Human epidermal growth factor receptor 2 positive rates in invasive lobular breast carcinoma: The Singapore experience
2020-06-19GaJingKeeRyanYingCongTanSultanaRehenaJoycelynJieXinLeeMaWaiWaiZawWeiXiangLianJoeYeongSuMingTanSweeHoLimBenitaKiatTeeTanYoonSimYapRebeccaAlexandraDentFuhYongWongGuekEngLee
Ga-Jing Kee, Ryan Ying-Cong Tan, Sultana Rehena, Joycelyn Jie-Xin Lee, Ma Wai-Wai Zaw, Wei-Xiang Lian,Joe Yeong, Su-Ming Tan, Swee-Ho Lim, Benita Kiat-Tee Tan, Yoon-Sim Yap, Rebecca Alexandra Dent,Fuh-Yong Wong, Guek-Eng Lee
Abstract
Key words: Lobular breast cancer; Invasive breast cancer; Human epidermal growth factor receptor 2 positive; Singapore; Clinicopathological characteristics; Prognostic value
INTRODUCTION
Invasive lobular carcinomas (ILC) represent about 5%-10% of breast cancer[1-3].Prevalence of overexpression of human epidermal growth factor receptor 2 (HER2) in breast cancer has been reported at 4.8%-5.1%[4,5]. The clinicopathological characteristics ofHER2positive (HER2+) invasive ductal carcinomas (IDC) are known to differ from that ofHER2negative (HER2-) IDC.HER2+ IDC is associated with estrogen receptor negativity (ER-), progesterone receptor negativity (PR-) and higher histologic grade[4,6]. A number of reports suggest that these associations are also present in ILC and thatHER2positivity may be a prognostic factor[7-13]. However, there remains a paucity of research examining the characteristics ofHER2+ as opposed toHER2- ILC,particularly in Asian populations. This study aims to investigate the prevalence and prognostic clinicopathological factors ofHER2+ ILC.
MATERIALS AND METHODS
Study design
A retrospective review of patients with ILC seen between January 1985 and July 2018 at National Cancer Centre Singapore, Singapore General Hospital, Changi General Hospital and KK Women’s and Children’s Hospital was conducted. We obtained the clinical and pathological data of ILC patients from the Joint Breast Cancer Registry,our prospective database. Clinical variables included patient demographic factors such as age at diagnosis, gender, ethnicity, disease factors such as tumour side, size,grade, stage, nodal status,ER,PRandHER2status, as well as treatment given such as chemotherapy, radiotherapy, surgery and anti-HER2therapy. The study was reviewed and approved by the SingHealth Institutional Review Board CIRB Ref:2019/2419.
Inclusion and exclusion criteria
From 1985 to 2018, 1095 patients were diagnosed with ILC. Of these, 242 patients with unknownHER2status were excluded from the study. Twelve patients with pathological stage 0 breast cancer were also excluded from the study. The remaining 864 patients were analysed (Figure 1).
Pathology assessment
Histopathological diagnoses of ILC were made by pathologists at various SingHealth medical institutions, namely Singapore General Hospital, Singapore; Changi General Hospital and KK Women’s and Children’s Hospital. Pathologic variables collected includedER,PRandHER2status. ASCO-CAP guidelines were used to define positivity cut-offs for the tumours as follows: A positiveER/PRresult was defined as the presence of at least 1% of tumour cell nuclei displaying unequivocal staining of any intensity, and forHER2, tumour positivity was defined as > 10% of tumour cells exhibiting 3+ membrane staining. AmbiguousHER2cases were tested and confirmed by fluorescencein situhybridization testing based on the ASCO-CAP guidelines[6-9]. In the Joint Breast Cancer Registry database, tumours were also classified into a molecular subtype as follows: Basal (ER-,PR- andHER2-);HER2+ (ER-,PR- andHER2+); Luminal A (ER- orPR- andHER2-); Luminal B (ER+ orPR+ andHER2+).
Statistical analysis
All demographic and clinicopathological characteristics were summarized in terms ofHER2status, asHER2+ andHER2- ILC. Categorical and continuous variables were summarized as frequency with percentage and median [interquartile range (IQR)]respectively. Differences betweenHER2+ andHER2- ILC were tested using chisquared test for categorical variables and Mann-WhitneyUtest for continuous variables.
