Alternative uses of lumen apposing metal stents
2020-06-17PrabinSharmaThomasMcCartyAnkitChhodaAntonioCostantinoCarolineLoeserThiruvengadamMunirajMarvinRyouChristopherThompson
Prabin Sharma, Thomas R McCarty, Ankit Chhoda, Antonio Costantino, Caroline Loeser,Thiruvengadam Muniraj, Marvin Ryou, Christopher C Thompson
Abstract The advent of lumen apposing metal stents (LAMS) has revolutionized the management of many complex gastroenterological conditions that previously required surgical or radiological interventions. These procedures have garnered popularity due to their minimally invasive nature, higher technical and clinical success rate and lower rate of adverse events. By virtue of their unique design,LAMS provide more efficient drainage, serve as conduit for endoscopic access,are associated with lower rates of leakage and are easy to be removed. Initially used for drainage of pancreatic fluid collections, the use of LAMS has been extended to gallbladder and biliary drainage, treatment of luminal strictures,creation of gastrointestinal fistulae, pancreaticobiliary drainage, improved access for surgically altered anatomy, and drainage of intra-abdominal and pelvic abscesses as well as post-surgical fluid collections. As new indications of endosonographic techniques and LAMS continue to evolve, this review summarizes the current role of LAMS in the management of these various complex conditions and also highlights clinical pearls to guide successful placement of LAMS.
Key words: Lumen apposing metal stents; Walled off necrosis; Gallbladder drainage;Biliary drainage; Gastric access temporary for endoscopy; Gastric outlet obstruction;Therapeutic endoscopy
INTRODUCTION
The introduction of novel lumen apposing metal stents (LAMS) over the past decade has ushered in a new era of therapeutic gastrointestinal endoscopy. Originally approved by the United States food and drug administration (FDA) in 2013, with a primary goal of managing pancreatic fluid collections, LAMS have become widely adopted as a preferred modality in the treatment of walled-off necrosis (WON), with even broader applications for multiple alternative conditions. Alternative uses that have not yet become FDA approved include treatment of luminal strictures, creation of gastrointestinal fistulae, achievement of pancreaticobiliary drainage, improved access for surgically altered anatomy, and drainage of intra-abdominal and pelvic abscesses as well as post-surgical fluid collections. In this review, we highlight both FDA approved as well as non-FDA approved uses of LAMS and provide a detailed summary of the current evidence to support the alternative uses for a variety of conditions.
DESIGN AND FUNCTIONALITY OF LUMEN APPOSING METAL STENTS
The advent of LAMS has radically changed the landscape of therapeutic endoscopy,allowing for multiple minimally invasive treatments for conditions previously thought to require surgical management. Among the various types of stents available,LAMS have revolutionized the field of therapeutic endoscopy given their unique advantages over traditional plastic stents. While variability in design exists between individual stent types, LAMS possess a barbell or saddle shape that allows for the ability to hold two luminal structures in apposition-leading to a lower risk of leakage.Additional benefits of LAMS include a large intraluminal diameter which is able to accomplish more efficient drainage. Furthermore, by virtue of this wider lumen,LAMS may serve as a conduit providing endoscopic access for interventions to various structures abutting the gastrointestinal tract. These bi-flange stents are covered by a silicone layer which helps prevent tissue ingrowth and thus facilitates easy removal. At present, different types of LAMS have been developed and used for transluminal interventions. While there are various similarities between the types,there are subtle differences in terms of their features and delivery mechanisms as summarized on Figure 1. The most commonly utilized LAMS, and the only stent approved in the United States, is the AXIOS stent (Boston Scientific, Natick, MA).Although four additional stents are commercially available, the majority of research and literature is based upon use with the AXIOS stent. However, we have included all available stents within this review to appeal to a more global audience who may be more familiar with alternative LAMS.
