transition
Fig.8 compares the true stress-strain curves of specimens under different sintering temperatures (Fig. 1(a)) by quasi-static compression tests.
With the increasing of the sintering temperatures, the compressive mechanical response of the cold-pressed reactive material gradually transits from brittle to elastic-plastic. The transition temperature ranges from 325°C to 350°C, which includes the melting temperature(327°C) of reactive material. Quasi-static compression reflects in 45°cracks of specimens sintered at a 275°C. With the increase of sintering temperature and when the specimens are compressed at a temperature below the melting temperature of reactive material matrix, they adopt a truncated cone shape with multiple 45°cracks. This shape indicates a difference in compaction from top to bottom in the cylindrical specimens. During long range pressings, the mandrel should be‘floating’ and the lower piston surface remains low flush with the mandrel and non-equilibrium compression profiles due to friction forces.Continuing rising the sintering temperature to above 325°C,the characteristic of ductile deformation appears. The specimens were pressed into drum shapes and no cracks and failure were observed. Fractographic observation of the three types of compressed specimens reveals that when the sintering temperature is higher than the collective crystallization temperature of matrix,fine PTFE fibers formed within the cracks. As the sintering temperature decrease, dense PTFE strips formed in the cracks instead.
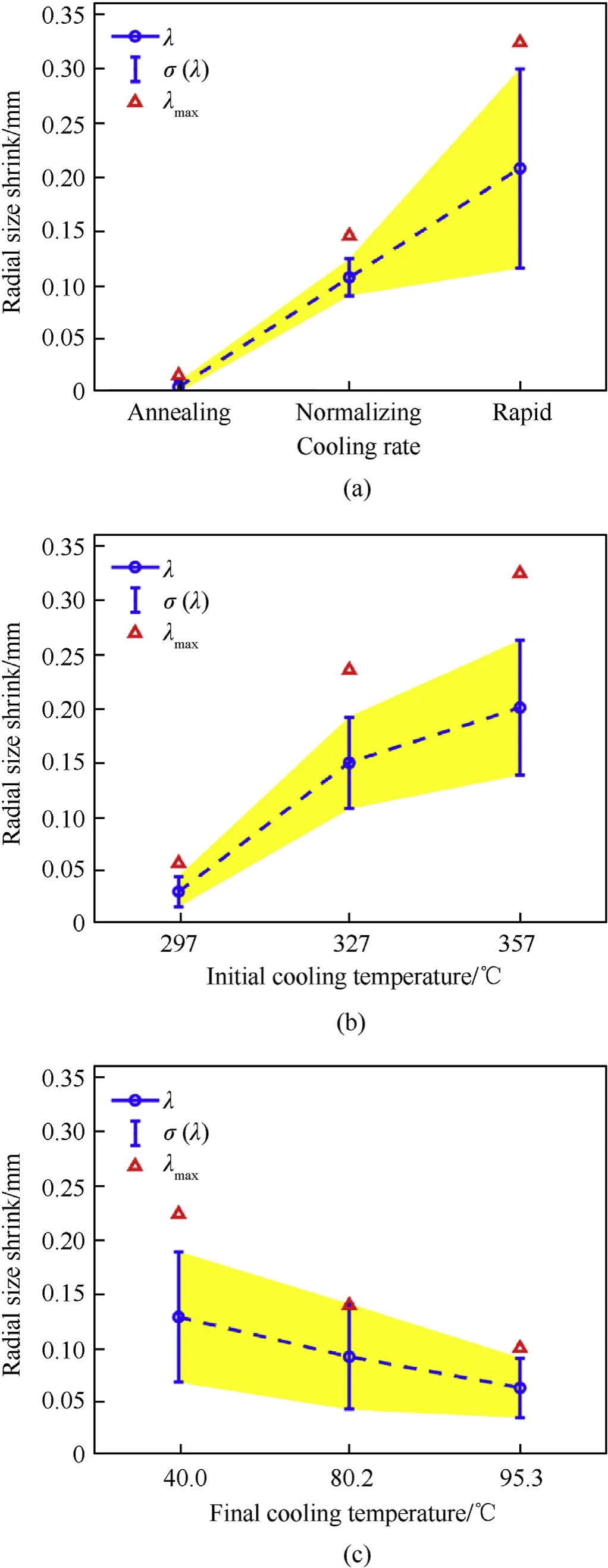
Fig. 7. Typical experimental results of radial size shrink of reactive materials.