The primary outcome overall survival (OS) was treated as time-to-event data and survival time was defined as time from date of diagnosis to date of death or date last seen. Secondary outcomes included disease-free survival (DFS) and breast cancerspecific overall survival (BCSS). DFS was treated as time-to-event data and duration of DFS was defined as duration from date of last treatment to date of relapse or date last seen or date of mortality. BCSS was treated as time-to-event data and duration of BCSS was defined as duration from date of last treatment to date last seen or date of mortality if cause of death was attributed to breast cancer. OS, DFS and BCSS were analysed forHER2+ andHER2- status using Kaplan-Meier survival analysis and were tested using log-rank test.
Univariate and multivariate Cox proportional hazard (CPH) regression analysis were used to find associations between OS and other prognostic factors in these patients with ILC. The following clinicopathological characteristics were investigated in the model: Age, ethnicity,ERstatus,PRstatus,HER2status, tumour size, stage,grade and treatment modalities such as chemotherapy, radiotherapy and surgery.Variables withP< 0.03 in the univariate CPH model were selected for multivariable model. Final multivariate CPH model was selected using stepwise, forward and backward variable selection method. Quantitative association from CPH regression model was expressed in terms hazard ratio with corresponding 95% confidence interval. Three separate CPH models were used for OS, DFS and BCSS. All statistical tests were two-sided andP< 0.05 was considered statistically significant. Analyses were performed using SAS Institute Inc 2013. SAS/ACCESS®9.4 Interface to ADABAS(SAS Institute Inc., Cary, NC, United States).
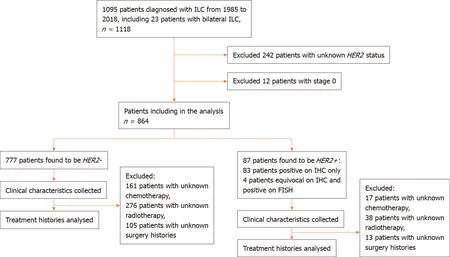
Figure 1 Consort flow diagram showing inclusion and exclusion of patients in study population. Human epidermal growth factor receptor 2 positive (HER2+)invasive lobular carcinomas was defined as an immunohistochemistry score of 3+ or an immunohistochemistry score of 2+ with a HER2 to chromosome 17 ratio ≥ 2.0 for samples after 1 January 2014 and HER2 to chromosome 17 ratio ≥ 2.2 for samples before 1 January 2014 on fluorescence in situ hybridization testing[4]. ILC:Invasive lobular carcinomas; HER2: Human epidermal growth factor receptor 2; IHC: Immunohistochemistry; FISH: Fluorescence in situ hybridization; HER2+: Human epidermal growth factor receptor 2 positive; HER2-: Human epidermal growth factor receptor 2 negative.
RESULTS
Clinical characteristics
A total of 864 patients with ILC were included in the analysis. Study population characteristics are shown in Table 1. Of note, a total of 87 (10.1%) were diagnosed withHER2+ ILC. Compared withHER2- ILC,HER2+ ILC was associated with a higher proportion ofER- (24.4%vs5.9%,P< 0.001),PR- negative (40.2%vs24%,P= 0.002)and grade 3 tumours (Grade 3, 29.0%vs10.2%,P< 0.001) (Table 1).
Treatment characteristics
Among the 87 patients withHER2+ ILC, 47 (54.0%) receivedHER2-directed therapy,12 (13.8%) did not receiveHER2-directed therapy and treatment data was not available for the remaining 28 (32.2%) patients. Of the patients who did not receiveHER2-directed therapy, reasons cited upon review of clinical charts included cardiac comorbidities, poor performance status, very early stage cancer, refusal of therapy or lack of access to therapy in the years prior to the availability ofHER2-directed therapy.
Survival outcomes
The median survival time was 2.95 (IQR: 1.89-8.87) years and 4.16 (IQR: 1.84-8.32)years respectively forHER2+ andHER2–ILC patients (P= 0.315). The 5-year and 10-year OS rates were 68.3% (59/87 patients) and 56.7% (49/87 patients) respectively inHER2+ patients and 83.4% (648/777 patients) and 72.9% (566/777 patients)respectively inHER2- patients (log-rankP= 0.004). The 5-year and 10-year BCSS and DFS rates inHER2+ andHER2- ILC patients are also shown in Figure 2.
