Role of regenerating islet-derived proteins in inflammatory bowel disease
2020-06-17JodiAnnEdwardsNicholasTanNadlieToussaintPeiqiOuCathyMuellerAlbertStanekVladimirZinsouSeanRoudnitskyMichelleSagalLisaDresnerAlexanderSchwartzmanChongminHuan
Jodi-Ann Edwards, Nicholas Tan, Nadlie Toussaint, Peiqi Ou, Cathy Mueller, Albert Stanek, Vladimir Zinsou,Sean Roudnitsky, Michelle Sagal, Lisa Dresner, Alexander Schwartzman, Chongmin Huan
Abstract Inflammatory bowel disease (IBD) is an inflammatory disorder of the gastrointestinal tract that affects millions of patients worldwide. It has a complex and multifactorial etiology leading to excessive exposure of intestinal epithelium to microbial antigens, inappropriate activation of the immune system and ultimately to the damage of intestinal tissues. Although numerous efforts have been made to improve the disease management, IBD remains persistently recurring and beyond cure. This is due largely to the gaps in our understanding of the pathogenesis of IBD that hamper the development of timely diagnoses and effective treatment. However, some recent discoveries, including the beneficial effects of interleukin-22 (IL-22) on the inflamed intestine, have shed light on a self-protective mechanism in IBD. Regenerating islet-derived (REG/Reg) proteins are small secretory proteins which function as IL-22’s downstream effectors.Mounting studies have demonstrated that IBD patients have significantly increased REG expressions in the injured intestine, but with undefined mechanisms and roles. The reported functions of REG/Reg proteins in intestinal homeostasis, such as those of antibacterial, anti-inflammatory and tissue repair,lead us to discuss their potential mechanisms and clinical relevance in IBD in order to advance IBD research and management.
Key words: Regenerating islet-derived proteins; Inflammatory bowel disease; Crohnʼs disease; Ulcerative colitis; Interleukin-22; Intestinal bacteria
INTRODUCTION
Regenerating islet-derived (REG/Reg) proteins are small secretory C-type-like lectins.In 1979, De Caroet al[1]found that pancreatic stones in patients with chronic calcifying pancreatitis were made largely of a type of proteins. This pancreatic stone protein was later independently reported as pancreatic thread protein by Grosset al[2]in 1985, and as regenerating islet-derived protein by Terazonoet al[3]in 1988. After the recognition of its different isoforms and the corresponding homologs across species in mammals,it was given the current name REG1α in a sequence and structure-based classification of the family proteins[4,5]. The tissue distributions and physiological activities of this protein family are studied primarily in humans and mice. The human REG family consists of REG1α, REG1β, REG3α, REG3γ and REG4, and these family members share between 30 to 80 percent homologous sequences. In mice, the family members of Reg1, Reg2, Reg3α, Reg3β, Reg3γ, Reg3δ and Reg4 share more than 50 percent homologous sequences, and are structurally and functionally conserved with their human counterparts[5]. Studies of REG/Reg proteins have focused on their functions in the pancreas and intestine since REG/Reg proteins are predominantly expressed in these two digestive organs. We and others have shown that in acute pancreatitis, the production of REG/Reg proteins is significantly induced in pancreatic acinar cells to inhibit inflammatory cell infiltration and to promote tissue repair[6-11]. In the inflamed intestine, REG/Reg expression is highly upregulated in different crypt cells including Paneth cells, deep secreting cells and enteroendocrine cells, and extend to other intestinal epithelial cells depending on the isoform[12-16]. Although the precise roles of REG proteins in human inflammatory bowel disease (IBD) have not been defined,mouse studies have confirmed that Reg proteins are required to maintain intestinal homeostasis in both physiological and pathological conditions.
Oseet al[13]reported that Reg1 deficient mice had reduced stem cell proliferation in the crypts and impaired epithelial migration along the villi in the small intestine,revealing the role of Reg1 in the preservation and renewal of intestinal villous structure. In line with these findings, Sunet al[17]showed that Reg1 protein protected against intestinal damage caused by indomethacin, a nonsteroidal anti-inflammatory drug. In the same disease model, Kitayamaet al[18]found that Reg1 deficiency severely attenuated the expression of tight junction proteins including claudins 3 and 4.Therefore, in addition to promoting the recovery of villous structure, Reg1 may enhance the integrity of the epithelial barrier during intestinal injury.
