Even-carbon predominance of Monomethyl branched alkanes in Humic coal from Junggar Basin,NW China
2020-06-22QingsongChengMinZhangGuanghuiHuang
Qingsong Cheng·Min Zhang·Guanghui Huang
Abstract A series of Monomethyl branched alkanes compounds were detected between nC14–nC36,in immature and low maturity Jurassic humic coal,Junggar basin.2-methyl alkanes and 3-methyl alkanes accounted for the vast majority of the compounds.It is worth noting that the 2-methyl alkanes in the humic coal samples show an obvious distribution of even carbon predominances rarely reported in the literature.The results show that with the increase of Pr/Ph (pristane/phytane),the even carbon dominance of 2-methyl alkanes is more obvious,while the odd carbon number distribution of 3-methyl alkanes is weakened.As Pr/Ph increases in the humic coal,the relative content of the hopanes increased,while the relative content of 2-methyl alkanes and 3-methyl alkanes increases first and then decreases.
Keywords Junggar basin·Humic coal·Even carbon predominance·2-methyl alkanes·3-methyl alkanes
1 Introduction
Monomethyl branched alkanes(MMAs)were first detected by Han and Calvin (1970) in blue-green algae.Then,Klomp(1986)and Fowler and Douglas(1987)detected the MMAs (source of the cyanobacteria) in the Precambrian crude oil of East Siberia and South Oman,and the distribution range of carbon number was C16–C21.In addition,Shiea et al.(1990) concluded that 2/3 cyanobacteria can generate MMAs compounds.Since then,a large number of studies have found that cyanobacteria are the source of monomethyl branched alkanes.Alongside algae (Ji et al.2009; Qian et al.2017) and higher plants (Heemann et al.1983; Kolattukudy 1969; He et al.2009; Huang et al.2011),these autotrophic organisms are the parent materials of the monomethyl branched alkanes,but heterotrophic bacterial (Tissot and Welte 1984; Connan et al.1986;Tegelaar et al.1989; Huang et al.1993,2003; Wang et al.1995a,b; Gelin et al.1999; Killops et al.2000; Sun et al.2015; Cheng et al.2019a) is also the biological parent of some monomethyl branched alkanes in some sediments and crude oil(especially high wax crude oil).In addition to direct biological contributions,monomethyl branched alkanes also have non-biological sources,such as the conversion product of the functionalized lipid precursors(such as carboxylic acids)in the diagenetic stage(Lu et al.2003; Kenig et al.1994; Kenig 2000; Thiel et al.1999;Summons 1987; Summons et al.1988),the long-term equilibrium product of isomers (Klomp 1986),the acidcatalysed (such as clay) product of the pyrolysis of olefins(Kissin 1987),and the transformation product of vulcanizing bacteria (Love et al.2008; Logan et al.1999,2001;Arouri et al.2000).
The distribution of 2-methyl alkanes and 3-methyl alkanes is the most prominent in the monomethyl branched alkanes.Some researchers have studied 2-methyl alkanes and 3-methyl alkanes in different humic coal samples.The accepted consensus is that the distribution of 2-methyl alkanes and 3-methyl alkanes is related to the terrestrial organic matter in coal or coal strata,and the abnormally high abundance of 2-methyl alkanes and 3-methyl alkanes are almost unexceptionally distributed in the coal series(Johns et al.1966; Philp 1987; Huang et al.1993; Wang et al.1995a,b; Cheng et al.2019b).The hydrocarbon generation properties of higher plant parent materials in coal strata terrestrial organic matter has been extensively studied,and the dominant position of higher plant parent material in coal-bearing strata is affirmed.At the same time,it is also considered that sedimentary organic matter in coal measures and that lower aquatic organisms in coal,especially bacteria and algae,contribute to hydrocarbon generation (Audino et al.2001; Wang et al.1997).Due to the influence of the sedimentary environment,the contribution of lower aquatic organisms in humic coal is very small.However in the Jurassic humic coal in the southern margin of the Junggar Basin,we detected a series of 2-methyl alkanes with an obvious distribution of even carbon predominances,as well as the 3-methyl alkanes with weak odd carbon dominance,and the distribution range of carbon number was C14–C36.It is worth noting that the 2-methyl alkanes in the humic coal samples show an obvious distribution of even carbon predominances rarely reported in the literature.The purpose of this paper is to study and analyze the geochemical characteristics of MMAs in humic coal organic matter and to explore the role of bacteria in the enrichment of organic matter in humic coal.