We performed a univariate and multivariate CPH regression analysis of OS in all 864 ILC patients. Based on the multivariate analysis, significant negative prognostic factors wereHER2+, age, ethnicity and stage.HER2+ and luminal B molecular subtypes also had also notably poorer OS compared to Luminal A subtype (Table 2,Figure 3). Additional univariate and multivariate CPH regression analyses of BCSS and DFS demonstrated thatHER2positivity remained a significant negative prognostic factor for BCSS and DFS on both the univariate and multivariate analysis(Tables 3 and 4).
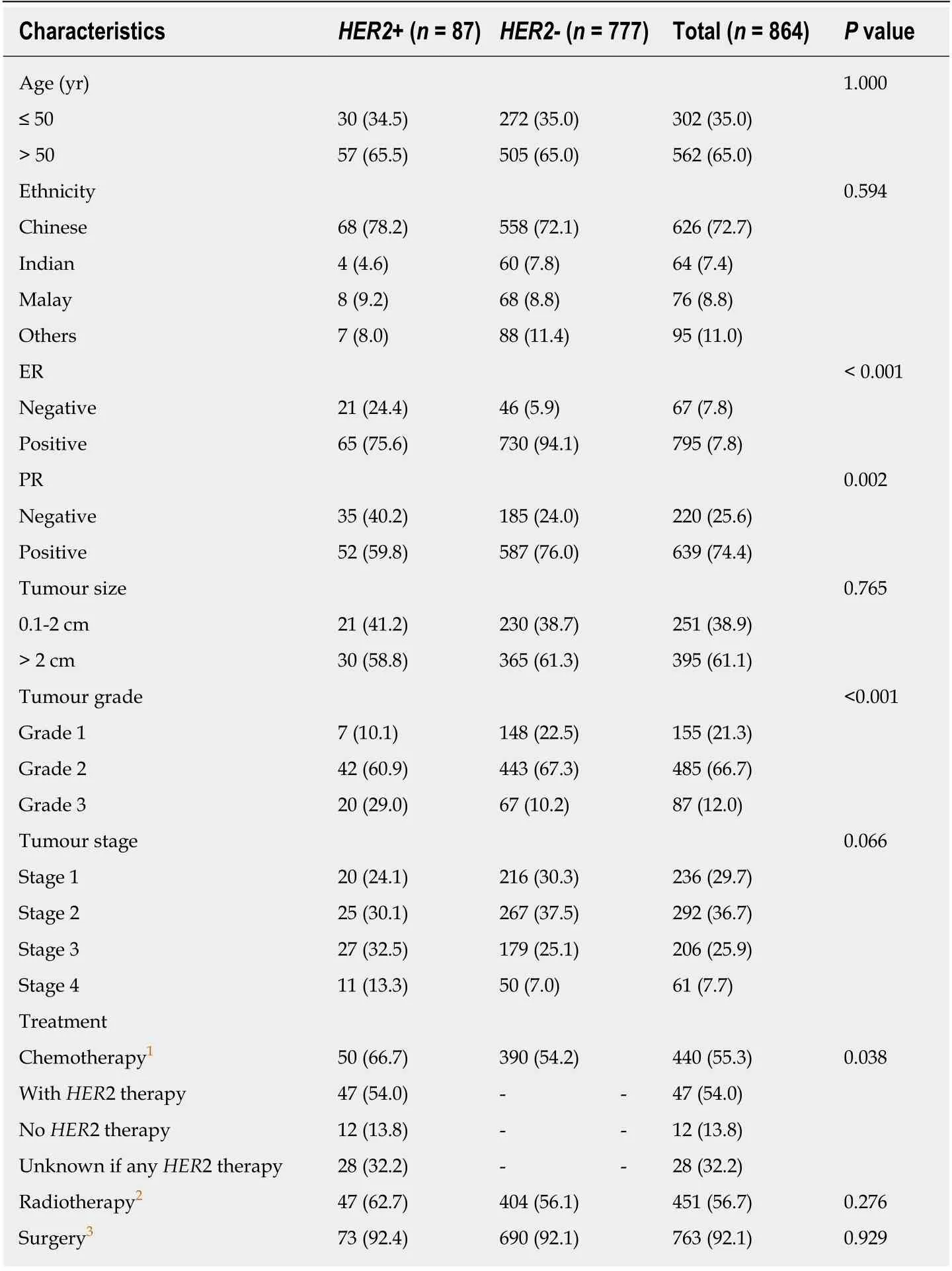
Table 1 Clinical and histopathological characteristics of human epidermal growth factor receptor 2 positive and human epidermal growth factor receptor 2 negative invasive lobular carcinomas patients, n (%)
DISCUSSION
Interestingly, although most ILC patients haveHER2- tumours, our cohort reports a higher prevalence ofHER2+ ILC (10.1%) as compared to some previous studies[1-5].The largest known study to date of 85048 ILC patients in the United States SEERS database found aHER2+ prevalence of only 4.8%[5]. Given that our study is one of the first few to describe prevalence ofHER2+ ILC in Asian populations, this may suggest differences across ethnic and geographical populations, although further studies are required to validate this finding.