Like Reg1 protein, Reg3 proteins also have a protective role in injured intestine.Studies showed that Reg3β and Reg3γ could promote the growth of cultured colonic epithelial cells[19], and their intestinal expression was highly upregulated in dextran sulfate sodium (DSS)- or pathogenic bacteria-induced mouse colitis[12,20,21]. Reg3β deficiency consistently exacerbated symptoms of colitis in DSS-treated mice[22].Similarly, in a mouse model of graft-vs-host disease, intestinal expressions of Reg3α and Reg3γ were upregulated in response to IL-22 signaling to enhance the survival of intestinal stem cells and Paneth cells[23,24]. Furthermore, compensation of Reg3γ deficiencies in IL-22 deficient mice by intraperitoneal injections of REG3γ or Reg3γ blocked the intestinal epithelial invasion of orally introducedC. rodentiumand saved the animals’ lives[25].
Notably, in addition to Reg3 proteins’ trophic effect on the epithelial barrier, the contribution of their direct inhibitory effect on bacterial invasiveness cannot be excluded[26,27]. Cashet al[28]showed that Reg3γ and its human counterpart REG3α are antimicrobial proteins that bind to the peptidoglycan carbohydrate on Gram-positive bacteria. Further studies showed that the interaction was mediated by a Glu-Pro-Asn motif in the long loop region of REG3α[29]. Upon interacting with peptidoglycan,REG3α oligomerizes to form hexameric transmembrane pores that kill bacteria by increasing their membrane permeability[30]. Consistently, Reg3γ deficient mice have more Gram-positive bacteria in the intestinal mucosa[31]. In contrast to REG3α/Reg3γ,Reg3β may attach to lipopolysaccharide to cause osmotic rupture in Gram-negative bacteria[26,27,32-34], in line with the increased proportion of intestinal Gram-negative bacteria in Reg3β deficient mice[35].
Unlike the expressions of Reg1/3 proteins in both the small and large intestines,Reg4 proteins are distributed predominantly in the colon. It has been shown that Reg4 is produced by deep crypt secretory cells and intestinal enteroendocrine cells, and expands to epithelial cells of the upper colonic crypts during inflammation[15,16].
INCREASED EXPRESSIONS OF REG PROTEINS IN IBD PATIENTS
IBD comprises two major disorders: Crohn’s disease (CD) and ulcerative colitis (UC).CD and UC share the features of chronic and destructive mucosal inflammation, but they are pathogenically and clinically distinct. CD patients have abdominal cramps,pain, diarrhea and weight loss, and their intestinal injury is characterized by transmural and segmental lesions that may occur at any level of the gastrointestinal tract “from mouth to anus”. UC patients frequently present with bloody stools and diarrhea, and their intestinal inflammation is localized to colonic mucosa. However,the mechanisms underlying the different pathogenesis in CD and UC remain poorly understood[36,37].
Over the past decade, increased REG expression has been detected by various techniques in both CD and UC patients[12,14,38-52](Table 1). Interestingly, the increased levels of different REG isoforms vary significantly in CD and UC, and in active and remissive phases of each disease, which could be attributed to the different transcriptional regulations of REG isoforms by inflammatory cytokines such as IL22 and TGFβ illustrated in Figure 1. CD and UC are characterized by different cytokine responses. In CD, the T-helper type 1 (Th1) cytokine interferon-γ and the Th17 cytokines IL-17/IL-22 are the main inflammation regulators. In contrast, UC has a Th2-like cytokine response that activates IL-13/IL-5, producing natural killer T cells[53]. These distinct cytokine patterns are associated with relatively high IL-22 levels in patients with CD compared to those with UC[54-56]. Animal studies have shown comparable results. For example, in the Th17 response-mediated mouse model of CD generated by the transfer of CD45RBhighT cells, IL-22 levels are high; but in the Th2 response-regulated UC model of T cell receptor α-chain deficient mice, IL-22 levels are low[56]. A recent study by Leunget al[57]provided an explanation. They showed that in active UC, a Th22 subset of helper T cells that produces IL-22, but not IL-17, was depleted by increased TGFβ in patients’ colonic tissues[57].
