Structure design and property adjustment of new cage rich-nitrogen pentazolyltetraazacubanes as potential high energy density compounds
2020-06-28QiongWuGaojieYanZewuZhangWeihuaZhu
Qiong Wu , Gao-jie Yan , Ze-wu Zhang , Wei-hua Zhu
a School of Materials Science and Engineering, Nanjing Institute of Technology,1 Hongjing Road, Nanjing 211167, China
b Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology,1 Hongjing Road, Nanjing 211167, China
c Institute for Computation in Molecular and Materials Science and Department of Chemistry, Nanjing University of Science and Technology, Nanjing 210094, China
Keywords:Pentazole Cubane High-nitrogen High-energy HEDCs
ABSTRACT In this study, based on two attractive energetic compounds pentazole (PZ) and tetraazacubane (TAC), a new family of high energy and high nitrogen compounds pentazolyltetraazacubanes were designed.Then, a different number of NH2 or NO2 groups were introduced into the system to further adjust the property.The structures,properties,and the structure-property relationship of designed molecules were investigated theoretically. The results showed that all nine designed compounds have extremely high heat of formation (HOF, 1226-2734 kJ/mol), good density (1.73-1.88 g/cm3), high detonation velocity(8.30-9.35 km/s), high detonation pressure (29.8-39.7 GPa) and acceptable sensitivity (ΔV: 41-87 Å3).These properties could be effectively positive adjusted by replacing one or two PZ rings by NH2 or/and NO2 groups, especially for the energy and sensitivity performance, which were increased and decreased obviously, respectively. As a result, two designed pentazolyltetraazacubanes were predicted to have higher energy and lower sensitivity than the famous high energy compound in use 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane,while two others have better combination property than 1,3,5-Trinitro-1,3,5-triazacyclohexane.In all,four new pentazolyltetraazacubanes with good combination performance were successfully designed by combining PZ with TAC, and the further property adjustment strategy of introducing a suitable amount of NH2/NO2 groups into the system.This work may help develop new cage energetic compounds.
1. Introduction
Since high energy density compounds (HEDCs) possess great potential used in the field of guns, high explosives, primary explosives,gas generants,solid rocket propellants and so on,making the development and obtain of new advanced HEDCs being very needed and crucial.One critical and alternative strategy is to design different kinds of HEDCs with novel and special structures, understand the structure-property relationship, and further adjust the performance sometimes. Information about these would be both useful for finding and providing appropriate targets for energetic materials experimenters,thus,decreasing the cost and risk,and also helpful for further developing new HEDCs with better combination properties.
Lately, cyclic high-nitrogen N-heterocycle compounds like tetrazoles [1,2], tetrazines [3,4] and pentazoles [5-11], and azides[12-14] have attracted lots of attention based on their many advantages like the green decomposition product (N2). Especially for the pentazole,who was predicted to possess huge energy and now is one significant research hotspot in the field of energetic materials,and significant progress on them have been made recently.For instance, lately, Zhang et al. [5] successfully synthesized a stable salt based on a negatively charged ring of five nitrogens(N5)6(H3O)3(NH4)4Cl. Then, Xu et al. [6,7] further successfully prepared four new energetic pentazole-based salts [M(H2O)4(N5)2]·4H2O (M=Mn, Fe and Co) and [Mg(H2O)6(N5)2]·4H2O, and one new one-dimensional metal-organic framework [Na(H2O)(N5)]·2H2O. Then, using high-pressure technology, Steele et al. [8] obtained CsN5. These mentioned studies indicated the potential of pentazole-metal complexes being a new class of high explosives,since N5-based energetic materials possess amazing high energy,which could be synthesized successfully nowadays,and also can be stable under some special external conditions.Besides,the amount of designed or synthesized N5-based compounds are limited until now,more studies are needed to further design and investigate the structure and properties of new different N5-based high energy compounds like its organic derivatives.
Except for N5-based energetic compounds, cage-based highenergy compounds[15-19]also attracted much attention of many researchers. Two existing outstanding synthesized successfully representatives are octanitrocubane (ONC) [16] and hexanitrohexaazaisowurtzitane(CL-20)[17],which are two extremely powerful CHNO high-energy compounds. Two other designed theoretically aza-cage ones hexanitrohexaazaprismane (HNHAH) [18] and tetranitrotetraazacubane [19] even possess better predicted detonation properties than CL-20 and ONC.These show the high value of energetic cage compounds.
In all,both pentazoles and aza-cages are attractive and valuable alternative HEDCs,and if incorporate them together,a new kind of energetic compounds may have good properties would be formed.Therefore, in the present study, first of all, a series of now highnitrogen high-energy compounds pentazolyltetraazacubanes (as shown in Fig.1: A1 pentazolyltetraazacubane; A2 dipentazolyltetraazacubane; A3 tripentazolyltetraazacubane; A4 tetrapentazolyltetraazacubane)were designed by combining different numbers of pentazoles(PZ)with tetraazacubane(TAC).Then,considering the designed new compounds may have some shortcomings like its large molecule structure and volume which would limit the density, two other small energetic groups NO2and NH2were also introduced to the system to further adjust the structure and optimize performance. Since two much NO2or NH2groups may weaken the advantage of PZ,thus the amount of NO2or NH2groups were restricted to less than two. Therefore, five more new compounds were further designed (Fig.1): A5 diaminodipentazolyltetraazacubane; A6 dinitrodipentazolyltetraazacubane; A7 aminonitrodipentazolyltetraazacubane; A8 aminotripentazolyltetraazacubane; A9 nitrotripentazolyltetraazacubane.Then, their structures and performance were simulated and predicted theoretically by employing different methods including the density functional theory (DFT) and electrostatic potential (ESP).