In our cohort,HER2+ ILC was significantly associated withERnegativity,PRnegativity and higher tumour grade. This affirms findings in a previous study which concluded thatHER2positivity had an inverse relationship withERandPRexpression in ILC[10]. In the same study,PRnegativity was notably more common thanERnegativity inHER2+ ILC. This was also seen in our study with the frequency ofPR- being nearly twice that ofER- in theHER2+ population. Our study reports a higher tumour grade inHER2+ ILC patients. This is not consistent with findings from previous studies which did not find significant associations withHER2positivity and tumour grade or size[11-14]. We hypothesize that this may be due the smaller sample sizes in those studies and the heterogeneity ofHER2+ ILC[15,16].
Our study also demonstrates poorer survival rates inHER2+ ILC as compared toHER2- ILC for OS, BCSS and DFS. On exploratory analyses of molecular subtypes,bothHER2+ and luminal B molecular subtypes reflected this poorer OS, corroboratingwith a separate study which showed similar survival outcomes for the different molecular subtypes of ILC[17]. One possible biological explanation for poorer survival rates inHER2+ ILC is a synergistic effect ofHER2and cadherin 1 mutations which promotes tumourigenesis and early relapses inHER2+ ILC[18]. The finding of Indian ethnicity being a poorer prognostic factor for ILC on multivariate analysis also deserves further validation in a larger sample size as they formed < 5% of patients in this cohort, making it challenging to draw definitive conclusions.
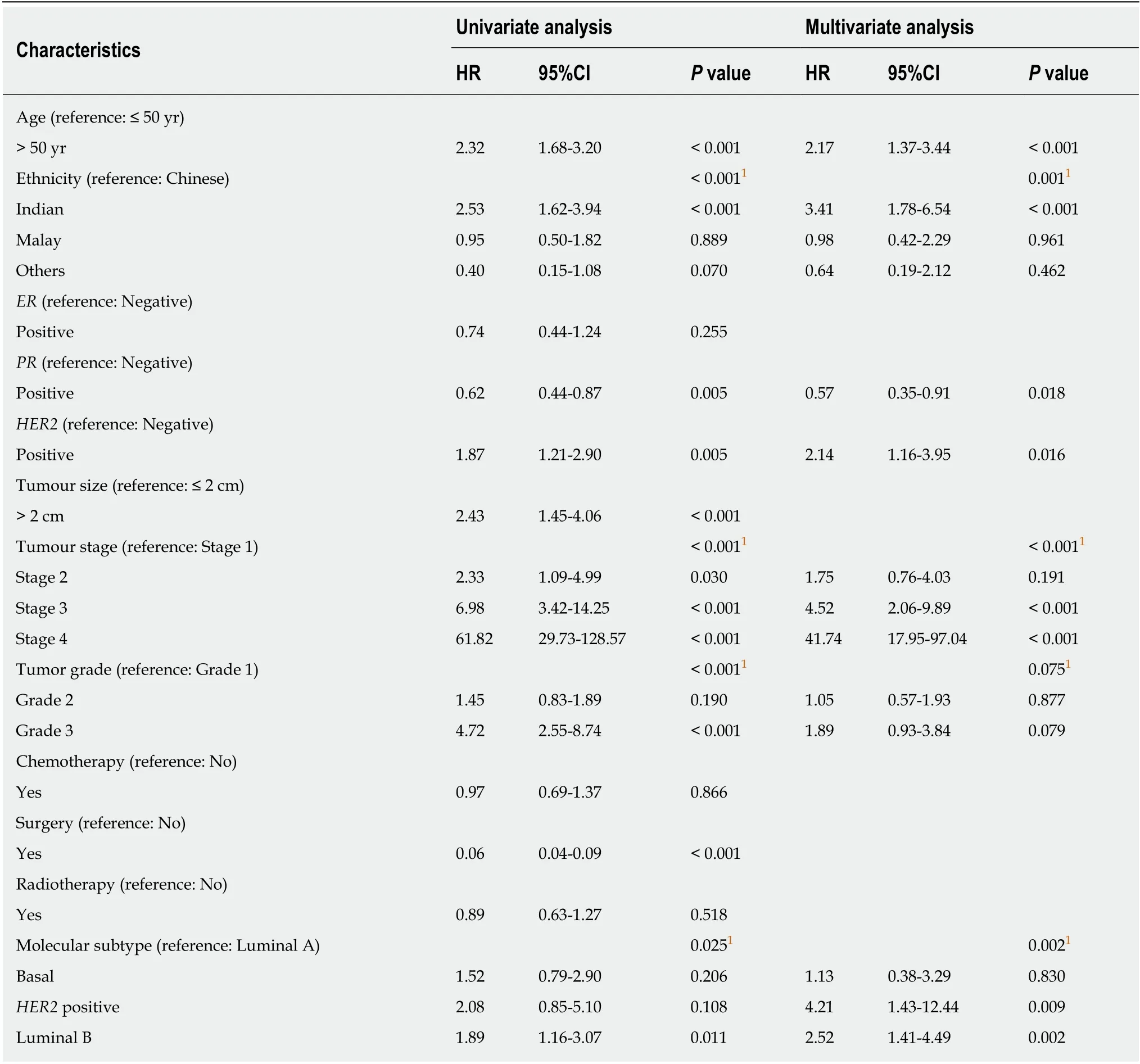
Table 2 Univariate and multivariate Cox proportional hazard regression analysis for overall survival among all 864 invasive lobular carcinomas patients
Due to the retrospective nature of this study, missing data limited our ability to perform analyses on treatments received with regards to survival outcomes.Prospective studies with larger long-term follow-up sample sizes are needed to validate our observations in this study.
In conclusion, our study demonstrates the prevalence ofHER2+ ILC to be 10.1%.HER2+ ILC patients were more likely to have poorer prognostic features such asER-,PR- and higher tumour grade. Lastly, patients withHER2+ ILC had poorer OS, BCSS and DFS compared to those withHER2- ILC. These findings warrant further prospective studies to validate observation and investigate the benefit of various treatment modalities to improve outcomes inHER2+ ILC.
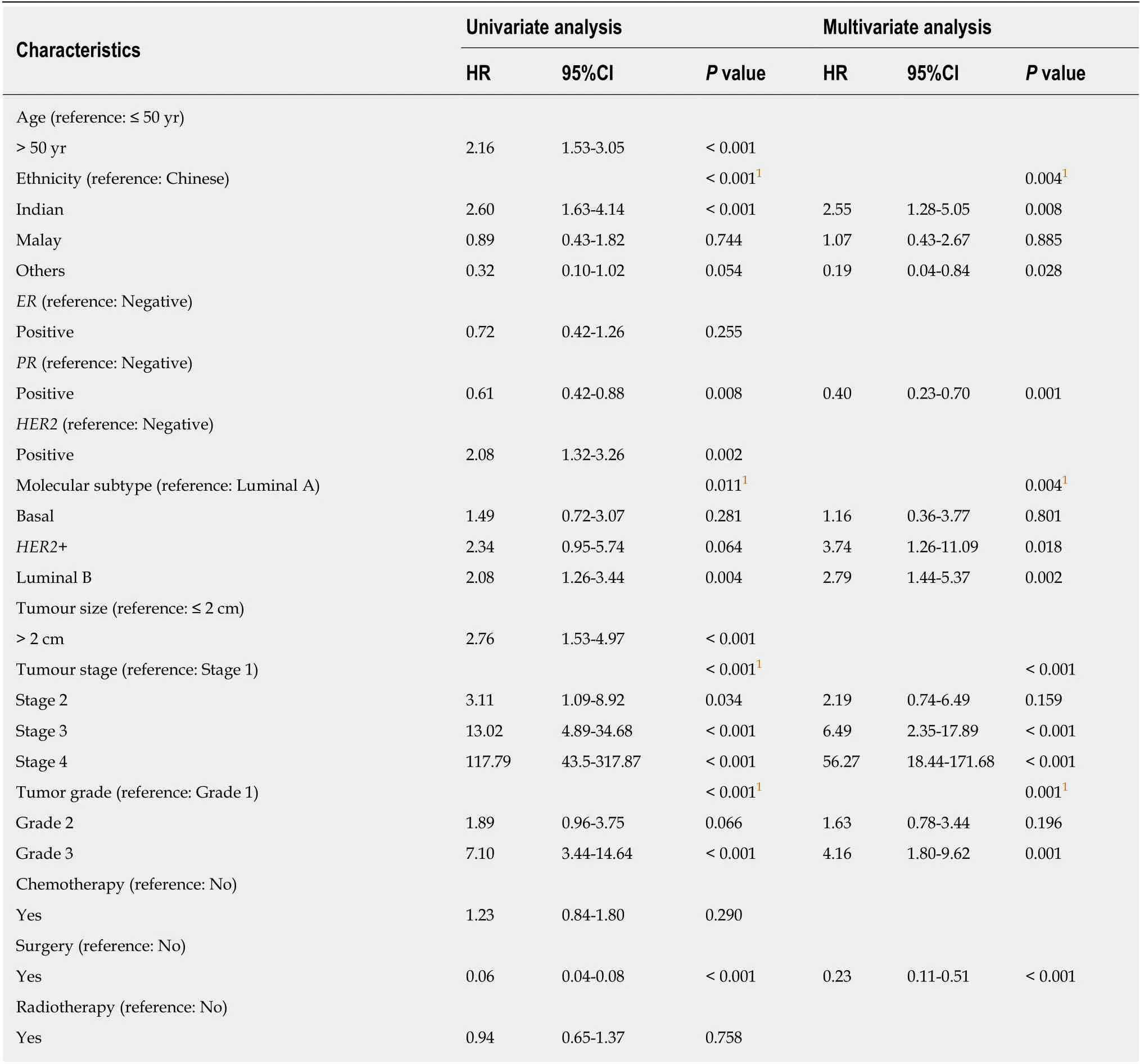