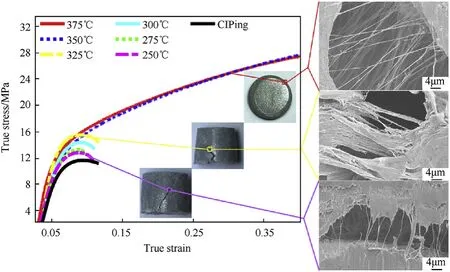
Fig. 8. Compressive response of specimens sintered at different temperatures and fractographic observations of the cracks.
Based on the stress-strain curves, elastic moduli for evaluating the effect of cooling process were obtained[29]As shown in Fig.9,elastic moduli fitted with a 95% confidence were determined for each type of materials. Stress-strain curves and elastic moduli for specimens following annealing, normalizing and rapid cooling processes are shown in Fig. 9(a). By increasing the cooling rate(from annealing to rapid cooling), elastic modulus reduces from 515.1 MPa to 203.4 MPa. Correspondingly, Fig. 9(b) and (c)demonstrate the significant effect of initial and final cooling temperatures on elastic response of materials. The elastic modulus increases from 227.5 MPa to 356.9 MPa, when the initial cooling temperature drops from 357.0°C to 297.0°C.When the final cooling temperature increases from 40.0°C to 95.3°C,elastic moduli of the reactive material increase from 240.1 MPa to 319.2 MPa. All the above results show that the cooling process has significant effect on the elastic response of reactive materials.
Further analysis on the change of elastic modulus reveals that,a reduction of 60°C of the initial cooling temperature corresponds to a reduction of 129.4 MPa of elastic modulus, compared with the reduction of 79.1 MPa resulted from final cooling temperature,demonstrating a more prominent effect of initial cooling temperature.And this is also consistent with the variation law of the radial size shrink.
4. Analysis and discussion
4.1. Cooling induced morphology change
Due to the sintering temperatures which are below the melting points of aluminum and aluminum oxide,dispersion phases of PTFE based reactive material, such as Al particle, maintain thermal stability and evenly disperse inside the material [31-33]. Moreover,dissimilar aluminum particles are only interest to reduce agglomeration of matrix, that is, degree of melting rate of the continuous phase matrix determines the overall particle flow of the specimens[34].Based on the thermodynamic properties of PTFE,the melting process of the reactive material can be divided into four stages:thermal stability stage, thermal softening stage, melting stage and fully melting stage, with increasing the sintering temperature in the sintering process, as qualitatively descripted in Fig.10(a-d).
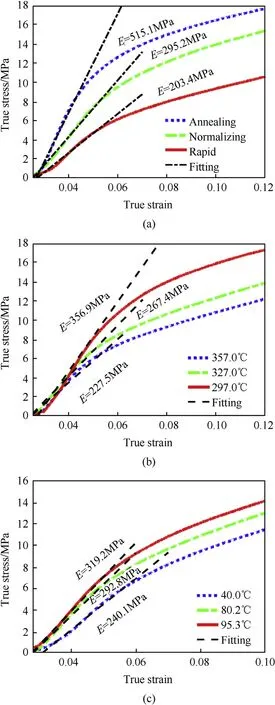
Fig. 9. Elastic modulus determination of PTFE/Al specimens following different: (a)cooling processes (b) initial cooling temperatures and (c) final cooling temperatures.
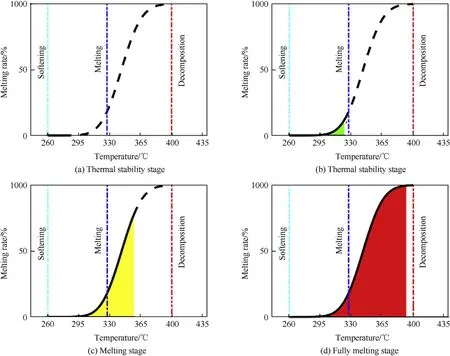
Fig.10. Description of the stages during sintering process: (a) thermal stability stage and (b) thermal softening stage (c) melting stage and (d) fully melting stage.