Tsuchidaet al[52]showed that REG1α, REG1β, and REG4 were all highly overexpressed in CD, while only REG4 was significantly overexpressed in UC. This could be caused by the limited IL-22 production in the colon because IL-22 signaling is essential for transcriptional activation ofREG1/3genes[25,46,52,58], whileREG4transcription could be driven by the mechanisms other than IL-22 signaling[52]. For example,REG4transcription is known to be activated by caudal type homeobox 2(CDX2), a transcription factor that regulates multiple genes for maintaining intestinal homeostasis in response to the Toll-like receptor signaling[59-62]. Interestingly in this regard, CDX2 expression has been found to be inhibited by phosphoinositide 3-kinase[63], which can be activated by exostosin-like glycosyltransferase 3 (EXTL3), a cell surface enzyme expressed in multiple organs including the intestine[64,65]. Since EXTL3 has been identified as a receptor for REG1/Reg1 and REG3/Reg3 proteins[64-67],it is possible that as illustrated in Figure 1, CDX2-activated colonicREG4transcription is inhibited in CD, but activated in UC, due to higher REG1/3 levels in CD than UC.In addition, GATA binding proteins (GATAs) could possibly regulateREGgenes transcription in an IL-22 independent manner. Studies have shown thatReg1/3transcriptions can be activated by GATA4, which is normally expressed in the proximal small intestine[68,69], but abnormally expressed in the inflammatory lesions of the distal small intestine and colon[70]. On the other hand,REG4transcription is specifically activated by GATA6[52,71], which is expressed in both the small and largeintestines[68]. Deficiency of GATA4 or GATA6 causes abnormal alterations in intestinal cells including Paneth cells, enteroendocrine and goblet cells[72,73]. Both GATA4- and GATA6-regulatedREGtranscriptions