2. Computational methods
First of all, the designed isodesmic reactions 1-9 were used to predict the HOF in gas phase of nine new compounds A1-A9 at 298 K,respectively.Based on this,the HOF of solid phase(equation(10), ΔHf,solid) at 298 K was predicted by the Hess’s law, in which heat of sublimation (ΔHsub) [18-21] was calculated by Eq. (11).
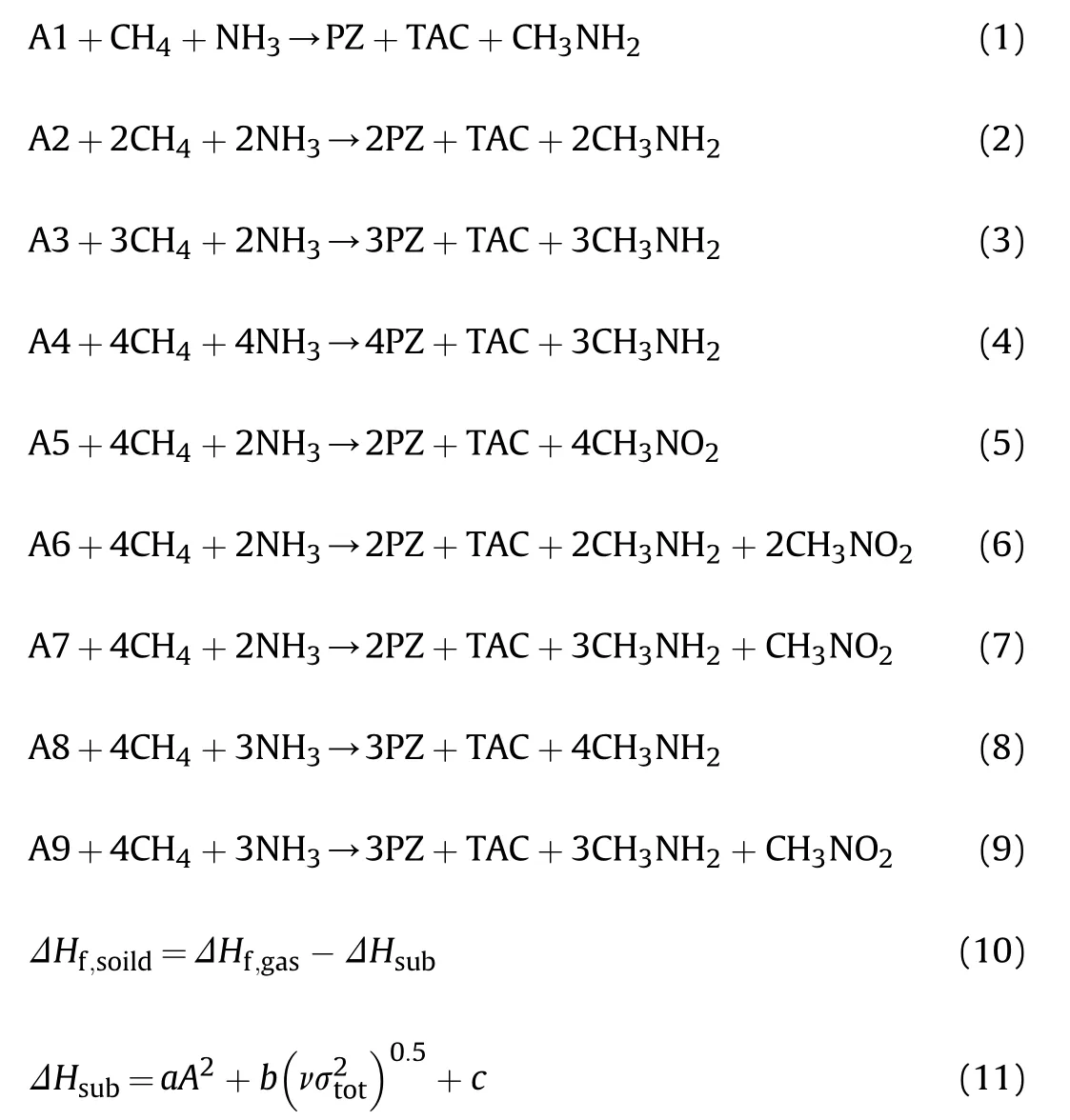
As for the two energy parameters detonation velocity (D) and detonation pressure(P), which were calculated by the famous and common Kamlet-Jacobs equations [22]:

Among them,the Density(ρ)in solid phase(Eq.(14))and impact sensitivity(ΔV,Eq.(15))were calculated by using the ESP methods[23-26]proposed by Politzer group at the same level they used in the corresponding literature:
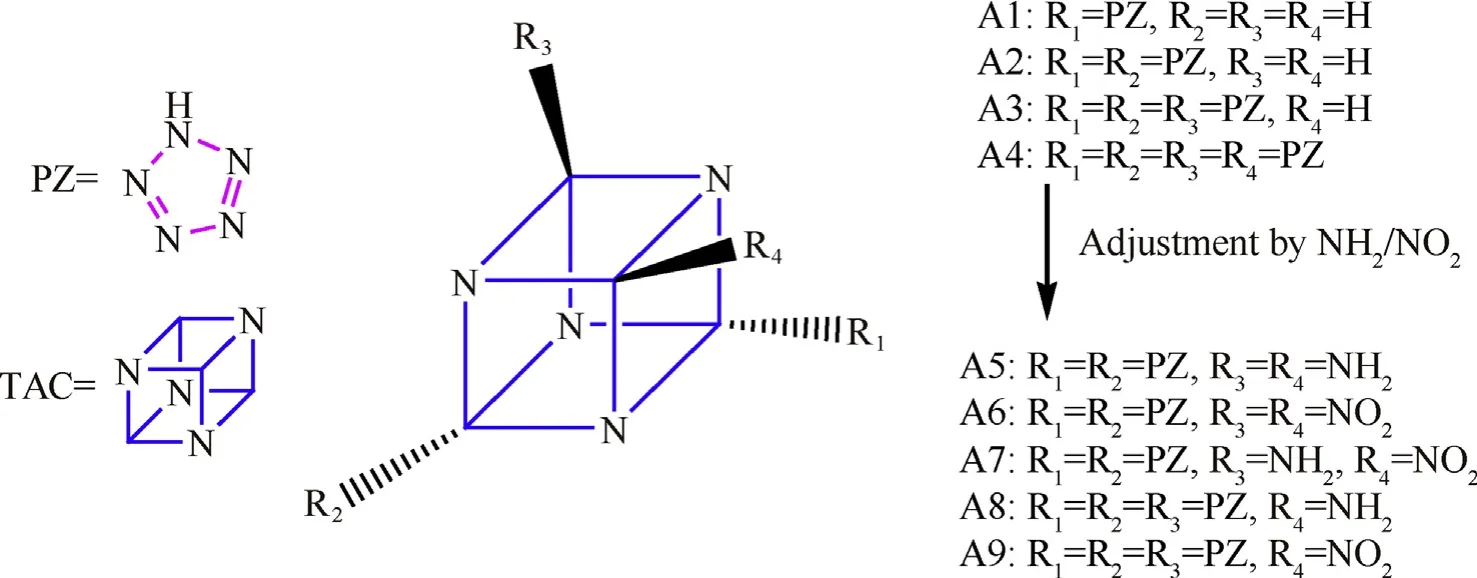
Fig.1. Molecular structures of designed pentazolyltetraazacubanes.
The structure calculations in this work were performed on the Gaussian program [27] at the B3LYP/6-31 + G(d,p) level. The detailed calculation parameters and data can be seen in Tables S1-S3.
3. Results and discussion
3.1. Molecular and electronic structures
Fig. 2 displays the optimized structures of designed molecules.As seen that both the ring of pentazole and the cage skeleton of tetraazacubane are maintained still, indicating that the combination of them did not destroy the original main structure. All nine molecules were predicted to have the C1 point group. The dipole moment of designed molecules decreases with the order A1 to A4(4.92-0.01 Debye), the NH2group could increase the dipole moment obviously(A5:8.15 Debye,A8:5.98 Debye)while the NO2group has the opposite effect (A6: 0.63 Debye, A9: 0.43 Debye).
