Tailored classification of portal vein thrombosis for liver transplantation: Focus on strategies for portal vein inflow reconstruction
2020-06-17FeiTengKeYanSunZhiRenFu
Fei Teng, Ke-Yan Sun, Zhi-Ren Fu
Abstract Portal vein thrombosis (PVT) is currently not considered a contraindication for liver transplantation (LT), but diffuse or complicated PVT remains a major surgical challenge. Here, we review the prevalence, natural course and current grading systems of PVT and propose a tailored classification of PVT in the setting of LT. PVT in liver transplant recipients is classified into three types,corresponding to three portal reconstruction strategies: Anatomical, physiological and non-physiological. Type I PVT can be removed via low dissection of the portal vein (PV) or thrombectomy; porto-portal anastomosis is then performed with or without an interposed vascular graft. Physiological reconstruction used for type II PVT includes vascular interposition between mesenteric veins and PV,collateral-PV and splenic vein-PV anastomosis. Non-physiological reconstruction used for type III PVT includes cavoportal hemitransposition, renoportal anastomosis, portal vein arterialization and multivisceral transplantation. All portal reconstruction techniques were reviewed. This tailored classification system stratifies PVT patients by surgical complexity, risk of postoperative complications and long-term survival. We advocate using the tailored classification for PVT grading before LT, which will urge transplant surgeons to make a better preoperative planning and pay more attention to all potential strategies for portal reconstruction. Further verification in a large-sample cohort study is needed.
Key words: Portal vein thrombosis; Liver transplantation; Portal reconstruction; Grading;Anatomical; Physiological; Non-physiological
INTRODUCTION
Portal vein thrombosis (PVT) generally refers to complete or partial obstruction of blood flow in the portal vein and/or its main branches due to a non-neoplastic thrombus. A malignant embolus is termed tumurous invasion of the portal vein,constituting another clinical entity with different pathogenesis, treatment and prognosis[1]. PVT has long been considered a contraindication for liver transplantation(LT), as adequate portal inflow cannot always be ensured. However, when PVT is removeden blocwith the liver or through thrombectomy, a routine porto-portal anastomosis can still be performed. For some complex PVTs, alternative approaches can redirect the portal venous flow into the graft to achieve physiological reconstruction. Under these circumstances, PVT has no significant impact on post-LT outcomes[2]. Therefore, the portal vein inflow reconstruction pattern, and not the PVTper se, determines the efficacy of LT in patients with PVT. Current PVT grading systems, which are mainly based on the location and extent of the thrombus and the degree of occlusion in the vascular lumen, correlate weakly with the adequacy of portal vein inflow after thrombectomy or compensatory collaterals due to the PVT,both of which are crucial for portal vein inflow reconstruction during LT. We aimed in this opinion review to propose a tailored classification of PVT specific for LT, with the primary consideration of strategies for PV inflow reconstruction.
PREVALENCE OF PVT IN LIVER TRANSPLANT CANDIDATES AND RECIPIENTS
A large epidemiologic study in northwest Italy identified 3535 new PVT cases from a total population of 13 million over an 11-year period, with an overall incidence of PVT of3.78 and 1.7 per 100000 inhabitants in males and females, respectively[3]. However,the prevalence of PVT in liver transplant candidates and recipients is much higher.Analysis of the Organ Procurement and Transplant Network database between 2002 and 2013 showed that PVT was reported in 2819 (3.3%) patients listed for LT and in 3321 (6.8%) patients intraoperatively[4]. A Swedish study based on 23796 consecutive autopsies found that 33.1% of 254 PVT cases were associated with cirrhosis and hepatic carcinoma[5], the main indications for LT. Specific for LT candidates and recipients, independent risk factors for preoperative PVT include older age, male sex,ethnicity, higher body mass index, longer waitlist time, autoimmune hepatitis, nonalcoholic steatohepatitis, diabetes mellitus, and transjugular intrahepatic portosystemic shunt[5-8]. With reference to ethnicity, African Americans had the lowest prevalence of PVT: 2.3% at registration and 4.9% at transplant, whereas Hispanic patients had a significantly higher prevalence: 4.6% at registration and 9.1% at transplant[6].