could be mediated by inflammation in IBD.Haveriet al[70]suggested the upregulation of GATA4 in inflamed intestine by activated signaling of TGFβ, whose expression is elevated in active but not remissive CD and UC[74,75]. This explains the significant increase of intestinal REG1α in active phase but not remissive phase of UC, which is in contrast to the disease status-independent increase of REG4 in UC[49]. As discussed before,REG4transcription could be activated by CDX2 in UC when IL-22 is diminished. In addition, the activation of GATA6 by inflammation also possibly contributes to REG4 expression in UC (Figure 1). In support of this view, Mustfaet al[76]showed that in IBD, inflammation globally
decreased SUMOylation, a post-translational modification that inhibitsGATA6transcription, in colonic cells[77].
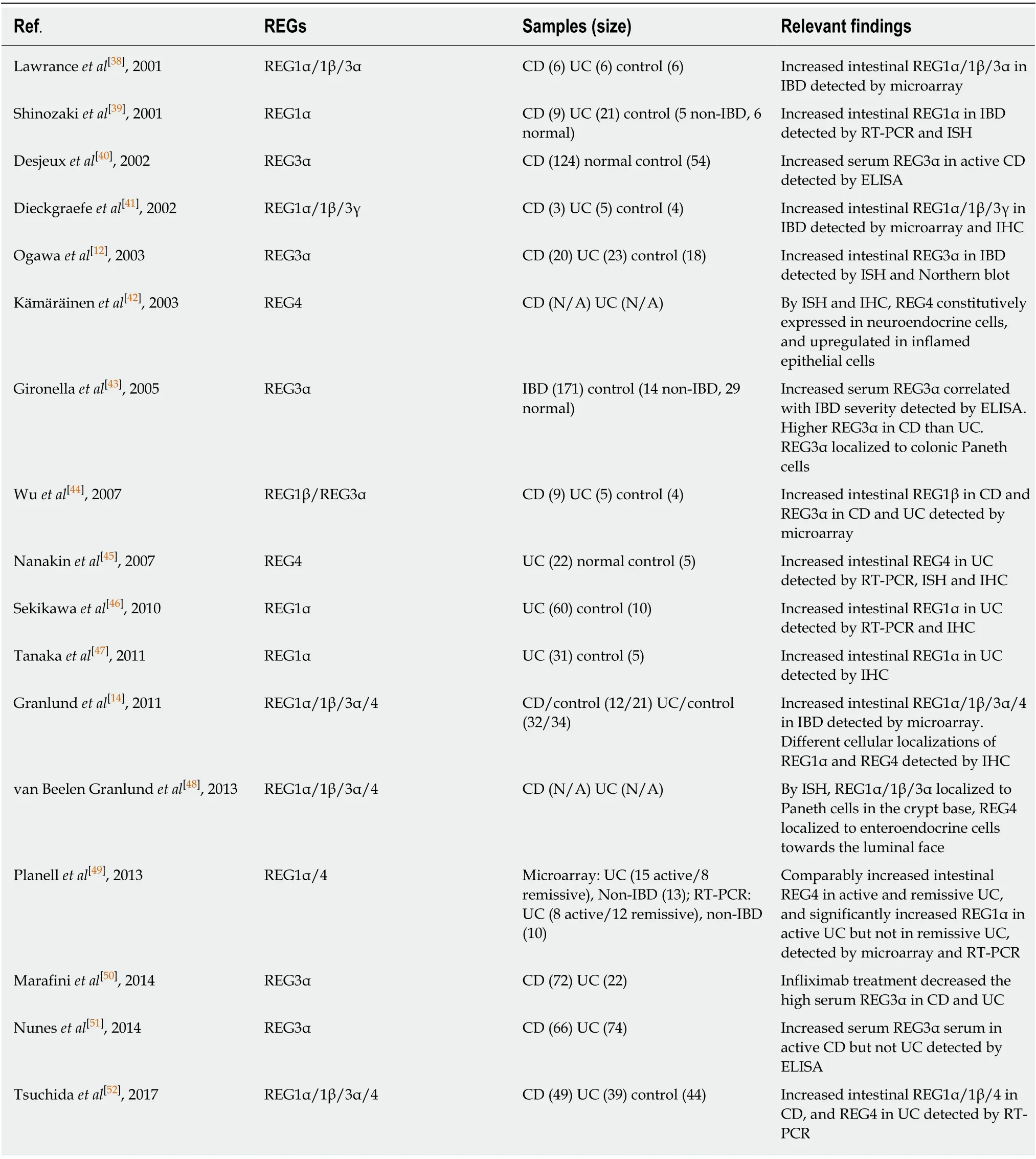
Table 1 Reported REG expressions in inflammatory bowel disease patients
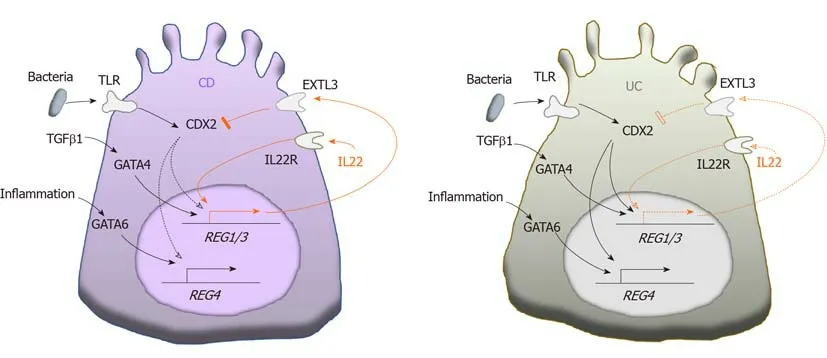
Figure 1 A model of the differential transcription of REG genes in colon crypt cells in Crohn’s disease and ulcerative colitis. Increased interleukin-22 induced transcriptions of REG1/3 in Crohn's disease (orange solid arrows) are relatively attenuated in ulcerative colitis (orange dotted arrows), leading to CDX2-activated REG transcriptions in ulcerative colitis (black solid arrows), but not in Crohn's disease (black dotted arrows). IL: Interleukin; TGF: Transforming growth factor;TLR: Toll-like receptor.
Of note, other factors, such as exclusive enteral nutrition diet commonly recommended for CD patients, influence the bacteria population, inflammation and mucosal healing in IBD[78]. Therefore, they could also have a direct and/or indirect regulatory effect on intestinal REG expressions, which should be investigated in future studies.