In the first stage(Fig.10(a)),the matrix of reactive material stays in a thermal stability stage when the sintering temperature of the reactive material is below 260°C. Due to the ineffective thermal conductivity during this process,material properties of the sintered reactive material are similar to unsintered specimens. Agglomeration of opaque continuous phase can be easily distinguished by the granular metal particles distributed upon the surface.
When the sintering temperature rises to temperature ranging from 260°C to 327°C, compared with the first stage, the material enters the thermal softening stage (Fig.10(b)). In this stage, slight thermal weight loss happens to the matrix. However, the reactive material does not melt or crystallize after sintering. Glossiness deterioration of the matrix can be observed corresponding to the increase of the sintering temperature.Then,the size of PTFE would shrink and be stiff and opaque again[33,35].
As the sintering temperature continues to rise across the melting point of the reactive material matrix, the temperature of fully heated PTFE is above its melting point of 327°C(Fig.10(c)).As the temperature drops,the PTFE would crystallize from the melting state again.The fully sintered reactive material samples undergo a non-oriented melt and the matrix turns into a transparent fused gel. The dispersed metal phases flows in the gel and cools to sufficient crystallization. The amorphous form of the molten reactive material undergoes phase change crystallization, and the fluidity increases with the ascending sintering temperature. The surface roughness of the rapidly cooled reactive material is further increased, but the surface gloss is uniform, compared with the annealing cooling and normalizing cooling of the samples.
The final stage is illustrated in Fig. 10(d). When the sintering temperature exceeds 370°C, matrix of the reactive material experienced thermal decomposition. Cooled from the temperature of 375°C, the reactive material converts from a molten state to a crystalline state,which is responsible for the morphology changes with crystallinity.The thermal instability of the material increase in addition, and the surface roughness improves as well.
4.2. Temperature induced distortion
In order to estimate the temprature distribution of the molten reactive material, reactive material specimen underwent heating and sintering stage was assumed to be a three-dimensional short cylinder under unsteady heat conduction conditions. From the symmetry of the cylinder, for any reactive material shaft section,the cooling process is only related to its position and cooling time.Also,the temperature at a certain point during the cooling process of the molten reactive material is only related to the spatial position of the material and the cooling time.
Considering one-dimensional unsteady heat conduction and temperature distribution at each point of the cylindrical reactive material specimens, a cylindrical coordinate system is established(Fig. 11). Corresponding relationship between temperature distribution and cooling duration can be expressed as Eqs. (5) and (6):

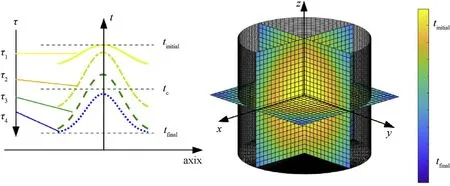
Fig.11. Schematic illustration of the temprature distribution of the specimen in cooling process.

Where t is the temperature of a certain reactive material unit, x denotes the position of the unit.a is a constant.tinitialand tfinalare the initial and final cooling temperatures. D and H are the initial diameter and height of the cylindrical specimen and τ is the cooling time.
When the sintering time develops from τ1to τ4,the temperature decreases gradually considering the build-up or formation mechanism of temperature induced stresses [35]. It is suggested that during the cooling, polymer chains are stretched and oriented in the flow direction. Accompanying the orientation process, these deformation relaxes. With the increase of oriented matrix chains,more relaxation results. However, relaxation of the most oriented chains in the outer layers is restricted by the less oriented inner layers,as shown in Fig.12,resulting in tensile residual stress in the outer layers and compressive residual stress in the inner ones.