More structure information can be seen in Table 1, which lists the predicted bond lengths of the pentazole, tetraazacubane, and designed molecules. First, it is seen that the difference of bond lengths of five N-N bonds of PZ in four designed compounds is bigger than that in parent PZ,and the more the PZ in the structures,the more obvious the difference is, showing that the conjugation effect in PZ weakens with the combination of PZ and TAC,and this weakening phenomenon is more remarkable with the increasing of the number of PZ.Similarly,comparing to PZ,then the introduction of NH2groups have few effects on the PZ, but NO2groups can weaken the conjugation effect in PZ. Since PZ is much weakrt and sensitive than TAC, and the conjugation effect decreases with the sequence of A1, A2, A3, and A4, thus, the sensitivity of designed compounds may also increase with the same order.The NO2groups will make the system being more sensitive. The Similar phenomenon could be seen from the changing tendency of the bond length of C-N bonds in TAC, indicating that the TAC cage skeleton also becomes weaker after the incorporation of PZ and TAC, and the introduction of NH2and NO2groups.Finally,the bond length of the CTAC-NPZbond (the bond linked with the TAC and PZ) decreases gradually with the order of A1 to A4,suggesting that the interaction between TAC and PZ increases with the same sequence,while this interaction could weaken both the PZ ring and TAC skeleton that further increase the sensitivity. Introducing NO2groups into the system leads to the shorter CTAC-NPZbond lengths,which may also add the sensitivity, but this is just the opposite for NH2groups.
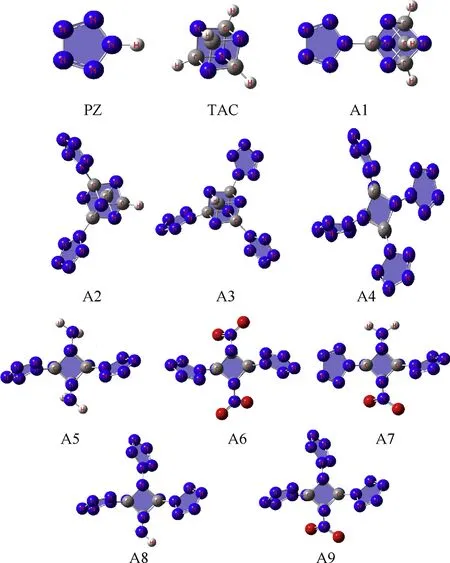
Fig. 2. Optimized structures of pentazole, tetraazacubane and designed pentazolyltetraazacubanes.
