肥胖对OSAHS患者日间嗜睡的影响
2020-06-08郑艳文邬海燕胡立红姜贻乾
郑艳文 邬海燕 胡立红 姜贻乾
【摘要】 目的:探讨肥胖与阻塞性睡眠呼吸暂停低通气综合征(OSAHS)患者日间嗜睡关系及对OSAHS患者影响。方法:经多导睡眠图(PSG)及体重指数(BMI)测评,分为OSAHS伴肥胖组40例,OSAHS不伴肥胖组38例,单纯肥胖组42例,测定三组体重指数(BMI)、呼吸暂停低通气指数(AHI)、夜间最低血氧饱和度、爱泼沃斯嗜睡量表(ESS)评分、血清瘦素(LEP)水平并进行对比,分析ESS评分与AHI及LEP水平相关性。结果:OSAHS伴肥胖组ESS评分、LEP水平均高于OSAHS不伴肥胖组及单纯肥胖组,差异均有统计学意义(P<0.05);OSAHS不伴肥胖组ESS评分高于单纯肥胖组,差异有统计学意义(P<0.05);OSAHS伴肥胖组ESS评分与LEP水平呈明显正相关性,差异有统计学意义(P<0.05)。结论:OSAHS伴肥胖患者日间嗜睡症状与肥胖有关,而日间嗜睡发病机制可能与LEP水平变化有关。
【关键词】 阻塞性睡眠呼吸暂停低通气综合征 肥胖 嗜睡 瘦素
[Abstract] Objective: To investigate the relationship between obesity and daytime sleepiness in patients with OSAHS and its effects on patients with OSAHS. Method: After polysomnography (PSG) and body mass index (BMI) evaluation, 40 cases in the OSAHS with obesity group, 38 cases in the OSAHS without obesity group, and 42 cases in the simple obesity group. Body mass indexes (BMI), apnea hypopnea indexes (AHI), nighttime minimum oxygen saturation, Epworth sleepiness scale (ESS) scores and serum leptin (LEP) levels were measured and compared among the three groups, and the correlation between ESS score and AHI and LEP levels was analyzed. Result: The ESS score and LEP level of the OSAHS with obesity group were higher than those of the OSAHS without obesity group and the simple obesity group, and the differences were statistically significant (P<0.05). The ESS score of the OSAHS without obesity group was higher than that of the simple obesity group, and the difference was statistically significant (P<0.05). There was a significant positive correlation between ESS score and LEP level in the OSAHS with obesity group, and the difference was statistically significant (P<0.05). Conclusion: The symptoms of daytime sleepiness in patients with OSAHS and obesity are related to obesity, and the pathogenesis of daytime sleepiness may be related to LEP level changes.