PANCREATIC FLUID COLLECTIONS AND PANCREATIC WALLED-OFF NECROSIS
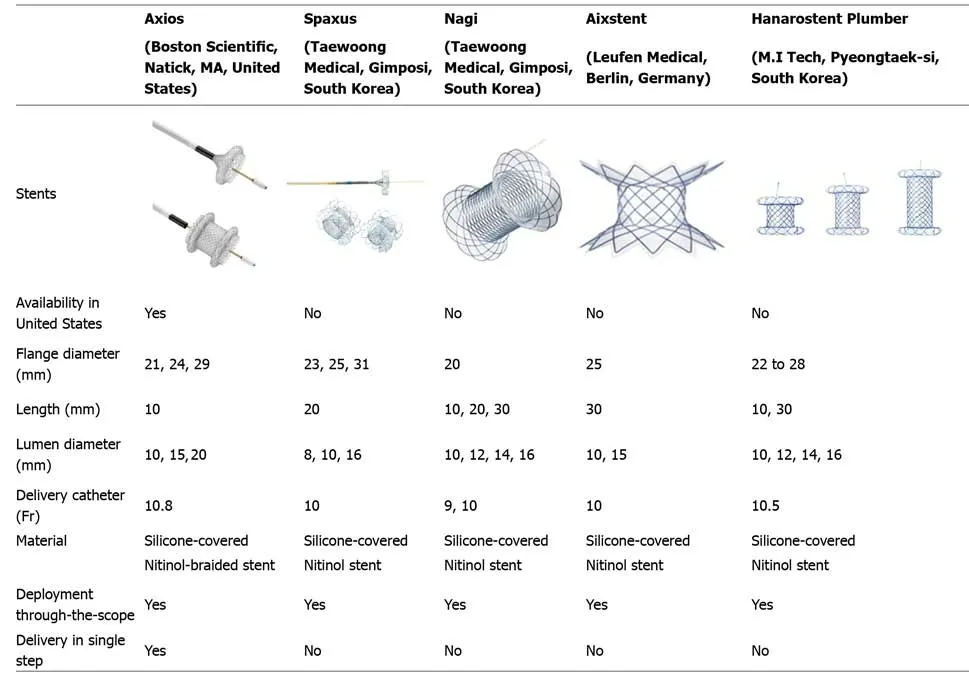
Figure 1 Types of lumen-apposing metal stents. (All stent images available on manufacturer website).
Pancreatic fluid collections typically develop as sequelae of acute pancreatitis or may arise from chronic injury to the pancreas. While these collections resolve spontaneously in most cases, patients with collections that persist beyond four weeks and develop a mature wall (i.e., pancreatic pseudocyst or pancreatic WON) may develop obstructive symptoms such as abdominal pain, early satiety, malnutrition, or infection and warrant drainage[1]. Traditionally, these collections have been drainedviaa percutaneous and surgical approach; however, these strategies may be associated with significant morbidity and high rates of adverse events. With the evolution and common adoption of endoscopic ultrasound (EUS)-guided drainage,the morbidity of drainage has decreased as have the associated adverse events-thereby becoming the preferred approach due to its superiority or comparability in terms of efficacy, cost-effectiveness, and safety[2-5].
Early EUS-guided drainage previously relied upon double pigtail plastic stents in an effort to create a communication between the bowel lumen and the cyst or fluid collection cavity. Plastic stents, however, have various limitations that include longer length and narrow luminal diameter predisposing to occlusion, ineffective drainage of solid debris, long procedure times, and frequent need for repeat intervention.Therefore, development of large diameter, bi-flanged LAMS were able to overcome the shortcomings of plastic stents with subsequent FDA approval in 2013 for treatment of WON with less than 30% solid necrosis. Appropriate patient selection and location of WON is very important to achieve successful LAMS placement as proximity to the gastric wall is ideal with the collection within approximately 1 to 1.5 cm of the bowel wall (Table 1). For this procedure, a transgastric approach is typically recommended; transduodenal route is also possible though may be associated with a longer course[6,7]. From experience, these authors also have noted that large collections extending into the paracolic gutters may not be ideal for endoscopic drainage though this approach may be preferred for patients with a history of gastric varices given EUS-guided visualization.
Treatment of pancreatic pseudocysts and WON were first described by Itoiet al[9]in 2012 and Gornalset al[8]in 2013, respectively. The first study reported outcomes of 15 patients with resolution of all pseudocysts after a single drainage procedure with a technical success rate of 100%[9]. In this study by Itoiet al[9], one stent migration was reported with a median time to removal of 35 d. In the Gornalset al[8]study evaluating pancreatic fluid collections, 9 patients were enrolled and technical success rate of 89%was reported. Two stent migrations occurred with 2 patients developing recurrence.Additional literature evaluating the efficacy and safety of LAMS for pancreatic fluid collections has shown impressive results[10]. A landmark prospective multi-center study was performed by Shah and colleagues in 2015, evaluating stent placement in 33 patients with symptomatic pancreatic pseudocysts or WON[11]. The mean size of these collections was 9.0 ± 3.3 cm with a technical success (defined as the ability to place LAMS successfully) of 91% and resolution of the pancreatic fluid collections in 27 of the 29 patients (93.1%). Additionally, these stents allowed for endoscopic debridement in 11 patients with mild-to-moderate adverse events reported in 15% of cases. Multiple systematic reviews and meta-analyses have since demonstrated similar efficacy and safety results for LAMS and support the notion that metal stents are advantageous compared to plastic stents for pancreatic fluid collections[12-14]. Given these impressive results for pancreatic fluid collections, it is no surprise that the use of LAMS is being increasingly adopted for the treatment of alternative conditions.