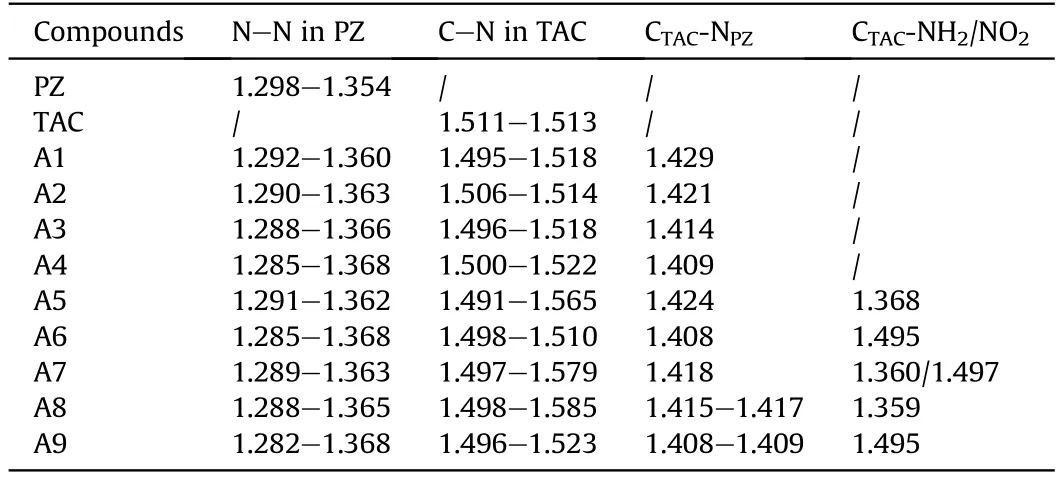
Table 1 The predicted bond lengths (Å) of the pentazole, tetraazacubane and designed pentazolyltetraazacubanes.
Fig. 3 displays the energies of the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) and energy gaps (ΔELUMO-HOMO) of designed molecules. First, for A1 to A4, Both the HOMO and LUMO energies decrease with the increasing number of PZ,indicating that the introduction of PZ into the TAC would make the electron acceptance and loss being easy and difficult, respectively. Since NH2is an electron-donor group while NO2is an electron-withdrawing group, thus, comparing to A4, the introduction of NH2or NO2groups into the structure both increase the HOMO and LUMO energies,while NO2groups have the opposite influence. In all, as a result,ΔELUMO-HOMOincreases gradually from A1 to A4,showing that the electron transfer is more and more difficult. While whatever introduces NH2or NO2into the structure, ΔELUMO-HOMOdecreases, thus making the electron transfer is easier.
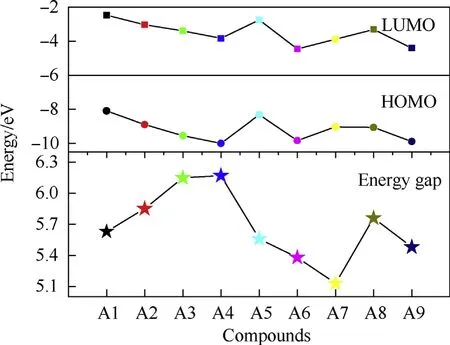
Fig. 3. Energies of HOMO, LUMO and energy gaps (ΔELUMO-HOMO) of designed pentazolyltetraazacubanes.
3.2. Heat of formation and density
Generally, a high heat of formation (HOF) or density value will set up a good basis for energetic compounds to obtain good detonation properties. For instance, the two potent organic energetic compounds CL-20 and ONC both have very high HOFs (≈600 kJ/mol) and densities (≈2.0 g/cm) [28-31]. First of all, the HOF and density of RDX are calculated to be 86 kJ/mol and 1.81 g/cm,respectively, which are close to its experiment values (relative deviations<3%) [32], suggesting the computing reliability of used methods.Then, the calculated HOF and density values of designed compounds are listed in Table 2.While Fig.4 displays a comparison of solid-phase HOFs and density of designed compounds,from this Figure, first, it can be seen that, based on the extremely high Ncontent(ranged from 71.2%to 87.5%,as listed in Table 2),high HOFs of parent PZ and TAC, all four designed pentazolyltetraazacubanes(A1-A4) also possess extremely high HOFs (1226-2734 kJ/mol),which are about 2-5 times more than ONC and CL-20 and even higher than the high-nitrogen compound 3,6-diazido-1,2,4,5-tetrazine (1101 kJ/mol, N%=74.5%) [33]. It can be found that introduce one PZ group into the system could increase the HOF by about 500 kJ/mol, and this increment effect would not weaken whatever introduces one or four PZ into the system.A5,A6 and A7 have high HOF values than A2, showing that both NH2and NO2groups can increase HOF. But this positive effect of NH2and NO2groups is weaker than that of PZ, since A5-A9 all have lower HOF than A4 to varying degrees. Despite this, the HOF values of A5-A9 are still maintain on the extremely high level (>1622 kJ/mol).
Then,the density increases with the order of A1 to A4,indicating that the introduction of PZ into the system could add the density also, but this increasing effect becomes weaker when drawing more PZ into the structure gradually.As a result,A3 and A4 have a higher density than a famous high energy compound in use RDX slightly. A5-A9 have a higher density than A2, showing that both NH2and NO2groups can improve density. Especially for the NO2group, whose related two compounds A6 and A9 possess better density than A4,suggesting that the positive effect of NO2group on the density is more evident than that of PZ. However, it should be notable that the density of all nine designed cage compounds are lower than that of CL-20 or ONC,which may due to their unfolded molecular structure(as seen in Figs.1 and 2)and big molar volume(102-312 cm3·mol-1). In all, the designed pentazolyltetraazacubanes have outstanding HOF values and good density property, thus, it may be expected that they would have good energetic properties. Moreover, the introduction of NH2and NO2groups into the system both have great and different effects on the HOF and density.