NATURAL COURSE OF PVT IN CIRRHOSIS
As a majority of liver transplant candidates and recipients have cirrhosis, it is crucial to obtain insight into the natural course of PVT in cirrhosis. All thromboembolic events can be traced pathophysiologically to the three fundamental components of Virchow’s triads - alterations of normal blood flow, hypercoagulability, and vascular endothelial injury. For PVT in cirrhosis, the first two components are more decisive.Cirrhosis is a chronic process complicated by remodelling of the intrahepatic architecture and portal hemodynamics and by rebalancing of pro- and anticoagulant activities[9]. Maruyamet al[10]performed an excellent study on the natural course of PVT in virus-related cirrhosis, with a rigorous design to exclude almost all interfering factors. None of the enrolled patients received any treatments related to PVT,including anticoagulants, vasoactive drugs, transjugular intrahepatic portosystemic shunt, surgery or even antiviral therapy. Over an 11-year period,de novoPVT developed in 28% of 150 patients with virus-related cirrhosis but without PVT at baseline. Moreover, the prevalence of PVT increased along the course of cirrhosis,with cumulative incidences of 12.8%, 20% and 38.7% at 1, 5 and 8-10 years,respectively. The baseline flow volume of the largest collateral vessel was the only independent risk factor for PVT, though collateral vessels were comparably common in the PVT and non-PVT groups, with baseline incidences of 93% and 96%,respectively. Follow-up of the 42 patients with PVT revealed PVT improvement in 47.6%, no change in 45.2%, and worsening in only 7.2%. These findings were consistent with the Organ Procurement and Transplant Network database analysis,which showed that 40% of the listed 1603 patients with PVT did not report PVT at LT[4]. Overall, these results are very interesting, indicating that PVT is a multifactorinduced event during the course of cirrhosis but tends to be stable or to resolve.
Portal pressure is a suitable surrogate reflecting remodelling of the intrahepatic architecture and portal hemodynamics. Portal pressure usually increases with the progression of cirrhosis, with a tendency to decrease from its peak value when portal blood flow is partly diverted by collateral vessels. Rebalancing of pro- and anticoagulant activities can be embodied as the intensity of thrombophilia, which increases during the early stage of cirrhosis and decreases during the decompensation period. We believe that the relationship between portal pressure and thrombophilia elucidates the three types of natural courses of PVT: Never occurring, occurring but stable or improved, and worsening (Figure 1).
CURRENT GRADING SYSTEMS FOR PVT
There are approximately ten grading systems for PVT, as reviewed in two major articles[1,11]. Essentially, these grading systems can be grouped into three categories.The early grading systems only considered the PVT’s location, obstruction and extension, with the Yerdel grading system being the most representative and well recognized[12]. The collaterals and cavernous transformation were added in the later grading systems, among which the Jaimeson grading system has been the most instructive[13]. The third category of PVT classification involved additional indicators,including duration, presentation and underlying liver disease, and was more complicated[1,14]. Nonetheless, in the setting of LT, these grading systems have limited value because a PVT located in the branches and distal trunk can be resected together with the liver but a more advanced PVT can also be removed through thrombectomy.Cases with insufficient portal blood inflow after thrombectomy and how to use alternative vessels for portal reconstruction are major challenges. Bhanguiet al[11]proposed a dichotomy for PVT, which included complex PVT and noncomplex PVT,grouping Yerdel grade 4 or Jamieson grades 3 and 4 into the former and others into the latter. This classification was used as a guide for portal flow reconstruction during LT, which was also defined by a dichotomy of non-physiological and physiological reconstruction. However, a complex PVT has not always been assigned to a nonphysiological reconstruction, and the relationship between a non-complex PVT and physiological reconstruction appears in a same manner.
TAILORED CLASSIFICATION OF PVT WITH REFERENCE TO PV INFLOW RECONSTRUCTION
Portal vein inflow reconstruction in LT is a considerable challenge for advanced PVT complicated with structural and hemodynamic abnormalities of the portal venous system, not only with regard to surgical techniques but also with regard to postoperative complications and patient survival. Therefore, PV inflow reconstruction patterns can stratify patients with PVT undergoing liver transplantation. We propose a tailored classification of PVT with reference to three patterns of PV inflow reconstruction, mainly based on the vascular sources providing the portal inflow for the liver graft.