2 Sample and analytical methods
2.1 Sample
8 Jurassic humic coal samples (Fresh outcrop samples)were collected from a shallow mountain zone of the Sangong River valley.The area belongs to the Fukang fault zone in the North Tianshan foreland thrust belt,southern margin of the Junggar Basin.Fukang fault zone western end is adjacent to the Southern Fukang sag,and the eastern extent is adjacent to the Southern Jimsar sag(Fig.1).It is a favorable oil and gas accumulation structure (Yang et al.2004).The Jurassic source rocks in the study area are mainly developed in the middle and lower Jurassic(Badaowan formation-J1b,Sangonghe formation-J2s and Xishanyao formation-J2x).It is a set of swamp facies and shallow lacustrine facies coal deposits,with high organic matter abundance,and the organic matter type is mainly type III,and it has good hydrocarbon generating potential.
2.2 Methods
2.2.1 Organic petrology
Organic petrology was carried out on selected polished whole-rock samples that were prepared according to the ISO 7404-2 (2009) standard.Reflectance measurements followed the ASTM standard D7708 (2014) and the ISO 7404-5 (2009) standard.A reflected light microscope system equipped with fluorescent light sources were used for petrological observation under standard condition.Maceral analysis was performed using the point-counting method(300 points of organic matter were counted in each sample—the mineral matter was excluded) under both white and UV light.The classification of the liptinite group macerals was followed,as described in Pickel et al.(2017).
2.2.2 Organic geochemistry
Samples were crushed to 100 meshes and then put through Soxhlet extraction with copper sheets for 72 h(removal of sulfur).The asphaltene precipitated in the extract was first removed,and then washed with n-hexane,n-hexane/dichloromethane and trichloromethane/anhydrous hexanol in the alumina chromatographic column to obtain saturated hydrocarbons,aromatics,and non-hydrocarbons.Finally,the saturated hydrocarbons were analyzed by Agilent 6890 N/5995MSD gas chromatography-mass spectrometry.The GC was fitted with an HP-5 capillary column(100 m × 0.32 mm × 0.25 μm,Agilent,USA).With helium as carrier gas and pulse non-shunt injection,the flow rate was 1 mL/min.The following oven temperature program was performed:an initial temperature of 50 °C for 1 min,an increase in temperature to 100 °C at a rate of 20 °C/min,an increase in temperature to 310 °C at a rate of 3 °C/min and holding at this temperature for 15 min.The temperature of the Injector was 300 °C,the ionization energy was 70 eV,and the detection mode was SCAN/SIM.
2.3 Analysis
Method for calculating the relative content and absolute concentration of each compound:
After GC–MS analysis,the peak area of each compound and the peak area of the standard sample can be calculated respectively on the software of GC–MS Data Analysis.Then the relative content and absolute concentration of each compound are obtained by using the following formula.
Formula for calculating the relative content of compounds
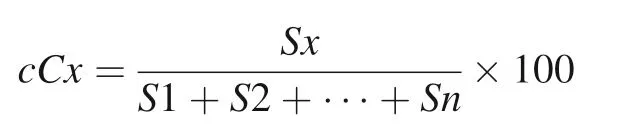
cCx:Relative content of compounds to be determined(%),Sx:Peak area of compounds to be determined,S1,S2….Sn:Peak area of each identified compound
Formula for calculating absolute concentration of compounds

Fx=fx/fs.Relative correction factor Fx is the ratio of an absolute correction factor of x component to standard sample in this paper,assuming the Fx=1,that the absolute concentration is semi-quantitative (Zhang et al.2008; Zhu 2012).
sCx:Absolute concentration of compounds to be determined (μg/mg),Sx:Peak area of compounds to be determined,Cs:Concentration of standard sample (μg/μL),Vs:Volume of standard sample (μL),Ss:Peak area of standard sample,M:Weight of experimental sample (mg).