3.3. Energetic properties and sensitivity
Detonation velocity(D)and detonation pressure(P)are two key and common energy parameters often employed to survey the energetic properties of HEDCs, and also calculated in the present work, as listed in Table 2.
The D and P of RDX are calculated to be 8.7 km/s and 34.5 GPa,respectively, which are close to the experiment values (relative deviations<3%)[28],showing the accuracy of used methods in this work. Then, a comparison of D and P of designed pentazolyltetraazacubanes with RDX and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX) is displayed in Fig. 5. First, it can be seen that, because the introduction of PZ into TAC could enhance the HOF and density monotonously.Thus,both D and P increase gradually from A1 to A4,showing that PZ is helpful for improving energetic properties.Furthermore, similar to that of density, this positive effect is weakened gradually when introducing more and more PZ into the structure.Then,A3 and A4 both have higher D and P than RDX,A2 also has close D and P to RDX, while A1 has comparative D and P with another famous energetic compound triaminotrinitrobenzene, these show the good energetic properties of designed pentazolyltetraazacubanes. But due to low oxygen balance (OB,ranged from -85.9% to -12.1%) that decrease the heat of detonation, and the relatively mediocre density, all designed pentazolyltetraazacubanes have lower energy than HMX. Fortunately,the energy is effectively positive adjusted by replacing PZ by an appropriate amount of NH2or NO2groups.Since both NH2and NO2groups can improve the HOF and density, especially for the NO2group,which can increase the density and OB efficiently,A6,A7,A8 and A9 all have higher D and P obviously than A4,in which three of them(A6,A7,A9)have better energy than HMX.The D values of A6,A7, and A9 are even close to that of ONC (9.6 km/s).
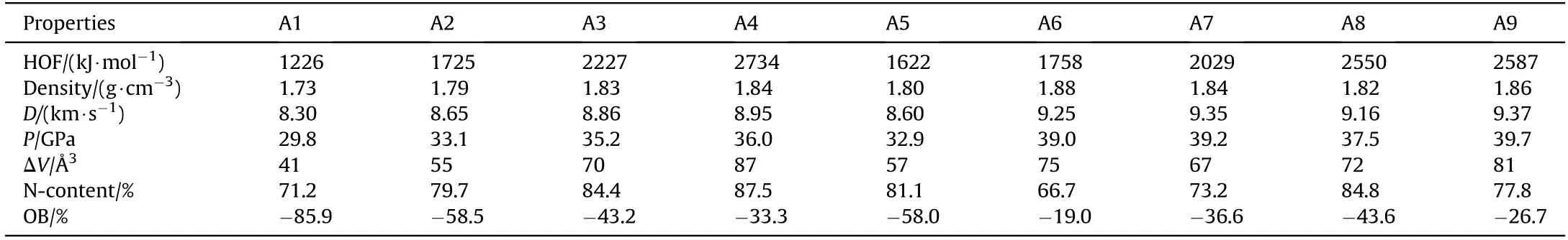
Table 2 The calculated values of solid-phase HOF,density, D, P,ΔV, N-content and OB.
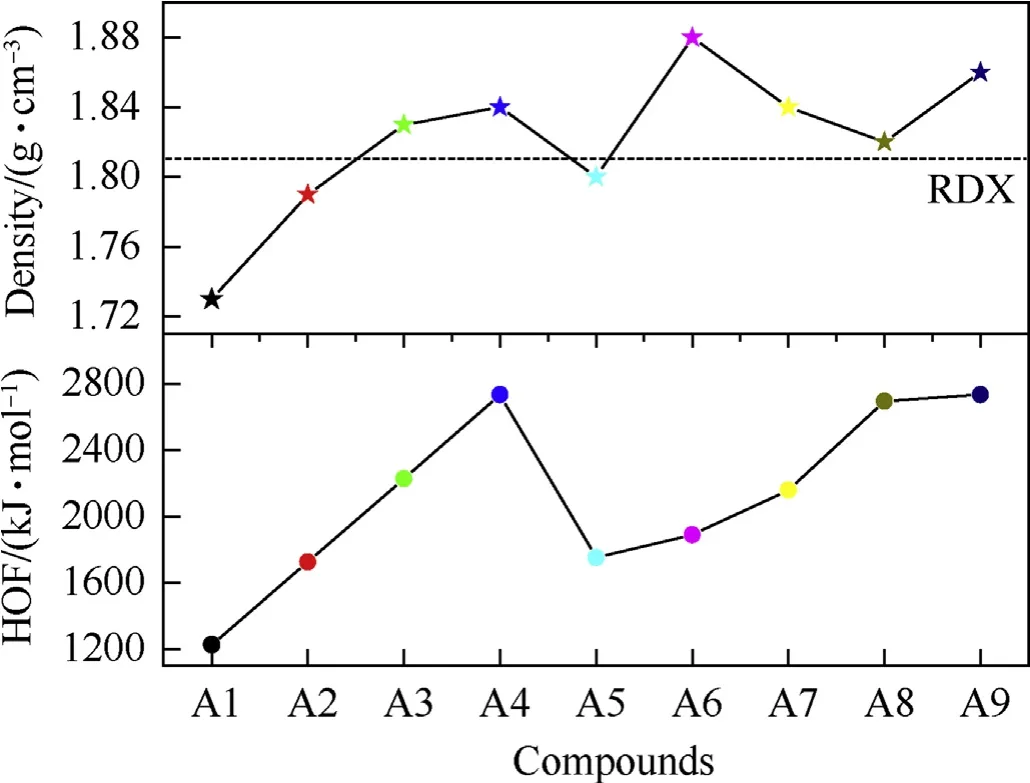
Fig. 4. A comparison of solid-phase HOFs and density of deigned pentazolytetraazacubanes.