Type I PVT: Anatomical porto-portal anastomosis with or without devascularization of the collaterals
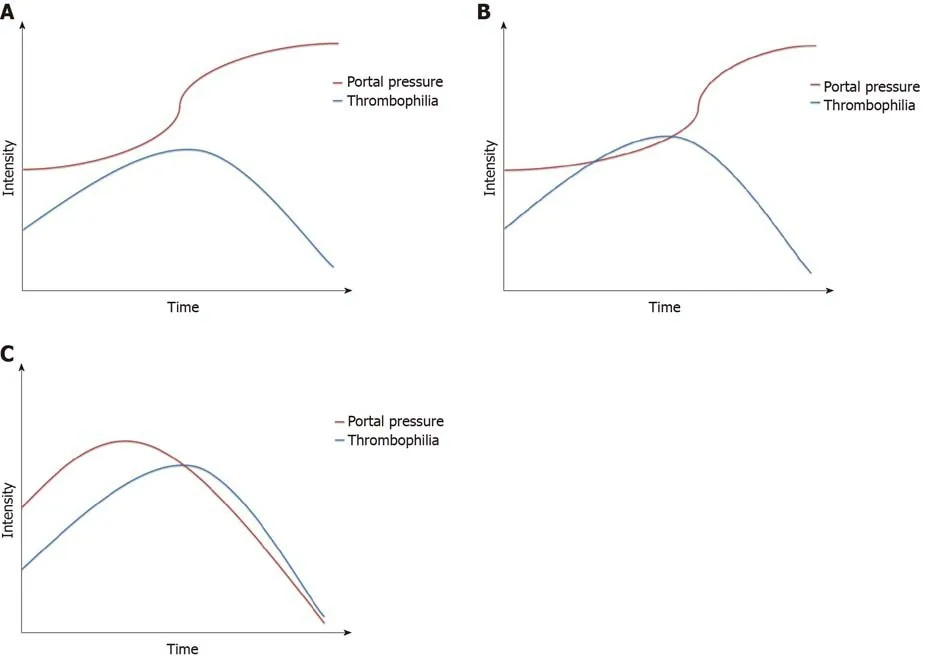
Figure 1 Relationship between portal pressure and thrombophilia in the natural course of portal vein thrombosis. A: Portal pressure increases gradually,while the intensity of thrombophilia increases and then decreases. No crossover of the two curves means that portal vein thrombosis (PVT) never occurs; B: The two curves cross each other, but portal pressure continues to increase, and its curve is separate from the curve of thrombophilia; thus, PVT occurs but stabilizes or improves; C: Portal pressure increases at first and then decreases due to diversion of the blood flow by the collaterals. The two curves cross and remain close to each other, meaning that PVT occurs and worsens.
Type I PVT is defined as a PVT located in the branches and distal trunk that can definitively be resected with the liver or removed through thrombectomy to recover adequate portal vein blood flow. This type is the most common in LT recipients with PVT. According to a systematic review of the surgical resolution of 1957 patients with PVT,PVT was resected through low dissection of the PV in 5% of the patients and removed through thrombectomy in 75% during LT[15]; both conditions would be assigned to type I PVT in our tailored classification, as an end-to-end donor-recipient portal anastomosis was performed thereafter.
Thrombectomy should always be maintained as the first option for any PVT,regardless of location, obstruction and extension; even for Yerdel grade 3 or 4 PVT,thrombectomy can still be performed successfully with appropriate approaches in experienced centres. The key points for thrombectomy include the following: (1) Low dissection of the PV as proximal as possible to reach the superior border of the pancreas; (2) Right operating spaces between the thrombus and the vascular wall; and(3) Appropriate Pringle manipulation with fingers to control bleeding and guide handling. Panet al[16]introduced an improved eversion thrombectomy without cutting off the thrombus to obtain persistent traction from the diseased liver through the PVT.Using this technique and simple or eversion thrombectomy, all the Yerdel grade 1 or 2 PVTs in 218 cases were removed successfully, and the success rates for grades 3 and 4 PVT were 79.3% (23/29) and 50% (3/6), respectively. Kasaharaet al[17]and Mizunoet al[18]also independently reported a pull-out technique for thrombectomy in 6 PVT patients. All inflow branches to the PV above the confluence of the superior mesenteric vein (SMV) and splenic vein (SV) were ligated and transected. The PV trunk was dissected posterior to the pancreas, pulled out inferior to the pancreas, and then transected at the confluence of the SMV and SV. After thrombectomy, the donor PV was placed posterior to the pancreas where the PV was used, and portal reconstruction was performed with or without an interposed vascular graft.