Table 3 Univariate and multivariate Cox proportional hazard regression analysis for breast cancer-specific survival among all 864 invasive lobular carcinomas patients
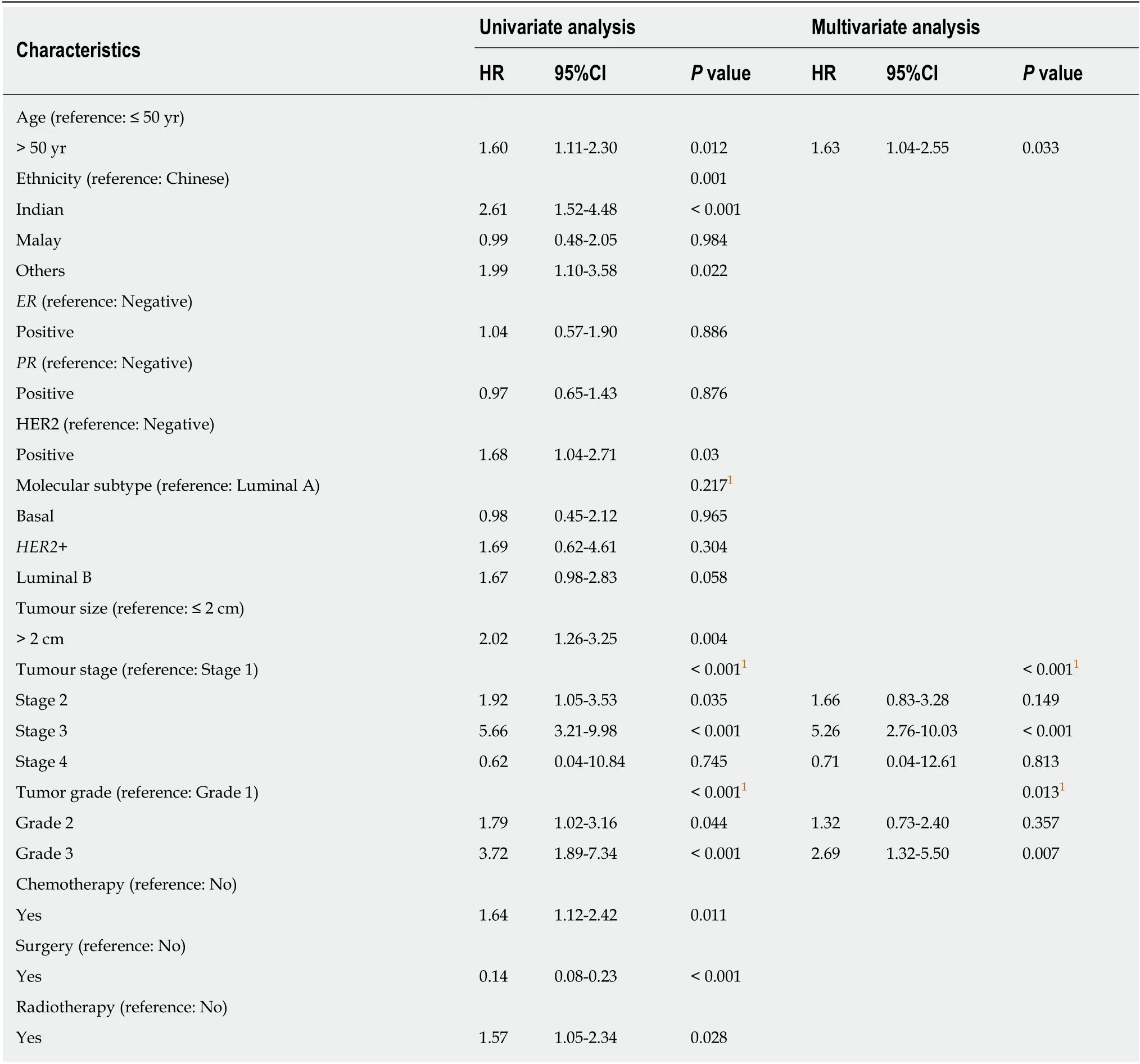
Table 4 Univariate and multivariate Cox proportional hazard regression analysis for disease-free survival among all 864 invasive lobular carcinomas patients
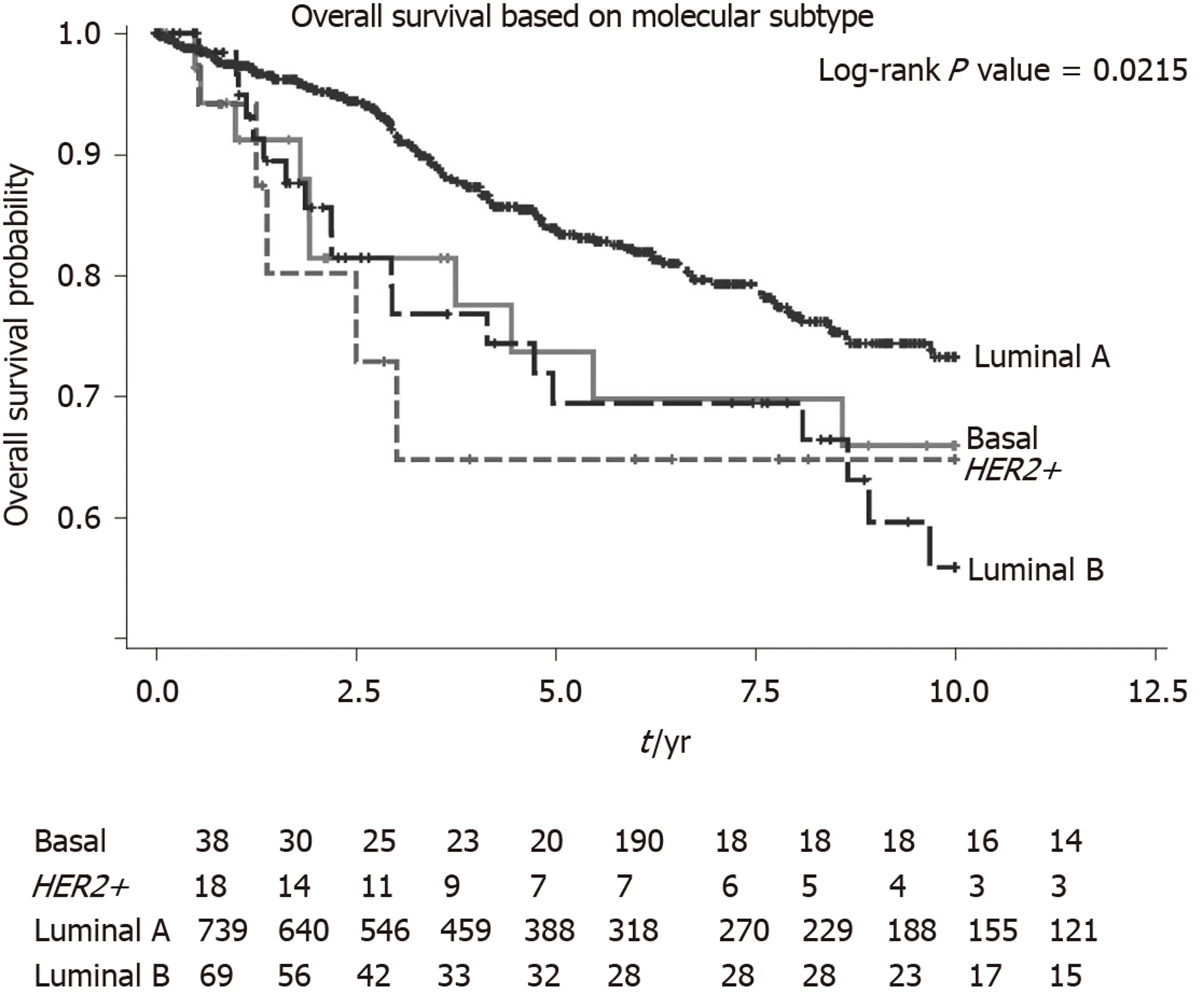
Figure 3 Overall survival of all Invasive lobular carcinomas patients by molecular subtype. Basal: Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) negative; HER2+: ER, PR negative and HER2 positive; Luminal A: ER or PR positive and HER2 negative; Luminal B: ER or PR positive and HER2 positive. HER2+: Human epidermal growth factor receptor 2 positive.
ARTICLE HIGHLIGHTS
Research background
Invasive lobular carcinomas (ILC) represent about 5%-10% of breast cancer. Prevalence of overexpression of human epidermal growth factor receptor 2 (HER2) in breast cancer has been reported at 4.8%-5.1%. The clinicopathological characteristics ofHER2positive (HER2+) invasive ductal carcinomas are known to differ from that ofHER2negative (HER2-) invasive ductal carcinomas. However, there remains a paucity of research examining the characteristics ofHER2+ as opposed toHER2- ILC, particularly in Asian populations.