The standard samples of saturated hydrocarbons and aromatic hydrocarbons in this study are 5α-androstane and D10-anthracene.
3 Results
The basic geochemical information of the sample is shown in Table 1.Macerals in the coal consist of vitrinite 57.46–90.00 wt.%),followed by Inertinites (0.00–37.37 wt.%) and liptinite (0.51–20.79 wt.%).

Table 1 The basic geochemical information of the samples
3.1 Identification of monomethyl branched alkanes(MMAs)
The main means to identify the MMAs (Monomethyl branched alkanes) compounds are based on the retention time on TIC,the contrast of characteristic ions,and the characteristics of mass spectra compared with the literature data.We also compared the sample mass spectrum with the mass spectrogram in the standard spectral library and assessed the similarity of the fragments.A series of compounds with a similar structure were detected between nC14–nC38in the humic coal samples of the study area.Under the condition of EI,the branched alkanes broke first at the branch point,leading to the formation of odd-mass number (even electron) and even-mass number (odd electron) ion fragments,and the abundance of the even-mass fraction was greatly improved.Even-mass ions are caused by hydrogen transfer (Mc Carthy et al.1968; Fowler and Douglas 1987; Summons 1987).

Fig.2 C22 monomethyl alkanes series mass spectrogram
The results of mass spectrometry identification,as shown in Fig.2,show that the peak of the mass spectrum of characteristic ions is higher than that of adjacent ions on mass spectra.The carbon substitution of methyl methanes from second carbon atoms to intermediate carbon atoms showed a decreasing trend.At the same time,the closer the position of the substituent was to the middle of the main chain,the more difficult it was to separate,and the closer the methyl substituent was to the intermediate carbon atom,the closer the peak time of the adjacent methyl alkanes was.This was because,with the increase of the number of carbon in the main chain and the elevation of the methyl substituent position,the physical properties of the monomethyl alkanes of the adjacent methyl substituents became increasingly similar,making them more difficult to differentiate (Krkošová et al.2007).
3.2 Distribution characteristics of monomethyl branched alkanes
Abundant methyl branched alkanes were detected in Jurassic humic coal in the southern margin of the Junggar Basin.Their typical mass chromatogram features are shown in Fig.3 below(The samples in the map are arranged according to the order of Pr/Ph from small to large).In monomethyl branched alkanes,2-methyl alkanes,and 3-methyl alkanes have an absolute predominance.The abundances of 4-methyl and 5-methyl and X-compounds are lower,the 2-methyl alkanes show obvious even carbon predominances,and the 3-methyl alkanes exhibit odd carbon predominances.When Pr/Ph is 1.77 and 2.19(Fig.3A/3B),the abundance of 2-methyl alkanes and 3-methyl alkanes is the same.The carbon number distribution range is C19–C30,and the main peak carbon is C26.The peak signal of monomethyl branched alkane compounds is weak in the mass chromatography,indicating that their compound abundance is low.When Pr/Ph is 2.71(Fig.3C),the peaks of the monomethyl alkanes are clear in the mass chromatogram,the distribution range of carbon number increases to C13–C33,the peak of 2-methyl alkanes is obviously higher than that of the 3-methyl alkanes.The 2-methyl alkanes form a normal distribution and the main peak carbon is C22;the 3-methyl alkanes appear to be the front peak type distribution and the main peak carbon is C16.When Pr/Ph is 3.15(Fig.3D),the distribution range of carbon number is still C13–C33,2-methyl alkanes show a post-peak distribution,and the main peak carbon is C28,and the abundances of the 3-methyl alkanes are further below the 2-methyl alkanes.When Pr/Ph is 3.15(Fig.3E),the carbon number of the monomethyl alkanes is further increased to C13–C36,and the 2-methyl alkanes show a post-peak distribution and the main peak carbon is C28.When Pr/Ph is 5.19(Fig.3F),the carbon number distribution range of monomethyl branched alkanes are reduced to C17–C30,and the 2-methyl alkanes are still the post-peak type distribution,and the main peak carbon is C26.As a whole,the carbon number distribution range of monomethyl branched alkanes increases first and then decreases with the increase of Pr/Ph.2-methyl alkanes show an even carbon dominant distribution,and 3-methyl alkanes exhibit odd carbon predominances.With the increase of Pr/Ph,the even carbon predominance of 2-methyl alkanes is more obvious,while the odd carbon predominance of 3-methyl alkanes is weakened.