REG PROTEINS ARE POTENTIALLY PROTECTIVE IN IBD
Studies have supported that IBD progression is driven by defective bacterial clearance, aberrant immune responses, and impaired epithelial barrier[37]. Notably,REG proteins appear to have the corresponding protective effects that can counteract these defects in IBD as illustrated in Figure 2.
In line with REG/Reg proteins’ bactericidal activities, mice carrying genetically modifiedREG/Reggenes have altered compositions of intestinal bacterial microbiota[16,31,35,79,80](Table 2), indicating the regulatory roles played by REG/Reg proteins in the gut microbiome. Notable among these are that mice with REG3α overexpression and mice with Reg4 deficiency were resistant to DSS-induced colitis,suggesting the importance of REG/Reg-regulated intestinal microbiota in IBD pathogenesis[16,79]. Furthermore, mice with Reg3γ overexpression had an enriched fraction of beneficialLactobacilliin the gut microbiome, suggesting the positive selection of “good bacteria” by Reg3γ[80]. In support of the importance of an altered intestinal microbiome in IBD pathogenesis, a systematic review and meta-analysis of CD and UC patients showed different intestinal microbiome compositions in patients with active disease as compared to those in remission[81]. Given the fact that patients with active IBD have higher levels of REG proteins, further studies are needed to define the significance between increased REG expression and altered microbiome composition observed in these patients.
In addition to their bactericidal activities, REG3/Reg3 proteins are antiinflammatory since they can inhibit proinflammatory cytokine secretion,inflammatory cell activation and infiltration in inflammatory diseases including IBD[8-10,12,82-84]. REG3α incubation inhibited the proinflammatory cytokine secretion in intestinal mucosa harvested from patients with active CD in a dose dependent manner, and decreased the adhesive molecules, such as E-selectin, ICAM-1 and VCAM-1, which were found to be upregulated on endothelial cells to promote inflammatory infiltration[12]. Additionally, REG3/Reg3 proteins regulate the activities of macrophages, which regulate inflammatory injury in IBD[84-87]. It is thus possible that REG3/Reg3 proteins may also alleviate IBD inflammation via macrophages.

Figure 2 A mechanistic model of REG proteins’ protective activities in inflammatory bowel disease. IBD:Inflammatory bowel disease.
As previously mentioned, REG/Reg proteins have trophic effects on intestinal epithelium in both physiological and pathological conditions. These trophic effects have been attributed to the activation of pro-survival and pro-proliferative signaling pathways, such as MEK1/2, ERK1/2, phosphoinositide 3-kinase-Akt and JAK2-STAT3[5]. Therefore, the direct tissue protection and repair of mucosa in IBD by REG/Reg proteins cannot be excluded. Indeed, transgenic overexpression of REG3α or intrarectal administration of REG3α alleviated the epithelial damage in 2,4,6-trinitrobenzene sulphonic acid-induced mouse colitis[79], while Reg3β deficiency worsen DSS colitis in mice[22]. Similarly, administration of REG3γ /Reg3γ improved epithelial integrity inC. rodentium-induced mouse colitis[25]. Additionally, Reg4+deep crypt secretory cells promoted the formation of organoids derived from Lgr5+colonic stem cells, and Reg4 stimulated the growth of colonic organoids isolated from mice with DSS-induced colitis[15,16].
REG PROTEINS’ PROSPECTIVE CLINICAL RELEVANCE IN IBD
Despite the recent advancements, there are unmet needs in current IBD management,particularly including disease activity detection and treatment[88,89]. REG proteins have been considered as potential diagnostic markers and/or therapeutic targets for immune-mediated diseases[90]. The recognized upregulation of REG proteins in IBD and REG proteins’ beneficial activities provide unique opportunities to address some of these unmet needs for improving IBD management, such as serving as biomarkers for disease activity, shifting the composition of bacterial microbiota, and enhancing the repair of intestinal epithelium.