Occasionally, adequate portal vein flow or pressure is not regained even when the PVT is removed completely, probably due to diversion of blood flow by the collaterals. Under these circumstances, devascularization of the collaterals should be considered after restoration of blood inflow to the graft. The dominant collaterals were compensatory enlarged gastric coronary veins and splenorenal shunts, as other collaterals, such as the pericholedochal vascular plexus and umbilical vein, were usually ligated and cut off during resection of the diseased liver, and mesentericocaval shunts in rare cases. The gastric coronary veins are usually easy to deal with in the operative field, though spontaneous or surgical splenorenal shunts are more complicated and require careful evaluation of preoperative images[19]. For splenorenal shunts that are difficult to dissect, ligation of the left renal vein might be an alternative strategy that does not affect renal function or recipient survival[20,21].Diffuse and fine collaterals communicating portal and vena cava systems in the retroperitoneal region, which are common in pediatric recipients, can be thermally devascularized using bipolar electrocoagulators.
Type II PVT: Veins belonging to the recipient portal system used for portal inflow reconstruction
Type II PVT is defined as PVT that cannot be removed successfully through thrombectomy to achieve adequate portal vein flow; thus, a substituted inflow vessel is used instead of or in addition to porto-portal anastomosis. The substituted vein belongs to the recipient portal system. Therefore, the reconstruction of portal inflow is still physiological, albeit not anatomical. The main substituted vessels used for patients with type II PVT are mesenteric veins and compensatory enlarged collaterals.
Recipient mesenteric veins as portal influx: Vascular graft interposition between recipient mesenteric veins and the donor portal vein was found to be the second most commonly used technique after thrombectomy, accounting for 8.4% of all PVT cases[15]. An interposed vascular graft can be an autologous vein such as the external iliac vein, ovarian vein or internal jugular vein, a donor vessel, a cadaveric cryopreserved vessel, or an artificial vessel. The SMV is the main portal influx source because it collects most of the hepatopetal blood from the gut and is closer to the portal vein. The vascular graft interposition has been performed using the jump method, by which the vascular graft is brought to the portahepatis through a transmesocolic route, anterior to the pancreas and posterior to the pyloric antrum.When the SMV distal to its confluence with the splenic vein is not available due to thrombus, hypoplasia or any other reasons, an enlarged inferior mesenteric vein can be used as a portal influx source with a jump vascular graft interposition[22,23].
Enlarged collaterals as portal influx: Enlarged collaterals have been used as portal influx in 2.4% of all PVT cases[15]. For example, spontaneous splenorenal shunts were the most common collaterals in decompensated cirrhosis patients who underwent evaluation for liver transplantation, with an incidence of 23%[24]. Nevertheless, use of the left renal vein to graft PV anastomosis was preferred due to its technical simplification, which is discussed below for the type III PVT. A splenorenal shunt was directly used as portal influx when its confluence to the left renal vein was identified and sectioned, and then the splenorenal shunt was cautiously dissected and brought behind the stomach[25]. Among the collaterals, the enlarged left gastric vein or gastric coronary vein was the most commonly used portal influx, as it was superficial, close to the porta hepatis and easy to dissect. Pooled analysis for the left gastric vein or gastric coronary vein as portal influx showed that 92% of 24 patients were alive with a patent portal reconstruction at the last follow-up visit[11]. In some cases, a large pericholedochalvarix was the only collateral available for portal reconstruction, but a subsequent Roux-en-Y hepaticojejunostomy was reasonable for biliary reconstruction,as the dissection of recipient bile duct was abandoned beforehand to avoid injury to the pericholedochalvarix[26,27]. Nine of 10 (90%) patients with a pericholedochalvarix as portal influx were reported to be in good condition with a patent portal reconstruction. In case reports, other enlarged mesenteric vein tributaries, such as the right gastroepiploic vein and right and middle colic vein, have been used to reconstruct the portal flow to the graft[28-32].