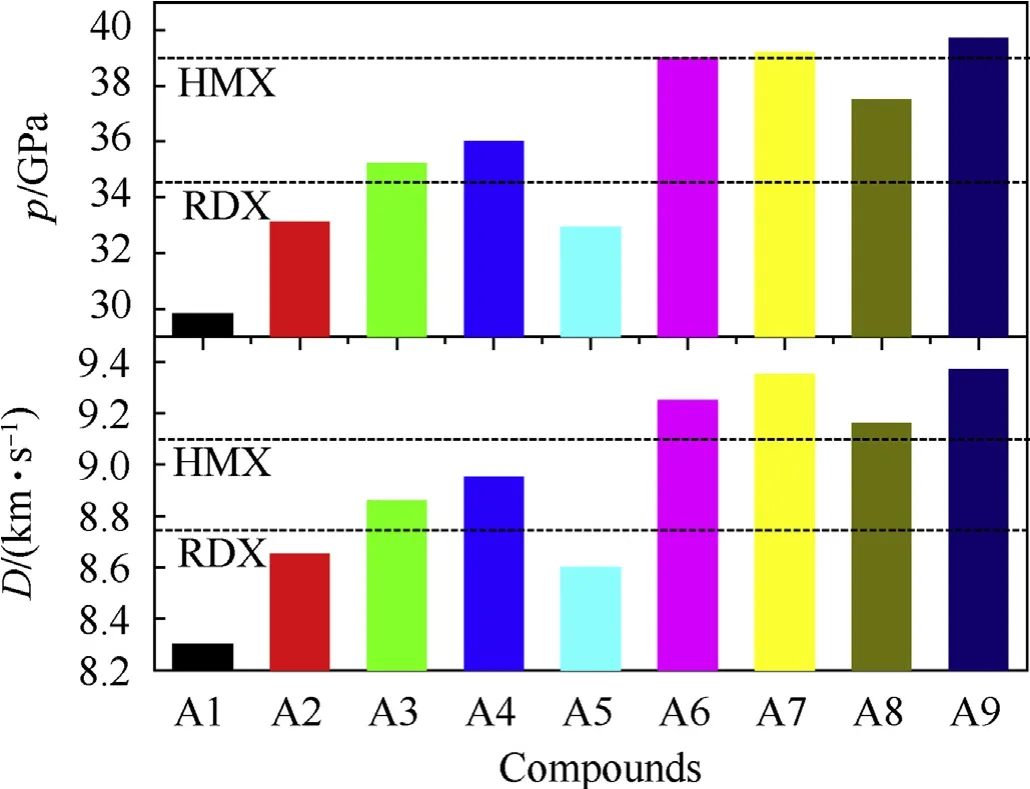
Fig. 5. A comparison of D and P of designed pentazolyltetraazacubanes with RDX.
3.4. Sensitivity
An acceptable sensitivity value is also necessary for a new high energy compound to be further investigated for practical application.In this section,a common value the free space per molecule in the unit cell (ΔV) [18,23,24] that has been used to compare the sensitivity of energetic compounds in many previous studies, and was predicted and used in our study also.The calculated ΔV values of designed compounds are displayed and compared in Fig. 6, it is seen that the ΔV value increases distinctly from A1 to A4, and this phenomenon becomes more obvious with the increment of the number of PZ,showing that introduce PZ into the structure increase the sensitivity and this effect is intensified with more PZ, since it was reported [18,23,24] that the sensitivity increases with the increment of ΔV value in general. The increased sensitivity is may due to the weakening phenomena of the cage skeleton of TAC and ring of PZ caused by their strong interactions, as discussed in the above section.The ΔV values of A3(70 Å3)and A4(87 Å3)are close to those of two cage energetic compounds ONC(74 Å3)[18]and CL-20 (87 Å3) [18], indicating that they have comparative sensitivity,respectively.But it should be notable that CL-20 is a very sensitive energetic compound and its sensitivity (h50=14 cm) [34] is obviously higher than RDX (h50=26 cm) [35] and HMX(h50=28 cm)[35],thus A4 is also very sensitive.Using NH2or NO2groups to replace the PZ in A4 decreases the ΔV obviously,as seen that A5-A9 all have lower ΔV values than A4 and CL-20.A5,A7 and A8 even have lower ΔV values than ONC,showing that they are less sensitive than ONC.The ΔV value of A6 is slightly higher than ONC.Furthermore,according to the Ref.[20],based on ΔV values,the h50values of A3,A6,A7 and A8 are predicted in the neighborhood of 45,40, 50 and 45 cm. Which are higher than that of RDX and HMX,showing that they are more insensitive than RDX and HMX. This above shows that the sensitivity performance has been effectively adjusted to the acceptable level by displacing PZ by different numbers of NH2or NO2groups properly, just similar to the adjustment situation of other properties. Besides, we also calculated the GIPF balance parameters (Table S3) [36] of designed compounds and CL-20, and found that the values (from 0.080 to 0.216) of designed compounds are higher than that of CL-20(0.072), indicating that these compounds are less sensitive than CL-20.