Table 1 Clinical pearls when performing procedures with lumen apposing metal stent
GALLBLADDER DRAINAGE FOR HIGH-RISK SURGICAL PATIENTS
Although laparoscopic cholecystectomy remains the mainstay of treatment for patients with acute cholecystitis, percutaneous transhepatic gallbladder drainage(termed cholecystostomy) performed by interventional radiology has been traditionally utilized for poor surgical candidates or in the setting of acute illness when surgical removal is contraindicated. Percutaneous drainage has been a preferred treatment strategy for symptomatic gallbladder disease among high-risk surgical candidates; however, it is limited by significant risk of inadvertent selfremoval of the catheter and risk of serious adverse events such as pneumoperitoneum, pneumothorax, and catheter leakage associated with this technique[15,16].To avoid or reduce some of the complications, endoscopic drainageviaa transpapillary or transmural approach has been devised (Figure 2).

Figure 2 Endoscopic drainage via a transmural or transpapillary approach. A: Endoscopic ultrasound-guided transmural gallbladder drainage using lumen apposing metal stent; B: Endoscopic ultrasound-guided transpapillary drainage of gallbladder. LAMS: Lumen apposing metal stent.
While transpapillary drainage is a well-established endoscopic technique for gallbladder drainage, achieved through an endoscopic retrograde cholangiography(ERC) approach, traversing the cystic duct can be challenging due to anatomy. A more novel approach, utilizing EUS-guided stent placementviaa transgastric or transduodenal approach, is also able to achieve drainage of the gallbladder. In the transmural approach, a cholecystogastric or cholecystoduodenal fistula is created using EUS-guided placement of LAMS (Figure 3). To accomplish this, the echoendoscope is advanced into the gastric antrum or duodenal bulb. Next, the decision should be made on how to create a fistulous tract from the stomach or duodenum to the gallbladder lumen. Once the route of access (i.e., transgastricvstransduodenal) is determined, two options are typically recommended: (1)performing freehand placement of an electrocautery-enhanced LAMS; or (2)placement of non-cautery enhanced LAMS over a wire after fine-needle injection and dilation of the tract (Table 1). The decision regarding these strategies is based primarily on the type of LAMS as well as individual provider expertise.
This technique with LAMS drainage, was first successfully described by Itoiet al[9]in 2012 with 5 patients with acute cholecystitis who underwent four cholecystoduodenostomies and one cholecystogastrostomy. Technical and clinical success was 100% with resolution of acute cholecystitis observed immediately after stent implantation at a follow-up period of 5 mo. Another pilot study by de la Serna-Higuera and colleagues utilized a transgastric approach in 12 patients and transduodenal in one patient[17]. Technical success in this initial series was 84.6% with two failures reported. Overall, clinical success was 100% for patients that underwent drainage with LAMS, and the stents were left in place in 10 of 11 patients without further symptom recurrence at a median follow-up of 100 d. In another patient with acute cholecystitis who had previously failed percutaneous cholecystostomy drainage, Teohet al[18], demonstrated the feasibility of a single-step EUS-guided puncture and delivery of a LAMS for gallbladder drainage using a novel cauterytipped stent delivery system. While use is limited to centers with expertise, Walter and others performed the first multi-center, prospective study of LAMS for EUSguided gallbladder drainage among high-risk surgical patients with acute cholecystitis[19]. Thirty patients were included in this study and demonstrated a technical success rate of 90% and clinical success of 96%; however, there were 4 LAMS-related adverse events reported. Despite this high adverse event rate,subsequent studies have not confirmed this data, with only one adverse event of a post-operative fever reported among 15 patients in a study by Iraniet al[20]. A metaanalysis of LAMS use in EUS-guided gallbladder drainage revealed a procedurerelated adverse event rate of 10.6% with a majority (5.7%) developing delayed events at a median follow-up of 6 mo[21]. These conflicting results underscore the importance of proper patient selection prior to EUS-guided LAMS placement for gallbladder drainage.
More recently, a meta-analysis was performed by Luket al[22]comparing EUSguided versus percutaneous transhepatic gallbladder drainage. On subgroup analyses of only studies utilizing LAMS, three studies were found and demonstrated no difference in pooled technical success [OR 0.21 (95%CI, 0.04 to 1.10)], clinical success[OR 1.43 (95%CI, 0.42 to 4.81)], or rate of adverse events [OR 0.42 (95%CI 0.14 to 1.28)].However, all three studies found that hospital length of stay was shorter (mean difference of 2.76 d;P= 0.03) with fewer readmissions [OR 0.14 (95% CI, 0.03 to 0.70)]and fewer reinterventions [OR 0.15 (95% CI, 0.02 to 0.98)] in the LAMS treatment arm compared to percutaneous cholecystostomy. Additional authors have concluded, that for patients with terminal diagnoses, EUS-guided gallbladder drainage may provide a better quality of life than other non-surgical techniques such as percutaneous cholecystostomy due to need for repeated intervention[23,24].