In the cooling process of the sintered molten reactive material,it can be simplified to free quenching without considering the material cooling crystal transformation stress,and only thermal stress is generated.During the rapid cooling process,cooling rate from the center to the outward of the molten specimen is not uniform[35],as shown in Fig. 12. When the outermost layer of the molten reactive material is cooled to a crystalline state, the specimen shrinks inward, and compressive stress is generated within the uncrystallized reactive material.Then the reactive material relaxes rapidly due to the viscous deformation of the reactive material.The crystals on both sides of the corner are hindered, and the equilibrium does not move under the compressive stress. As time progresses, the crystals accumulate at the corner and the material forms an inward distortion.
4.3. Strength decrease mechanism
The rate of cooling has great effects on the degree of crystallinity and mechanical properties of PTFE composites. Root causes of the mechanical properties changes of the PTFE matrix composites lie in the change of the crystallinity of PTFE. Rapid cooling process reduces the degree of crystallinity and hardness of PTFE composites[35,36]. The internal temperature of the reactive material rapidly decreases at the intersection of the end and the side faces,and the crystallization is first formed at the corners. A phase change thermal residual stress would then produce when the material crystallizes, as shown in Fig.13.
The residual stress caused by uneven cooling of the reactive material has significant effect on the mechanical properties(especially the elastic modulus) of the material. Strength and hardness of the matrix would be improved by increasing the crystallinity of the reactive material [33]. In reactive material, the PTFE-based body is crystallized to form a doped composite with Al granules. The mechanical properties are closely related to the crystallinity of the morphological structure and properties of the crystalline polymer. Under the condition of slow crystallization of PTFE, the crystal grain is arranged in a regular order, and the material strength is higher. In the non-isothermal crystallization kinetics of the polymer, the relative crystallinity and the relative crystallinity of the improved Ozawa method for isothermal cooling can be expressed as Eq. (7) [32,33]:
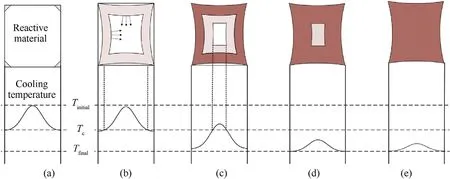
Fig.12. Cooling temperature induced distortion process reactive material specimen:(a)the edge temperature drops rapidly and crystallizes first,and(b-d)gradual crystallization process and (e) fully crystallized.
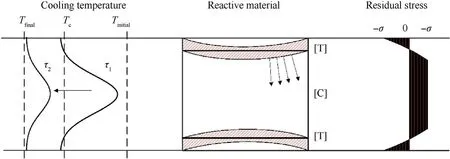
Fig.13. Cooling induced residual stresses of reactive materials.
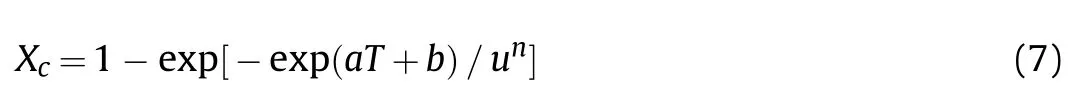
where Xcis the crystallinity of the polymer transformed at a temperature T,u is the cooling rate,and a and b are the cooling function which only varies as a function of the temperature,n is the Ozawa index which has the values of 1.49 for PTFE [37]. In this study,crystallinity of the polymer can be expressed by Eq. (8) [16]:

where ρ is the measured density of the specimen.
Fig.14 gives the crystallinity of reactive material matrix versus temperature at various cooling rates. Curves represents the crystallization curves and an asterisk represents experimental results based on Eq.(8).When the sintering temperature is lower than the crystallization temperature of the reactive material matrix, the relative crystallinity of the material is approximate to 0.0. When increasing sintering temperature to the initial crystallization temperature, the relative crystallinity of the material raises from zero and the crystallinity of the material gradually approaches 1.This is consistent with the trend of increasing compressive strength of the material in Fig.8.As the cooling rate increases,the reactive material is cooled from the high temperature molten state to ambient temperature, and the relative crystallinity is correspondingly reduced.