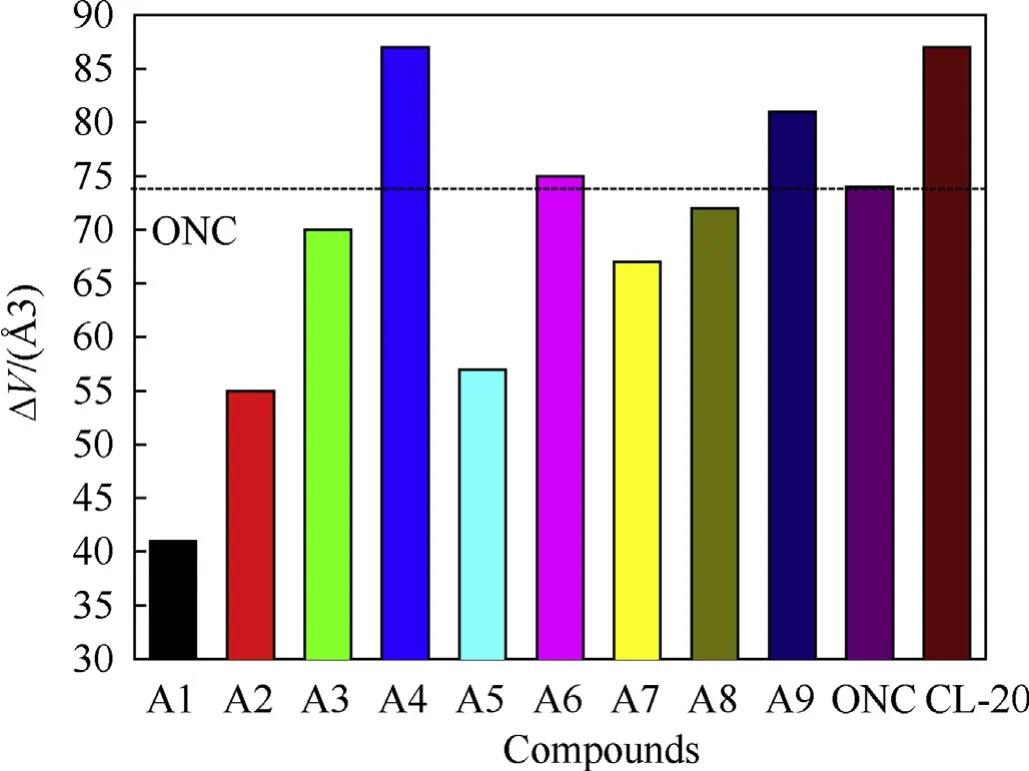
Fig. 6. A comparison of ΔV of designed pentazolyltetraazacubanes.
In all,A3 and A8 have higher energy and lower sensitivity than RDX, while A6 and A7 have higher energy and lower sensitivity than HMX, showing that these four new compounds have good combination performance, which further indicates the great potential of pentazolyltetraazacubanes used as HEDCs.Thus,four new pentazolyltetraazacubanes were successfully designed and obtained by combining PZ with TAC and the further property adjustment method of introducing a suitable amount of NH2or NO2groups into the system. Still, it should note that the energy performance of designed pentazolyltetraazacubanes is worse than ONC and CL-20 to some degree,more reasonable strategies will be needed and used to design new pentazolyltetraazacubanes derivatives with higher energy in the future studies.Besides,referring to the reported studies of pentazoles and cage energetic compounds the designed pentazolyltetraazacubanes may be synthesized under low temperature or high pressure.
3.5. UV-Vis spectrum
The ultraviolet-visible(UV-Vis)Spectrum in dimethylsulfoxide solution of designed molecules were predicted and depicted in Fig.7.First of all,in Fig.7.a,it is seen that for A1,two strong peaks occur at 215 and 278 nm,which are corresponds to the π→π*and n→π* transition, respectively. While for A2, A3 and A4, the strongest peak happens at 252, 242 and 239 nm, respectively,corresponding to n→π*transition all.This blue shift phenomenon is may be caused by the increasing steric hindrance with more PZ.While in Fig. 7.b, the strongest absorption peaks are at 257, 264,265, 248 and 249 nm. Comparing with A5 and A2, A8 and A3, it is found that the introduction of the NH2group into the TAC leads to the red shift and slightly weaker absorption strength. This hypochromicity effect is much more obvious for the NO2group,as seen that the absorption strength of A6 and A9 is clearly weaker than that of A5 and A8,respectively.No absorption peaks were observed in the visible light region for all nine designed molecules.