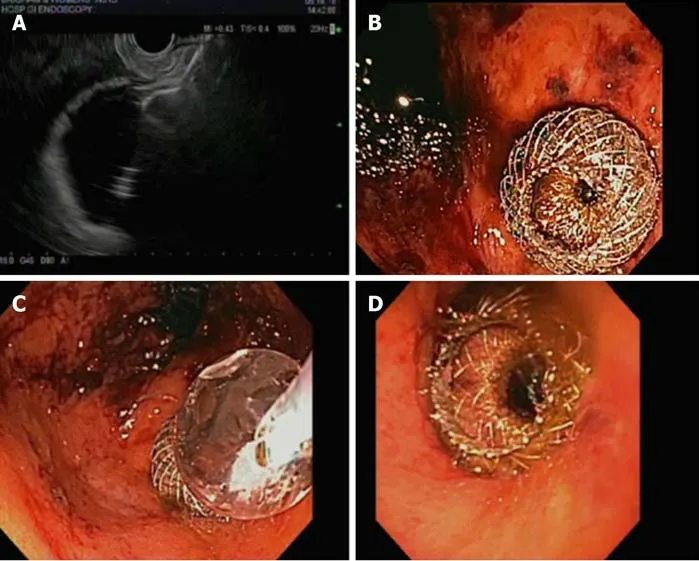
Figure 3 Transmural approach. A: Endoscopic ultrasound-guided gallbladder drainage with proximal lumen apposing metal stent (LAMS) deployment in gallbladder; B: Endoscopic view status post LAMS placement; C: Dilation of the LAMS with through-the-scope balloon; D: Successful endoscopic ultrasound-guided cholecystogastric fistula formation using LAMS.
BILIARY DRAINAGE FOR MALIGNANT DISTAL BILIARY OBSTRUCTION
Along with EUS-guided gallbladder drainage, drainage of the biliary systemviaEUSguided hepaticogastrostomy, choledochoduodenostomy, and cholecystostomy are feasible treatments with LAMS when or if ERCP is not feasible[25,26]. Although other non-lumen apposing metal stents may achieve choledochoduodenostomy, the biflanged design may improve drainage and decrease the risk of stent migration. From the same authors that originally described EUS-guided gallbladder drainage, Itoi and Binmoeller also first reported EUS-guided choledochoduodenostomy in a patient with pancreatic cancer who failed traditional transpapillary accessviaERCP[27]. In this study, LAMS was utilized to prevent leakage with the proximal and distal anchor flanges designed to hold the bile duct and the duodenal wall in apposition. The procedure was successful with subsequent studies demonstrating similar successful results. A multi-center study in Europe included 57 patients with malignant distal biliary obstruction[28]. In this study, cautery-enhanced and non-cautery LAMS were used with a technical success of 98.2% with a range of sizes employed. Clinical success was 94.7% with a low adverse event rate of 7.0%. A prospective multi-center study by Tsuchiyaet al[29]was recently performed to evaluate the long-term effectiveness of EUS-guided choledochoduodenostomy with LAMS placement. This study included 19 patients (all of which had failed prior ERCP) and found a technical success rate of 100% with no immediate adverse events reported. Moreover, 18 of the 19 stents remained in place at 6 mo follow-up period although there were four reported episodes of stent occlusion.
While promising, LAMS may not, in fact be required to achieve EUS-guided choledochoduodenostomy as traditional metal stents have shown similarly impressive results. Furthermore, use of LAMS typically requires a larger diameter bile duct and may increase the risk for sump syndrome after placement. We recommend the use of a pigtail stent through the LAMS to decrease this risk of sump syndrome post-choledochoduodenostomy (Table 1). Therefore, these authors typically reserve the use of LAMS for specific individuals that may be less optimal candidates for traditional metal stent placement.
ACCESS TO THE REMNANT STOMACH AND POSTBARIATRIC SURGICAL ANATOMY
Given the prevalence of obesity and subsequent bariatric surgery in the United States and worldwide, gastroenterologists may increasingly encounter difficult pancreaticobiliary conditions in patients with surgically altered anatomy. Rapid weight loss following bariatric surgery is associated with a higher incidence of cholelithiasis and risk for choledocholithiasis among patients with their gallbladders in situ. In patients with Roux-en-Y gastric bypass (RYGB) anatomy, access to the biliary and pancreatic tractviaERCP is not easily achieved with need to advance the endoscope distally from the gastric pouch to the jejunojejunostomy and then retrograde through the biliopancreatic limb to access the biliary system. While enteroscopy- and laparoscopy-assisted ERCP may provide alternative treatment options to access the pancreaticobiliary system, gastric access temporary for endoscopy (GATE) is a minimally invasive alternative. This procedure utilizes the unique LAMS design with large intraluminal diameter to create a fistulous tract and a working channel between the gastric pouch and remnant stomach.