The distribution range of the carbon number of the monomethyl branched alkanes from the cyanobacteria is C16–C21,and the abundance of 4-methyl and 8-methyl compounds is relatively high (Han and Calvin 1970;Klomp 1986;Fowler and Douglas 1987;Shiea et al.1990).The carbon number distribution of single methyl branched alkanes derived from lower algae and higher plants are C15–C35,which is close to the test results in this paper.However,as reported in the literature,the 2-methyl alkanes of monomethyl alkanes from algae and higher plant sources are generally distributed in odd carbon or without carbon dominance (Kolattukudy 1969; Heemann et al.1983;Ji et al.2009;He et al.2009;Huang et al.2011;Qian et al.2017).From Fig.3,the monomethyl alkanes in this paper have the absolute predominance of 2-methyl and 3-methyl compounds,the abundances of the 4-methyl and 8-methyl compounds are low,the carbon number is wide(the maximum C13–C36,the smallest also has C19–C30),and the 2-methyl alkanes show obvious even carbon predominances regardless of the high and low Pr/Ph.
3.3 Combination characteristics of biomarkers
Terpenes and steranes in coal measure strata can be systematically detected in a mass chromatogram.The composition and distribution characteristics of related compounds in humic coal in the study area are shown in Fig.4.
The tricyclic diterpene in humic coal in the study area were the main peaks of C19TT,C20TT,and C24TeT.And these compounds are derived from higher plants (Philp,1983).The relative content of tricyclic diterpene was 2.02–8.67%,and the absolute concentration was 2.18–22.41 μg/mg.The relative content of the sterane series was only 4.20–12.82%,and the absolute concentration was 5.92–46.24 μg/mg.C29αααR sterane was the main peak,and C27αααR–C28αααR–C29αααR regular sterane was distributed in the anti ‘‘L’’ type.The C27rearrangement sterane and C29rearrangement steranes were detected in the samples.The content of pregnane decreased with an increase of Pr/Ph.Among these compounds,C29sterane ββ/(ββ+αα) and C29sterane 20S/(20S+20R) are the maturity parameters suitable for Ro:0.5–1.0%.The ratio of C29sterane 20S/(20S+20R) of the humic coal was between 0.17 and 0.46,and the ratio of C29sterane ββ/(ββ+αα) was 0.21–0.37 (Table 2).Referring to the classification standard of Huang et al.(1991),we evaluated the maturity of humic coal in the study area by using C29sterane ββ/(ββ+αα) and C29sterane 20S/(20S+20R)parameters.The evaluation results of C29sterane ββ/(ββ+αα) and C29sterane 20S/(20S+20R) were consistent with those of Ro.The source rocks were in the immature to low maturation stage.
The content of the hopane in humic coal samples accounted for a majority of the sample,the relative content was 38.65–90.29%,and the absolute concentration was 41.22–529.46 μg/mg.A normal distribution of hopane and C27–C35series distribution integrity were found with C30hopane or C29hopane for peak and gradually decreasing C31–C35hopane content,respectively.The contents of diahopane and gammacerane were very low.The high abundance of C31hopane indicates a sedimentary environment of strong oxidation resistance (Peters and Moldowan 1991; Hou et al.1994).In addition,there was benzohopane from bacteria (Luo et al.1986; Sun et al.2015),benzohopane accounted for 0.26–2.06 μg/mg in the aromatics and the relative content was 0.14–4.60%.Overall,sesquiterpene was more developed in source rocks,with a relative content of 46.43%,and,usually,sesquiterpene is thought to come from bacteria (Alexander et al.1984; Ly et al.2017).The relative abundances of diterpenes,which reflect the contribution of higher plants(Hou et al.1992),were low,as shown in Fig.4.