The clinical diagnosis of IBD remission/relapse depends on the endoscopic biopsy,which has its own limitations including invasiveness, financial burden and inter-user variability. Non-invasive imaging such as ultrasound, and CT and MR enterography are useful modalities but also have drawbacks such as inter-operator variability,radiation exposure, and financial burden[91,92]. Therefore, efforts have been made to study potential IBD biomarkers in serum and feces including C-reactive protein(CRP), anti-Saccharomyces cerevisiae antibody, which is chiefly linked with CD, and perinuclear antineutrophil cytoplasmic antibody, which is linked with UC[93-96].However, these biomarkers have high specificities but limited sensitivities. Of note,inhibition of tumor necrosis factor alpha by Infliximab reduced serum REG3α levels in CD and UC patients[50], supporting the potential use of REG3α for evaluating the response of treatment. Furthermore, a multicenter prospective study showed increased serum REG3α with 94% specificity and 60% sensitivity for active CD[38].Similarly, a recent study showed increased serum REG3α in CD patients 3 mo prior to relapse, but with only 73% specificity and 50% sensitivity[51]. The authors explained that the lower specificity and sensitivity in their study compared with those from the previously mentioned report was likely due to the patients’ mild disease activity and low relapse rate. Given the functional redundancy among REG family members and confirmed upregulated REG1α/β in CD patients (Table 1), it is possible that CD patients with lower REG3α levels may have higher serum REG1α/β levels. If so,combining REG3α and REG1α/β measurements in each CD patient could collectively improve the sensitivity for evaluating the relapse. On the other hand, this study showed that serum REG3α levels were not correlative with UC activity[51]. This could be due to the relatively attenuated upregulation of REG3 in UC (Figure 1). Therefore,it would be more informative to assess the use of REG4 as a biomarker for UC, since it is more specific and highly upregulated in UC[45,49,52].
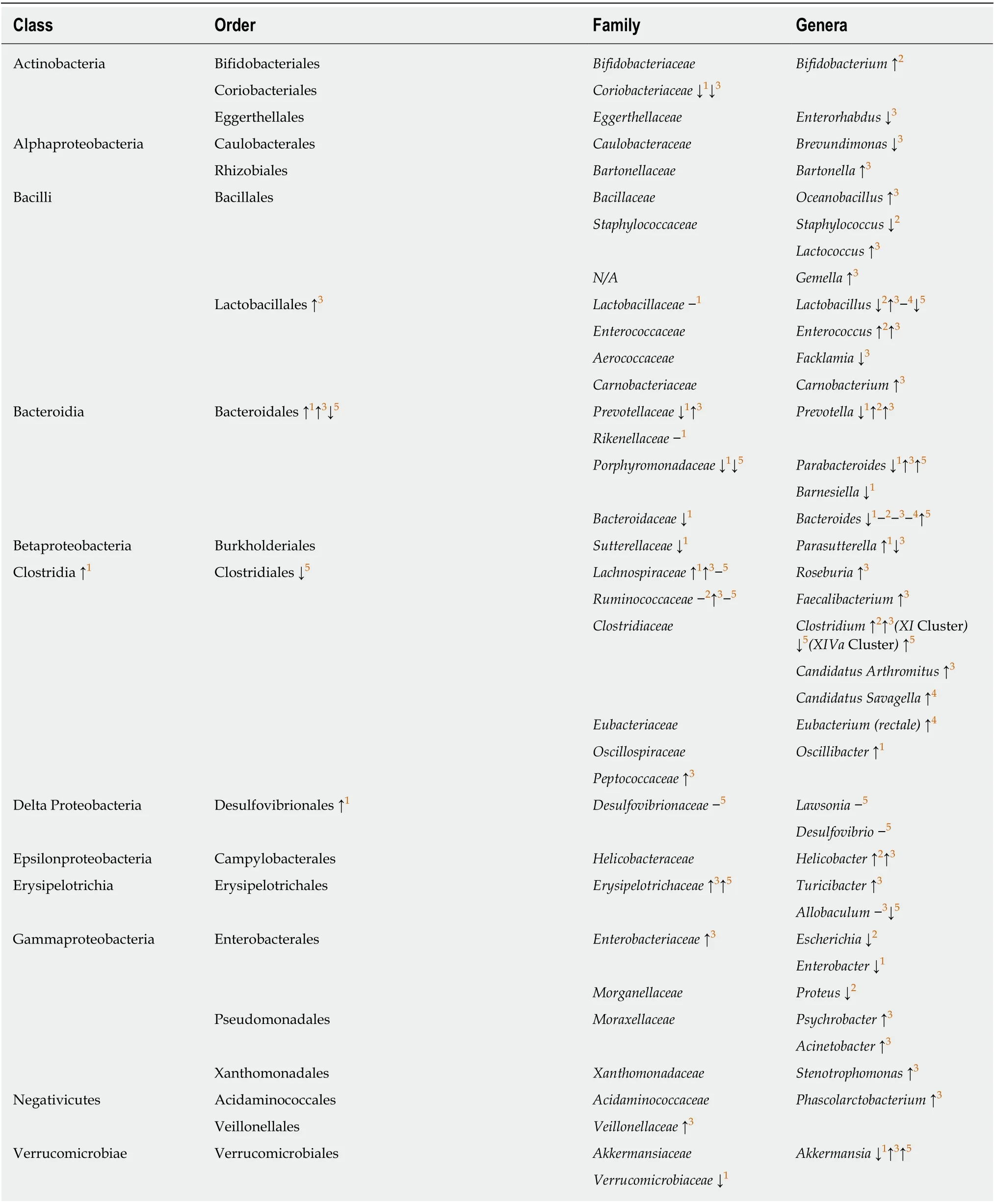
Table 2 Effects of genetically modified REG/Reg genes on the composition of intestinal bacterial microbiota in mice
Multiple clinical trials have shown that fecal microbiota transplantation is a promising treatment to induce remission in active UC[97-100]. However, the specific bacteria that protect against UC have not been identified. Studying altered intestinal bacteria populations in DSS colitis resistantREG3αtransgenic mice and Reg4 deficient mice therefore could help to identify the protective bacteria and associated REG/Reg regulation in UC[16,79].