Although multiple other descriptions or terminology have been proposed to describe this procedure [endoscopic ultrasound-guided transgastric fistula (EUS-TG),endoscopic ultrasound-guided gastro-gastric-endoscopic cholangiopancreatography(EUS-GG-ERCP), EUS-directed transgastric ERCP (EGDE), or EUS-directed transgastric intervention (EDGI)], these authors will use the common term GATE as this can be applied to procedures other than ERCP. The procedure entails EUS-guided access to the excluded stomach using the large diameter of the LAMS as a working channel-allowing access for not just ERCP but other procedures such as EUS,endoscopic mucosal resection, and endoscopic submucosal dissection of the foregut[30].
Using an echoendoscope, the excluded stomach is located to create a gastro-gastric fistula. We suggest accessing the remnant or excluded stomach from the proximal pouch to create a favorable angle and minimize chances of stent dislodgement when advancing the endoscope through the LAMS and pylorus. The LAMS is deployed under fluoroscopic and endosonographic guidance with the distal flange in the excluded stomach and the proximal flange in the gastric pouch. The lumen of the stent is then dilated up to the diameter of the stent lumen, thereby allowing for easy passage of any wider endoscope to access the remnant stomach to complete the desired procedure (Figure 4). It is important to avoid penetration of the diaphragm to minimize patient discomfort as well as avoidance of the gastric staple line to reduce the risk of a persistent gastro-gastric fistula (Table 1). Early LAMS removal with placement of a double pigtail stent to maintain the tract may also help to minimize subsequent gastro-gastric fistula. It may also be possible to create a fistulous tract from the jejunum to the remnant stomach; however, creation of a gastro-gastric connection is generally recommended when possible as a transjejunal approach may carry a higher risk of LAMS dislodgement.
Kedia and colleagues first performed this procedure in a single stage using a LAMS to create a gastro-gastric fistula in a patient with a history of RYGB[31]. Since the first successful report of this technique, there have not yet been large scale studies to date.However, a few case series have been published which have demonstrated the efficacy of the GATE procedure. In a multi-center case series of 13 patients using LAMS to create an EUS-guided gastro-gastric fistula to facilitate per-oral ERCP,technical success was reported to be 100% with LAMS dislodgement noted in 2 patients[32]. One retrospective study by these authors reported duodenal endoscopic submucosal dissection and sutured defect closure after performing GATE in a patient with RYGB[33]. In another study by authors of this review, 10 patients underwent the GATE procedure using a novel algorithmic approach using gastric and jejunal access points for LAMS deployment[30]. This study demonstrated a clinical and technical success rate of 100% with GATE and concluded that it was a safe and effective procedure to be considered as the preferred approach to ERCP in patients with RYGB anatomy at centers with LAMS experience. A comparator study was also performed by Bukhariet al[34]to compare GATE with ERCP versus enteroscopy-assisted ERCP.Technical success was significantly higher in the GATE group versus enteroscopyassisted group (100%vs60%;P< 0.001) with decreased total procedure time (49.8 minvs90.7 min;P< 0.001) and length of hospital stay (1 dvs10.5 d;P= 0.02) and no difference in adverse events (10%vs6.7%P= 1.0). A more recent study by Kedia and colleagues, sought to compare outcomes of GATE versus laparoscopy-assisted ERCP and found no difference in technical or clinical success, as well as adverse events;however, noted that GATE was associated with significantly shorter procedure times and length of hospital stay[35]. In 2018, a case report of successful drainage of a large,isolated fluid collection in the gastric remnant was described by Schulman and Thompson[36]. In this case, a GATE procedure was first performed followed by placement of a second LAMS to reconstitute dependent flow from the remnant stomach to the jejunum.