阻塞性睡眠呼吸暂停低通气综合征(obstructive sleep apnea hypopnea syndrome,OSAHS)是以在睡眠过程中反复发生呼吸暂停、低通气及微觉醒为特征的疾病[1]。OSAHS是睡眠障碍中最常見疾病[2]。肥胖为OSAHS患者首要危险因素[3]。日间嗜睡是OSAHS患者常见的临床表现之一,16%~22%的OSAHS患者存在日间嗜睡[4]。研究显示,OSAHS患者日间嗜睡与夜间缺氧程度及AHI相关,且患者BMI与AHI呈正相关[5]。但目前关于肥胖与日间嗜睡的独立关系的研究不多,且机制不清。肥胖程度与血清中瘦素浓度变化密切相关[6]。本研究通过对比是否伴肥胖的OSAHS患者日间嗜睡情况及血清瘦素水平,进一步探讨肥胖与OSAHS患者日间嗜睡关系及可能影响机制。
1 资料与方法
1.1 一般资料
研究对象均来自杭州市萧山区第一人民医院门诊、住院或健康体检中心,研究时间为2017年9月-2019年7月。OSAHS诊断标准:夜间7 h睡眠中呼吸暂停及低通气反复发作>30次或AHI≥5次/h,且以阻塞性呼吸暂停为主[7]。中国单纯性肥胖诊断标准:超重为BMI 25~26 kg/m2,肥胖为BMI>26 kg/m2。排除标准:心血管疾病、脑部疾病、老年痴呆、智力障碍、未控制的糖尿病、恶性肿瘤、甲状腺疾病、维生素缺乏、慢性阻塞性肺疾病、间质性肺疾病等。经多导睡眠图(PSG)及体重指数(BMI)测评,分为OSAHS伴肥胖组40例,OSAHS不伴肥胖组38例,单纯肥胖组42例。研究经医院伦理委员会同意,患者均签订知情同意书。
1.2 方法
嘱患者晚8点后禁饮食、饮水,次日清晨7点于卧位、空腹状态下抽取静脉血15 ml,置于试管内并加入EDTA抗凝,30 min内于2 ℃~8 ℃条件下以1 000 g离心15 min,取上清液置于-20 ℃冰箱中保存。采用化学发光酶免疫分析法测定血清瘦素(LEP)水平,试剂盒由上海康朗生物科技有限公司提供,所有标本均严格按照试剂盒说明书操作。
PSG监测:采用全数字多导睡眠监测系统(美国安波澜Embla N7000)对患者进行夜间连续7 h以上的监测。
1.3 观察指标及评价标准
(1)对比三组临床情况,包括一般情况(年龄、BMI)、AHI、ESS评分、最低血氧饱和度及LEP水平。AHI分级:轻度5~15次/h,中度15~30次/h,重度>30次/h。嗜睡程度采用ESS量表进行评估:询问患者过去2周中8种不同日间活动情景及打瞌睡或可能睡着的程度,总分0~24分,分数越高,嗜睡程度越重。(2)分析ESS评分与AHI及LEP水平相关性。
1.4 统计学处理
数据采用SPSS 23.0软件进行统计学分析,呈正态分布的计量资料以(x±s)表示,两组间比较采用t检验,多组间比较采用F检验,ESS评分与AHI及LEP水平相关性采用Pearson相关性分析,P<0.05为差异有统计学意义。
2 结果
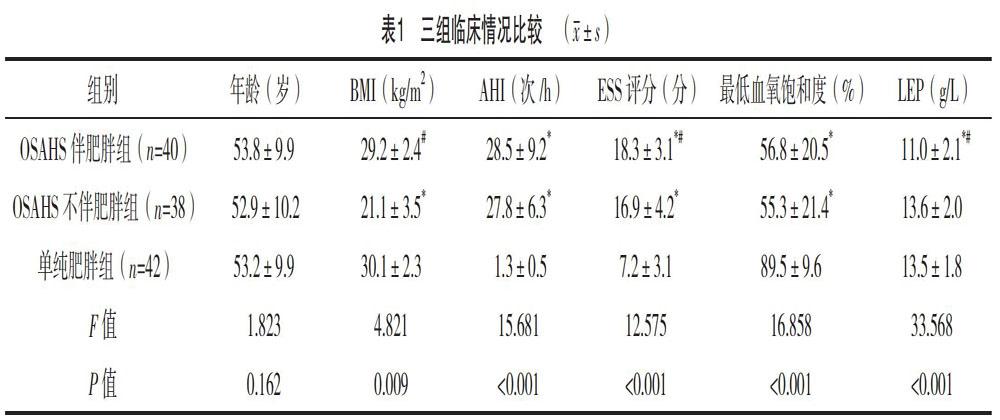
2.1 三组临床情况比较
三组年龄比较,差异无统计学意义(P>0.05);OSAHS伴肥胖组与OSAHS不伴肥胖组AHI、最低血氧饱和度比较,差异无统计学意义(P>0.05);OSAHS伴肥胖组与单纯肥胖组BMI比较,差异无统计学意义(P>0.05);OSAHS伴肥胖组ESS评分、LEP水平均高于OSAHS不伴肥胖组及单纯肥胖组,差异均有统计学意义(P<0.05);OSAHS不伴肥胖组ESS评分高于单纯肥胖组,差异有统计学意义(P<0.05),见表1。
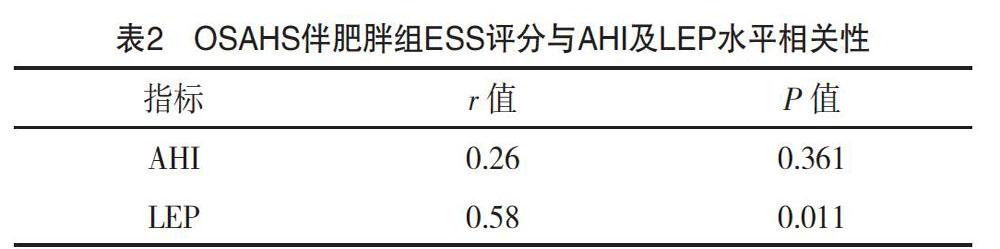
2.2 OSAHS伴肥胖组ESS评分与AHI及LEP水平相关性
ESS评分与LEP水平呈正相关性,差异有统计学意义(P<0.05);ESS评分与AHI无明显相关性,差异无统计学意义(P>0.05),见表2。
3 讨论
OSAHS是由間歇低氧导致的氧化应激和炎症反应,可对机体造成严重损伤[8]。由于肥胖患者上气道狭窄程度较严重,腹部及胸廓脂肪堆积,纵向气管牵张力及咽壁张力减弱,胸壁顺应性下降,致使肺容量进一步下降,加重阻塞程度[9]。目前研究认为,睡眠片段化及低氧血症与OSAHS患者日间嗜睡有一定关系[10]。瘦素是脂肪组织和中枢神经系统间网络联系的外周信号,可通过血-脑屏障与下丘脑的特异性受体结合,并作用于下丘脑弓状核,使摄食相关神经肽合成与分泌减少,从而降低食欲,增加能量消耗,加速脂肪分解,抑制脂肪合成,是预测肥胖敏感指标。研究显示,肥胖程度及间歇性缺氧时间等与血清中瘦素(Leptin)浓度密切相关[6]。肥胖伴OSAHS患者体内的血清瘦素水平比非肥胖伴OSAHS患者明显偏高[11]。