Research motivation
This study compares the clinicopathological characteristics ofHER2+ andHER2- ILC to assess the differences in survival probability between the two groups.
Research objectives
This study aims to investigate the prevalence and prognostic clinicopathological factors ofHER2+ ILC in an Asian population.
Research methods
A retrospective review of patients with ILC seen between January 1985 and March 2018 at various SingHealth medical institutions was conducted. Demographic and clinical data were collected from medical records. We examined clinicopathological characteristics and survival in relation toHER2status. Differences betweenHER2+ andHER2- ILC were tested using chisquared test for categorical variables and Mann-WhitneyUtest for continuous variables. Overall survival (OS), disease-free survival (DFS) and breast cancer-specific overall survival (BCSS) were analyzed forHER2+ andHER2- status using Kaplan-Meier survival analysis and were tested using log-rank test. All statistical tests were two-sided andP< 0.05 was considered statistically significant.
Research results
Interestingly, although most ILC patients haveHER2- tumours, our cohort reports a higher prevalence ofHER2+ ILC (10.1%) as compared to some previous studies. The median survival time was 2.95 (interquartile range: 1.89-8.87) years and 4.16 (interquartile range: 1.84-8.32) years respectively forHER2+ andHER2- ILC patients (P= 0.315). Based on the multivariate analysis,significant negative prognostic factors wereHER2+, age, ethnicity and Stage.HER2+ and Luminal B molecular subtypes also had also notably poorer OS compared to Luminal A subtype.Additional univariate and multivariate Cox proportional hazard regression analyses of BCSS and DFS demonstrated thatHER2positivity remained a significant negative prognostic factor for BCSS and DFS on both the univariate and multivariate analysis.
Research conclusions
In conclusion, our study demonstrates the prevalence ofHER2+ ILC to be 10.1%.HER2+ ILC patients were more likely to have poorer prognostic features such as estrogen receptor negativity, progesterone receptor negativity and higher tumour grade. Lastly, patients withHER2+ ILC had poorer OS, BCSS and DFS compared to those withHER2- ILC.
Research perspectives
The findings from our study warrant further prospective studies to validate observation and investigate the benefit of various treatment modalities to improve outcomes inHER2+ ILC.