Bowel resection is considered for the patients that are refractory to medical therapy or with serious complications of the medications[101-103]. However, in addition to the risk of short bowel syndrome, septic complications are commonly associated with anastomotic leaks[103]. The early diagnosis of anastomotic leaks enables a timelier intervention essential for a better outcome. It has been shown that a low CRP on postoperative day 4 is a reliable biomarker for excluding postoperative infectious complications in abdominal surgery, while high CRP levels should prompt aggressive imaging for possible anastomotic failure[104]. Like CRP, REG/Reg proteins have been considered to be acute phase proteins that could be used as markers of septic complications in patients including those undergoing abdominal surgery[105-110].Therefore, determining whether REG proteins can serve as a more sensitive and specific sensor of postoperative anastomotic leak could have significant clinical potential.
It is also worthy of note that Reg proteins do not lead to immune suppression or immunogenicity, the side-effects that are associated with risks of infection and immune dysregulation in some agents used in IBD treatment[88,89]. Given that REG proteins have bactericidal, anti-inflammatory and tissue repair functions in the inflamed intestine (Figure 2), the use of REG proteins as an adjunct to reduce the doses of current medications for IBD could potentially minimize complications.Despite these benefits, however, concerns of long-term application of REG proteins remain. For example, REG/Reg proteins may potentially overactivate the oncogenic STAT3 signaling pathway[5,27], even though the gastrointestinal tract administration may decrease the oncogenic risk in other organs. Additionally, CD patients are prone to bowel stricturing/stenosis formation[111]. Based on our finding of Reg1 as an activator of stellate cells, the predominant producer of collagen in pancreatitis[67], the potential of REG1 for bowel stricturing/stenosis should be clarified, which could argue against its use in patients with CD.
CONCLUSION
IBD remains a significantly challenging disorder due to as yet unresolved issues in its pathogenesis, diagnosis and clinical management. In this review, by discussing the expressions and activities of REG proteins in the inflamed intestine, we have attempted to illuminate potential applications of these REG proteins that may help to improve detection and treatment of the disease, but further comprehensive studies are necessary to clarify and confirm these benefits in IBD.
杂志排行
World Journal of Gastroenterology的其它文章
- Tailored classification of portal vein thrombosis for liver transplantation: Focus on strategies for portal vein inflow reconstruction
- Alternative uses of lumen apposing metal stents
- lnnate immune recognition and modulation in hepatitis D virus infection
- Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine
- Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signalregulated kinase phosphorylation
- Periportal thickening on magnetic resonance imaging for hepatic fibrosis in infantile cholestasis