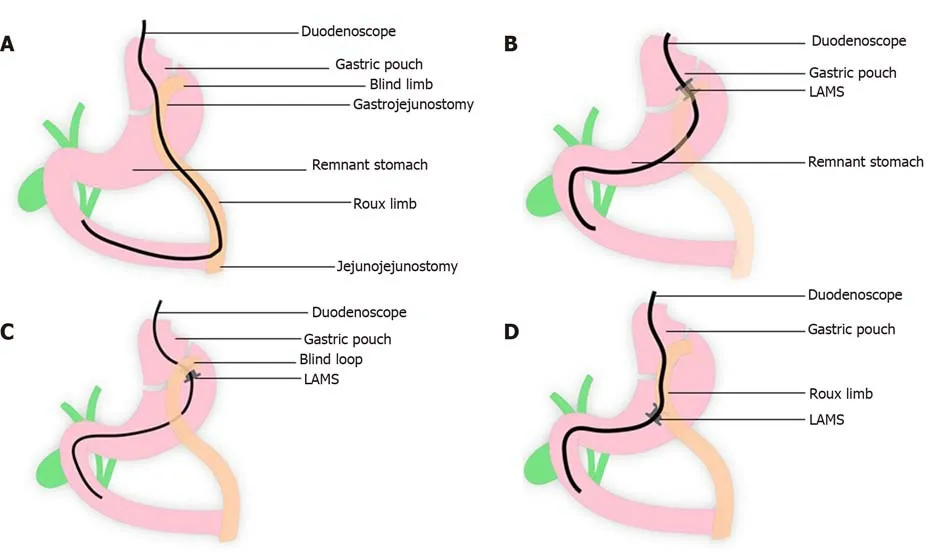
Figure 4 The lumen of the stent is dilated up to the diameter of the stent lumen, thereby allowing for easy passage of any wider endoscope to access the remnant stomach to complete the desired procedure. A: Normal Roux-en-Y gastric bypass (RYGB) anatomy showing long endoscopic route to be traversed to access the biliary system; B: Lumen apposing metal stent (LAMS) placement between gastric pouch and remnant stomach in RYGB anatomy; C: LAMS placement between blind limb and remnant stomach in RYGB anatomy; D: LAMS placement between Roux Limb and remnant stomach in RYGB anatomy. LAMS: Lumen apposing metal stent.
These results are no doubt promising; however, similar to EUS-guided gallbladder drainage, LAMS use for GATE is limited to centers with expertise. Furthermore, it is important to understand that the two potential ramifications of this procedure are the potential for weight regain due to creation of a gastro-gastric fistula, as well as the potential for stent migration. However, despite these concerns, this procedure improves access to the remnant stomach, provides the ability to perform the procedure in a single session, and is a minimally invasive alternative to traditional laparoscopic-assisted techniques.
MANAGEMENT OF GASTRIC OUTLET OBSTRUCTION
Traditionally, surgical gastrojejunostomy has been the primary treatment for both benign and malignant gastric outlet obstruction despite the procedure being associated with a high complication rate approaching nearly 40%[37,38]. Given this significant adverse event profile, enteral stenting has been widely utilized, though stent occlusion and migration have also resulted in an increased need for repeat intervention[39]. As such, EUS-guided gastroenterostomy (EUS-GE) has emerged as an attractive procedure to treat patients with gastric outlet obstruction as an alternative to surgery (Figure 5)[40,41].
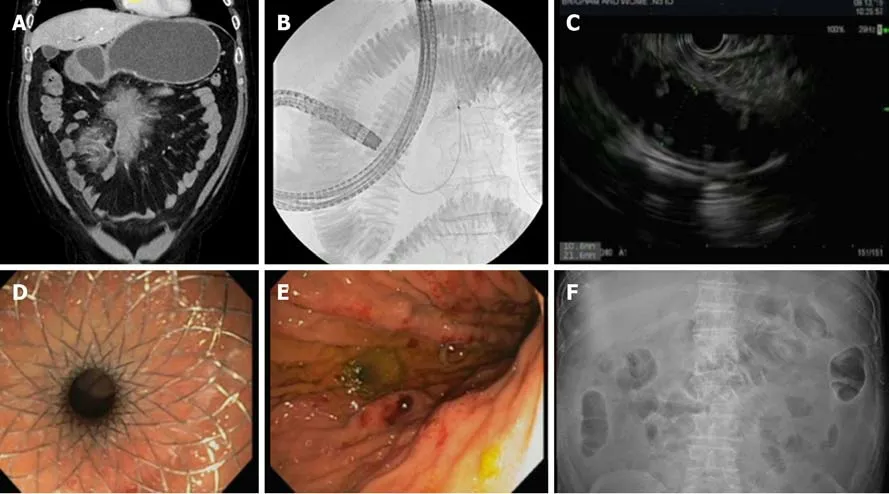
Figure 5 An attractive procedure to treat patients with gastric outlet obstruction as an alternative to surgery. A: Initial computed tomography demonstrating gastric outlet obstruction; B: Fluoroscopy with duodenal stenosis and distal filling with contrast diluted in sterile water; C: Endoscopic ultrasound-guided gastroenterostomy demonstrating filling of distal bowel; D: Successful placement of lumen apposing metal stent (LAMS); E: Endoscopic image of gastroenterostomy placement with LAMS; F: Follow-up radiograph demonstrating successful LAMS placement to achieve gastroenterostomy.