The splenic vein as portal influx: Heterotopic liver transplantation in splenic fossa after splenectomy has been proven to be feasible in technique and long-term results[33,34], therefore providing an option for portal reconstruction in PVT patients using the splenic vein. For patients receiving heterotopic liver transplantation in the splenic fossa, the conditions are usually complicated not only by PVT but also by other problems, such as failure to remove the diseased liver. In addition to portal reconstruction, reconstructions for graft outflow, hepatic artery and bile duct are different from orthotopic liver transplantation. Three cases of PVT were reported using heterotopic liver transplantation in the splenic fossa, and two of the patients were in good condition with normal liver function and patent vessels at 60 months and 18 months after the operation[35,36].
Type III PVT: Vessels that did not belong to the recipient portal system used for portal inflow reconstruction
In patients with type III PVT, recovered portal inflow to the graft is partly or completely from vessels that do not belong to the recipient portal system, making the portal reconstruction a non-physiological pattern. The strategies for type III PVT include cavoportal hemitransposition (CPHT), renoportal anastomosis, portal vein arterialization and multivisceral transplantation.
CPHT: Cavoportal transposition was performed in early animal experiments to determine the effects of portal blood on liver regeneration, as well as the diversion of systemic blood to the liver, and was then used to treat glycogen storage disease clinically in the 1960s[37]. CPHT, in which only one anastomosis between the donor PV and recipient inferior vena cava was performed either in an end-to-end or end-to-side way, was first described in liver transplant recipients with diffuse PVT in 1998[38]. As a novel and simplified resolution for diffuse PVT, CPHT has been performed in many transplant centres, mainly in the 2000s. However, the results of CPHT were suboptimal and seemed unpredictable. Pooled analysis showed that 63% of 86 patients who underwent CPHT were alive at the last follow-up, but ascites were almost inevitable, with an incidence of portal or cavomesenteric thrombosis of 28%and an incidence of intra-abdominal bleeding of 30%-50%[11]. An eventless course after CPHT largely depends on two aspects, namely, proper perfusion to the graft and potential cavoportal shunts. The caval flow directed into the graft should be quick enough to avoid re-thrombosis, but not be too quick to avoid hyperperfusion.Potential cavoportal shunts guarantee the gradual correction of portal hypertension,reducing the risk of intra-abdominal bleeding and refractory ascites. Nonetheless,there is uncertainty in both aspects. Conversely, better results under the same circumstances have been achieved with renoportal anastomosis and multivisceral transplantation. Thus, CPHT has rarely been reported in diffuse PVT in the last decade.
Renoportal anastomosis (RPA): RPA was first described in 1997 in a liver transplant recipient with PVT and a surgical splenorenal shunt[39]. A total of 64 cases of RPA among PVT patients have been reported[11,40-44]. Among 57 patients with available longterm results, 46 (80.7%) were alive with a patent portal reconstruction at the last follow-up visit; complications related to RPA included ascites (40.4%), renal dysfunction (28.1%), portal thrombosis or stenosis (5.3%) and variceal bleeding (3.5%).The left renal vein was observed to be comparable to the native PV in reference to the vessel size and blood flow. In addition, the proximal left renal vein close to the inferior vena cava was easy to dissect, which ensured the operability of RPA. This technique is particularly reasonable for PVT patients with a pre-existing splenorenal shunt, spontaneous or surgical. RPA drained the splanchnic blood through the splenorenal shunt, effectively decompressing portal hypertension and delivering portal trophic factors to the graft. A major concern for RPA lies in whether it is practicable for diffuse PVT without a splenorenal shunt. To date, 5 cases of RPA in the absence of a splenorenal shunt have been reported; 3 of the patients died, though none of the deaths were directly related to the procedure or complications of RPA.Performing a surgical splenorenal shunt followed by RPA appears to be a solution when RPA is the only option[11].