4. Conclusions
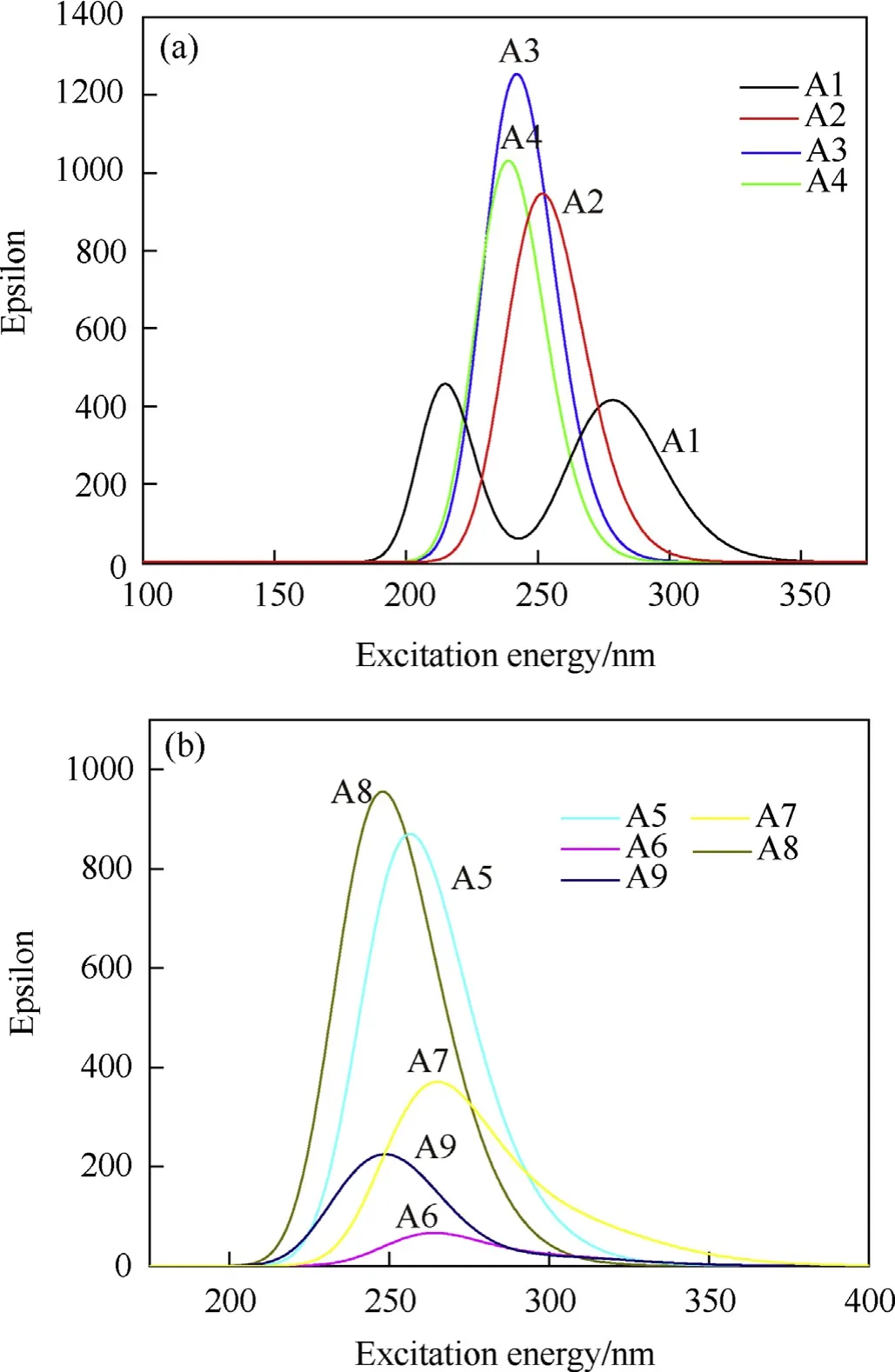
Fig. 7. The calculated UV spectrum of designed pentazolyltetraazacubanes.
In this work, we designed four new energetic pentazolyltetraazacubanes by combining two attractive high-energy parent compounds PZ with TAC together.Further property adjustment was performed by introducing a proper amount of NH2or NO2groups into the system and yielded another five new compounds. The results shown that, first of all, the structures of TAC and PZ could be influenced by each other, and the interaction between them strengthens with the increasing number of PZ, while this interaction would weaken both the PZ ring and TAC skeleton that further increase the sensitivity.Secondly,the designed compounds have extremely high HOF and good density, introducing one PZ group into the system would increase the HOF by about 500 kJ·mol-1, and this increment effect would not weaken whatever introduce one or four PZ into the system.The introduction of PZ into the system could add the density also, but different from that of HOF,this increasing effect is weakened when drawing more PZ into the structure gradually.Both NH2or NO2groups are helpful for increasing the HOF and density, especially for the NO2group,which can adjust and improve the density effectively.Then,A3 and A4 have higher D and P than RDX, but they are less powerful than HMX. While the energy can be further obviously improved by the adjustment of NH2and NO2groups,as A6,A7,A9 has higher energy than HMX.Finally,A4 has the highest energy among A1-A4,but it is very sensitive. The sensitivity can be effectively adjusted and decreased by NH2and NO2groups also, as A5,A7 and A8 are even less sensitive than ONC.In a word,four new compounds(A3,A6,A7,and A8) with good combination performance were successfully designed and obtained by combining PZ with TAC and the further property adjustment method of introducing a suitable amount of NH2or NO2groups into the system.A3 and A8 have higher energy and lower sensitivity than RDX, while A6 and A7 have higher energy and lower sensitivity than HMX,indicating the great potential of pentazolyltetraazacubanes used as HEDCs. The present work may help develop new N5-based or cage high-energy compounds.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The present work was supported by the Natural Science Foundation of Nanjing Institute of Technology(CKJA201603),the Natural Science Foundation of Jiangsu Province(BK20170761,BK20160774),the Jiangsu Key Laboratory Opening Project of Advanced Structural Materials and Application Technology (ASMA201707), Science Innovation Project for Undergraduates of Jiangsu Province(201811276023Z), Outstanding Scientific and Technological Innovation Team in Colleges and Universities of Jiangsu Province, and Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2019.09.014.
杂志排行
Defence Technology的其它文章
- Statistical variability and fragility assessment of ballistic perforation of steel plates for 7.62 mm AP ammunition
- Texture evaluation in AZ31/AZ31 multilayer and AZ31/AA5068 laminar composite during accumulative roll bonding
- Local blast wave interaction with tire structure
- Research and development of training pistols for laser shooting simulation system
- Summed volume region selection based three-dimensional automatic target recognition for airborne LIDAR
- A novel noise reduction technique for underwater acoustic signals based on complete ensemble empirical mode decomposition with adaptive noise, minimum mean square variance criterion and least mean square adaptive filter