This EUS-GE procedure first requires precise location of the small bowel distal to the gastric outlet obstruction (either distal duodenum or jejunum) endosonographically from the gastric antrum or body. Typically, a guidewire is passed beyond the area of obstruction under direct fluoroscopic guidance as well as the use of a catheter to fill the distal small bowel with a mixture of contrast and sterile water. Then using fluoroscopy and EUS-guidance, the bi-flanged LAMS is placed from the stomach to the small bowel - thus creating a newly formed fistulous tract and thereby bypassing the point of gastric outlet obstruction. The positioning of the patient in a prone/swimmer’s position and use of fluoroscopy is essential. Distention of the bowel with dilute contrast with or without methylene blue and use of water (not saline), and use of glucagon to decrease motility of the bowel is also key. A freehand technique may also be adopted as placement of a wire may push small bowel away from the stomach (Table 1).
Technical feasibility of EUS-GE was first demonstrated by Binmoeller and colleagues using 5 porcine models in 2012[42]. Since that initial animal study,translation to humans has been achieved with more widespread adoption of the EUSGE procedure. The first report of LAMS associated gastroenterostomy was performed by Ikeuchiet al[43]in 2015. Subsequent literature with variable techniques has suggested EUS-GE is safe and effective for the treatment of gastric outlet obstruction.Kashabet al[44]reported outcomes for 10 patients with gastric outlet obstruction. This study showed technical success rate of 90% with no associated adverse events. A similarly high technical and clinical success rate was observed in a multi-center study of 26 patients by Tyberget al[45]at 92% and 85% respectively. Itoi and colleagues also demonstrated similarly impressive results and demonstrated the efficacy of EUS-GE predominantly as palliative treatment of malignant gastric outlet obstruction[46]. In a patient with both biliary and duodenal obstruction, a case report by Abidi and Thompson described successful choledochoduodenostomy and gastrojejunostomy with LAMS as well. In this case, initial ERCP and EUS-guided rendezvous were unsuccessful, prompting placement of a LAMS to create a choledochoduodenostomy and subsequent electrocautery-enhanced LAMS without an initial needle or wire access to create an endoscopic gastrojejunostomy to relieve biliary and duodenal obstruction in an 84-year-old gentleman[47].
A recent meta-analysis including benign and malignant gastric outlet obstructions by the authors of this review showed a technical success rate of 92.90% and clinical success of 90.11%[41]. More importantly, serious adverse events occurred in 5.61% of cases with a reintervention rate of 11.43%. In a study by Manuel Perez-Mirandaet al[48]comparing surgical and EUS-guided strategies, technical success was not different between an EUS-GE cohort and patients undergoing laparoscopic gastrojejunostomy(88%vs100%,P= 0.11); however, EUS-GE was associated with a significantly lower rate of adverse events (12%vs41%,P= 0.0386). Another study by the authors of this review examined EUS-GE versus enteral stenting and found EUS-GE was associated higher rate of initial clinical success (95.8%vs76.3%;P= 0.042) and a lower rate of stent failure requiring repeat intervention (8.3%vs32.0%;P= 0.021)[49]. Not only does the current literature support the feasibility, efficacy, and safety of EUS-GE as a treatment for gastric outlet obstruction, but it also appears to suggest EUS-GE may be a preferred treatment strategy compared to surgical gastrojejunostomy and enteral stenting.
TREATMENT OF BENIGN GASTROINTESTINAL STRICTURES
Current management options for benign gastrointestinal strictures include endoscopic balloon dilation, incisional treatment, steroid injection, and self-expandable metal stents (SEMS)[50-54]. These endoscopic management techniques are effective but limited by recurrence rates requiring repeated interventions[55]. Additionally, due to the biflanged design, LAMS have a lower risk of stent migration compared to fully-covered SEMS[56,57]. When performing the procedure, it is recommended to first traverse the entire length of the stricture with a smaller diameter endoscope (if possible) to ensure that the one cm length of the LAMS is adequate. Use of a guidewire is also important to prevent trauma and reduce the risk of perforation as the LAMS deployment catheter is relatively rigid and may navigate tortuous downstream bowel without wire guidance (Table 1).