Portal vein arterialization (PVA): PVA is more commonly used as a salvage technique when hepatic artery reconstruction is deemed impossible in liver transplantation or hepatopancreatobiliary surgery to increase the oxygen supply to the liver and alleviate ischaemic biliary necrosis[45]. This technique was first reported as a solution for portal reconstruction in pre- and post-LT-confirmed PVT in 1995[46]. A total of 18 cases of PVA for PVT in liver transplantation have been reported, mainly in the 1990s and 2000s[46-56]. In a few of them, PVA was performed to augment portal vein flow after native porto-portal anastomosis but contributed predominantly to portal inflow to the graft over the long term[52,55]. Among the 18 patients, 6 (33.3%) died postoperatively, and 2 underwent embolization of the diverting arteries due to aggravating portal hypertension with related complications. The main drawback of PVA is the impossibility of relieving portal hypertension, which usually leads to an eventful postoperative course. Another disadvantage is overarterialization, which may result in liver fibrosis. Therefore, the arterial flow should be calibrated, by using either medium-sized arterial vessels or partial ligation around the arterial side.
Multivisceral transplantation (MVT): MVT, whichen blocengrafts the liver, small intestine, stomach, pancreaticoduodenal complex and sometimes a segment of the colon, represents the last surgical option for diffuse PVT, replacing the entire splanchnic venous system of the recipient. Due to the major challenges in this technique, immunosuppression and postoperative management, most MVTs have been performed by a few experienced teams, though there has been tremendous progress over the past decades[57-60]. The first case of successful MVT for diffuse portomesenteric thrombosis, which resulted from protein C deficiency, was reported in 2002[61]. The Indiana group reported 34 cases, the largest single-centre series of MVT for diffuse PVT, and patient survival was 80% at 1 year and 72% at 3 and 5 years postoperatively, with a median follow-up of 2.78 years[58,62]. However, surgical complications occurred in 56% of the patients. The steep learning curve and additional risks of the intestinal component of MVT, such as rejection, sepsis,malnourishment and post-transplant lymphoproliferative disorder, remain major obstacles for the routine adoption of MVT in diffuse PVT.
ROLES OF THE TAILORED PVT CLASSIFICATION IN THE SETTING OF LIVER TRANSPLANTATION
Type I, II, and III PVTs strictly correspond to three patterns of PV inflow reconstruction in LT: Anatomical, physiological, and non-physiological reconstruction. This tailored classification can stratify PVT patients by surgical complexity and risk of postoperative complications, as well as long-term survival.Although it is not a preoperative classification, the PVT type can be predicted before liver transplantation through rigorous evaluations via abdominal CT angiography and symptoms of portal hypertension. A proposed algorithm for the tailored PVT classification and PV reconstruction strategy is illustrated in Figure 2.
We advocate using this tailored classification for PVT grading before LT, even though the determined type may change during the operation. The tailored PVT classification allows for better preoperative planning, urging transplant surgeons to pay more attention to all potential strategies for portal reconstruction. In addition to the conditions of the recipients, the procurement of donor organs and interposed vascular grafts, as well as the technical capacity of MVT, should be fully considered.Only in this way can portal reconstruction be performed as planned rather than passively. Furthermore, a large-sample retrospective study should be performed with regard to the development of a model to accurately predict the tailored PVT type before LT, which should be verified in an independent cohort.
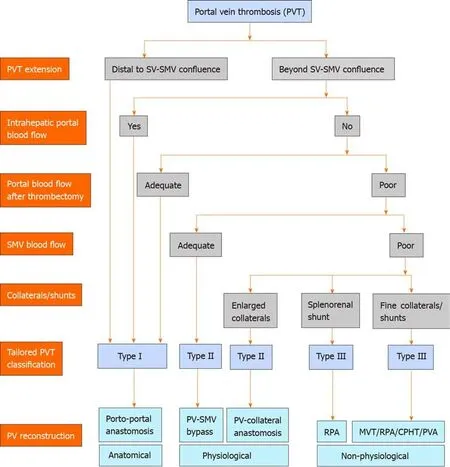
Figure 2 A proposed algorithm for the tailored portal vein thrombosis classification and portal vein reconstruction strategy. CPHT: Cavoportal hemitransposition; MVT: Multivisceral transplantation; PV: Portal vein; PVA: Portal vein arterialization; RPA: Renoportal anastomosis; SMV: Superior mesenteric vein;SV: Splenic vein.
杂志排行
World Journal of Gastroenterology的其它文章
- Alternative uses of lumen apposing metal stents
- lnnate immune recognition and modulation in hepatitis D virus infection
- Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine
- Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signalregulated kinase phosphorylation
- Periportal thickening on magnetic resonance imaging for hepatic fibrosis in infantile cholestasis
- Cost of postoperative complications: How to avoid calculation errors