When the material is rapidly cooled from different initial temperatures(357°C,327°C,297°C),if the temperature of the reactive material is higher than the crystallization temperature,the relative crystallization degree of the material gradually increases with the initial temperature.However,when the initial temperature is lower than the crystallization temperature, the reactive material would completely crystallize. This is consistent with the fact that the rapidly cooled reactive material from the initial temperature of 297.0°C in Fig. 4(c) is not distorted. This indicates that in the process of the rapid cooling of the fully crystalline reactive material,crystallization degree and thermal residual stress affects the material geometry distortion.
When the final cooling temperature is lower than the crystallization temperature, the relative crystallinity is affected by the cooling rate. As the final temperature rises (Fig. 14(c)), the crystallization increases and remains stable. It shows that the rapid cooling of the crystallization temperature and the final cooling temperature has a significant effect on the mechanical properties of the reactive materials.
5. Conclusions
In this research, the effect of the sintering and cooling process on geometry distortion and mechanical properties transition of PTFE/Al reactive material is studied. Six particularly selected sintering temperatures, three different cooling modes (annealing cooling, normalizing cooling and rapid cooling), three different initial cooling temperatures, as well as six different final cooling temperatures were designed to compare the effects of sintering temperature, cooling rate, initial cooling temperature and final cooling temperature on the properties of reactive materials. The main conclusions are as following:
1) Sintering and cooling process have significant effect on the macroscopic geometry, microscopic morphology and mechanical properties of PTFE/Al reactive material. Rapid cooling has a significant effect on the morphology transformation of reactive materials,while initial cooling temperature has more significant effect on the dimensional distortion than final cooling temperature.
2) From the mechanical properties point of view,by increasing the sintering temperature, a mechanical response transition could be observed by quasi-static compression tests. Obvious transition from brittle to ductile is found at the sintering temperature above 325°C. Analysis on the elastic modulus also shows that cooling process have significant effect on the elastic response of reactive materials, and also a more prominent effect of initial cooling temperature than cooling rate and final temperature is revealed.
3) Mechanisms of cooling induced morphology change, temperature induced distortion and strength decrease, is analyzed.Crystallinity and morphology of the PTFE matrix paly vital role on the geometry distortion and mechanical properties transition. Sintering and cooling process determines the geometry and mechanical response by determining the crystallizing process and crystallization of PTFE matrix.
The research included in this manuscript would provide indepth understand on the effect of sintering process on material properties,and would promote the optimization of the fabrication method of fluoropolymer matrix reactive materials. More work would be conducted to study the effect of fabrication method on the thermal-chemical properties,including the reaction and energy release properties.
Declaration of competing interest
All the authors declare no conflict of interest.
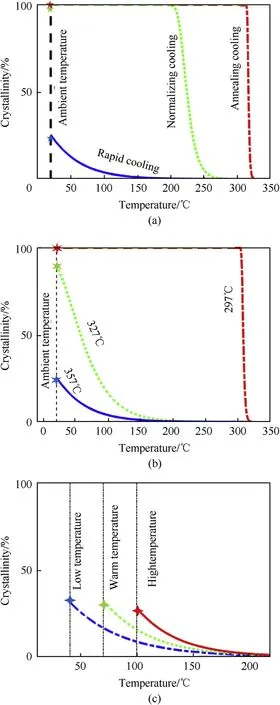
Fig.14. Crystallinity of reactive material matrix versus temperature at various cooling rates: (a) cooling processes (b) initial cooling temperatures and (c) final cooling temperatures.
Acknowledgements
The authors are very grateful for the support received from the National Natural Science Foundation of China (No.11202030) and State Key Laboratory of the State Key Laboratory of Explosion Science and Technology(QNKT19-03).
杂志排行
Defence Technology的其它文章
- Statistical variability and fragility assessment of ballistic perforation of steel plates for 7.62 mm AP ammunition
- Texture evaluation in AZ31/AZ31 multilayer and AZ31/AA5068 laminar composite during accumulative roll bonding
- Local blast wave interaction with tire structure
- Research and development of training pistols for laser shooting simulation system
- Summed volume region selection based three-dimensional automatic target recognition for airborne LIDAR
- A novel noise reduction technique for underwater acoustic signals based on complete ensemble empirical mode decomposition with adaptive noise, minimum mean square variance criterion and least mean square adaptive filter