Current literature has demonstrated LAMS use for the management of multiple types of strictures including refractory esophageal strictures, pyloric stenoses, and gastrojejunostomy stricturing after RYGB[58]. Successful placement of LAMS for esophageal or gastric strictures as well as small bowel and colonic stenoses have been reported in numerous case series to date[59-66]. A retrospective study by the authors of this review demonstrated that non-electrocautery enhanced LAMS could be an effective treatment for RYGB patients with persistent gastrojejunal anastomosis stenosis[67]. This study examined 18 patients with a technical success rate of 100% and clinic success reported in 94% of patients with 6 patients developing adverse events.In another multi-center study, a total of 49 patients underwent 56 LAMS procedures with a technical success rate of 100% and clinical success rate of 96.4%[68]. Despite these impressive results, stent migration occurred in 17.9% of procedures, notably more likely to occur for strictures in the lower gastrointestinal tract. Although LAMS were well-tolerated by patients in this study, symptom recurrence at the time of removal was also common. In a systematic review of 8 studies (n= 192 patients)evaluating LAMS for benign gastrointestinal strictures, LAMS demonstrated statistically better outcomes in regards to stent migration and post-procedure pain when compared with fully-covered SEMS and biodegradable stents. In our practice,we typically recommend removal of LAMS after approximately 3 mo with determination at that time whether a second LAMS placement is needed based upon response.
DRAINAGE OF POST-SURGICAL FLUID COLLECTIONS AND PELVIC ABSCESSES
Post-surgical fluid collections are a common cause of morbidity and mortality in postoperative patients[69-71]. Common causes of collections may include peripancreatic fluid collections after pancreatic surgery, bile leaks post-cholecystectomy, and pelvic fluid collections or abscesses after low anterior resection, colectomy, appendectomy, and gynecologic surgeries[72]. Though these collections can be drained surgically, for over a decade the mainstay of management has been percutaneous drainage under radiologic guidance. A percutaneous approach has classically been preferred due to lower mortality and morbidity compared to surgical drainage and has a lower associated procedure cost. However, percutaneous drainage requires external percutaneous drain placement that may remainin situfor weeks to months and may be associated with an increased risk of fluid and electrolyte loss, catheter or drain dislodgement, as well as formation of a cutaneous fistulae[69,70].
More recently, with the advent of EUS-guided drainage and the innovation of LAMS, there has been a transition in the management of post-surgical fluid collections from a percutaneous to endoscopic-guided approach. The advantage of EUS-guided transmural drainage lies in the ability to internally drain the fluid - minimizing the risk of infection as well as fluid and electrolyte derangement associated with percutaneous drainage. This has been further associated with higher clinical success rates, lower costs, and an overall improvement in the quality of life[73,74]. Favorable locations for endoscopic drainage with LAMS and avoidance of percutaneous or surgical drainage include collections that are located adjacent to the stomach,duodenum or rectum (Table 1). Prior case series have also demonstrated successful drainage of distal pancreatectomy-related collections as well as pelvic abscesses using EUS-guided placement of plastic stents[71,75,76]. However, the literature on the use of LAMS for drainage of post-surgical fluid collection was lacking until 2017 when Mudireddyet al[72]published the first study on the role of LAMS for this purpose. In this retrospective study, they reported technical and clinical success rates of 93.6%and 89.3%, respectively. Further studies on the use of LAMS for this purpose are still scarce. In a recent multi-center study evaluating the safety and efficacy of the use of LAMS in the management of postsurgical fluid collections, technical success rate was 96.8% and clinical success rate was 91.9% with no procedure-related mortality but intraoperative adverse events of 1.6% and postoperative adverse events of 11.3%[77]. To date, there is a paucity of literature available regarding EUS-guided LAMS drainage of post-surgical fluid collections and pelvic abscesses, though available literature suggests a promising role for LAMS.
CONCLUSION
In conclusion, LAMS have revolutionized the role of interventional endoscopists in the management of a variety of complex conditions. These expanded indications include not only treatment of pancreatic fluid collections, but also broader applications to treat acute cholecystitis, distal malignant biliary obstruction, postbariatric surgery complications, benign gastrointestinal strictures, gastric outlet obstruction, and intra-abdominal, pelvic, and post-surgical fluid collections. Current literature suggests the use of these novel stents may significantly improve treatment response and provide a much needed minimally invasive therapeutic option to patients in need. We have provided a comprehensive literature review of current LAMS indications as well as clinical pearls to achieve a successful placement. We anticipate that the next decade will see expanded applications for LAMS providing minimally invasive treatment options for more patients.
ACKNOWLEDGEMENTS
All authors approved the final version of the manuscript.
杂志排行
World Journal of Gastroenterology的其它文章
- Tailored classification of portal vein thrombosis for liver transplantation: Focus on strategies for portal vein inflow reconstruction
- lnnate immune recognition and modulation in hepatitis D virus infection
- Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine
- Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signalregulated kinase phosphorylation
- Periportal thickening on magnetic resonance imaging for hepatic fibrosis in infantile cholestasis
- Cost of postoperative complications: How to avoid calculation errors