4 Discussion
The abnormally high abundance of MMAs is almost exclusively distributed in coal measures strata(Johns et al.1966; Philp 1987; Huang et al.1993; Wang et al.1995a;Audino et al.2001).In the Jurassic coal measures of the Turpan-Hami basin,Wang et al.(1997)detected a series of long-chain high substitution dimethyl branched alkanes with even carbon dominance,which was believed to be derived from bacteria and algae and preserved in a partial reduction environment of the coal marshes.The distribution of 2-methyl alkanes and 3-methyl alkanes in the source rocks of the Shahejie Formation in the Banqiao sag of Dagang Oilfield(East China) has been found to be unique,that is,there is a strong even carbon predominance in 2-methyl alkanes,and there is a weak odd carbon predominance in the 3-methyl alkanes(Wang et al.1995b).At the same time,under microscopy,the activity of bacteria in the source rocks with higher plants as the main source is stronger,and we observed that the sporophyte is obviously degraded by bacteria.Sun et al.(2015)detected a series of 2-methyl alkanes with weak even carbon predominances in the carbonaceous mudstones of the Oligocene Shahejie Formation in the eastern sag of Liaohe basin (NE China).In addition,the C32–C34benzohopane presence in TIC high carbon number area is believed that have come from bacteria.It is presumed that the even carbon number 2-methyl alkanes should be derived from the transformation of higher plant bacterial metabolites.Table 2 shows that the biomarkers of bacterial origin (sesquiterpenes,hopane)account for 68.31–90.71% of the samples.In addition,previous studies have shown that bacterial wax is the biological parent material of monomethyl branched alkanes in some sediments and crude oil (especially in high waxy crude oil).Therefore,bacteria are an important source of hydrocarbon generation in humic coal.
Hopane is generally considered to be from the bacterial membrane.The relative content of hopanes increases With the increasing of Pr/Ph (Fig.5).That is,with the enhancement of environmental oxidation,the activity of bacteria is enhanced.With the increase of Pr/Ph,the relative content of 2-methyl alkanes and 3-methyl alkanes,first increase and then decrease (Fig.6).This suggests the source of MMAs may be bacterial metabolites.At first,with the increase of oxidability,the input of bacterial metabolites increased.When Pr/Ph is greater than 3.5,with the further enhancement of oxidability,the metabolites of bacteria are oxidized and decomposed,and their relative content decreases.

Fig.5 Correlation between Hopanes,Tricyclic diterpenes and Pr/Ph in humic coal of the study area

Fig.6 Correlation between 2-methyl alkanes,3-methyl alkanes and Pr/Ph in humic coal of the study area
5 Conclusion
A series of 2-methyl alkanes with even carbon dominance were detected in the humic coal of the study area.The relative content of hopanes,sesquiterpenes are high.The relative content of diterpenes,long-chain tricyclic terpenes,and sterane are low.
2-methyl alkanes,hopanes,and sesquiterpenes are derived from the bacterial metabolites.Bacterial activities in low-rank coals and their transformation from humic material to bacterial by-products,which increases the hydrocarbon generation capacity of coal as a source of gas.Acknowledgements This research was financially supported by the National Natural Science Foundation of China (No.41772124) and National Science and Technology Major Project (No.2016ZX05007001-002).
杂志排行
Acta Geochimica的其它文章
- In-situ nitrogen fate in the vadose zone of different soil types and its implications for groundwater quality in the Huaihe River Basin,China
- Organic geochemistry of the Lower Permian Tak Fa Formation in Phetchabun Province,Thailand:implications for itspaleoenvironment and hydrocarbon generation potential
- Mantle plume:the dynamic setting of the origin of Early Paleozoic mafic dykes in Ziyang,Shaanxi Province,Southern Qinling Block,China
- Using Sr isotopes to trace the geographic origins of Chinese mitten crabs
- Geochemical characteristics and origin of the Neoproterozoic high-K calc-alkaline granitoids in the northern part of Mandara hills,northeastern Nigeria
- A re-assessment of nickel-doping method in iron isotope analysis on rock samples using multi-collector inductively coupled plasma mass spectrometry