既往研究显示,OSAHS严重程度与日间嗜睡有明显相关性[12]。本研究显示,OSAHS伴肥胖患者日间嗜睡症状较OSAHS不伴肥胖及单纯肥胖患者明显,同时LEP水平明显升高;此外,相关分析显示,LEP水平与ESS评分呈明显正相关。说明肥胖为OSAHS患者日间嗜睡的影响因素。可能机制:(1)肥胖患者尤其颈部肥胖患者可增加低氧血症发生风险,引起睡眠-唤醒过程中交感神经过度兴奋,导致夜间深睡眠不足,日间嗜睡加重。(2)OSAHS伴肥胖患者体内存在瘦素抵抗,可导致中央呼吸控制机制的缺陷及周围和中央化学感受器的不良反应,导致呼吸节律发生紊乱,影响机体氧合状态,从而造成白天嗜睡[13]。Lecube等[14]描述,胰岛素抵抗及瘦素抵抗可产生更严重的睡眠呼吸暂停症状及更高的睡眠呼吸暂停低通气指数,从而导致白天嗜睡。(3)其他因素,如代谢异常、炎症反应、氧化应激引起睡眠片段化等[15-16]。此外,肥胖可加重OSAHS患者白天嗜睡程度。
本研究发现,瘦素水平与日间嗜睡程度呈正相关,说明肥胖为OSAHS患者日间嗜睡的相关因素。但本试验样本数量未能达到大样本数量要求,且未考虑缺氧程度的影响作用,因此日后还需进一步实施相关试验。
参考文献
[1]中国医师协会睡眠医学专业委员会.成人阻塞性睡眠呼吸暂停多学科诊疗指南[J].中华医学杂志,2018,98(24):1902-1914.
[2] Peromaa-Haavisto P,Tuomilehto H,K?ssi J,et al.Obstructive sleep apnea:the effect of bariatric surgery after 12 months.A prospective multicenter trial[J].Sleep Medicine,2017,7(35):85-90.
[3] Carneiro G,Zanella M T.Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events[J].Metabolism,2018,7(84):76-84.
[4] Kapur V K,Baldwin C M,Resnick H E,et al.Sleepiness in patients with moderate to severe sleep-disordered breathing[J].Sleep,2005,28(4):472-477.
[5] Magne F,Gomez E,Marchal O,et al.Evolution and predictive factors of improvement of obstructive sleep apnea in an obese population after bariatric surgery[J].Journal of Clinical Sleep Medicine,2019,15(10):1509-1516.
[6] Pamuk A E,Süslü A E,Yalcinkaya A,et al.The serum leptin level in non-obese patients with obstructive sleep apnea[J].Auris Nasus Larynx,2018,45(4):796-800.
[7]中华医学会呼吸病学分会睡眠呼吸障碍学组.阻塞性睡眠呼吸暂停低通气综合征诊治指南(2011年修订版)[J].中华结核和呼吸杂志,2012,35(1):19-22.
[8]郑艳文,钦光跃,张颖,等.经鼻气道持续正压通气对OSAHS伴夜尿增多患者肾损伤影响作用[J].实用医学杂志,2018,34(24):4120-4122.
[9] Jeong J I,Gu S,Cho J,et al.Impact of gender and sleep position on relationships between anthropometric parameters and obstructive sleep apnea syndrome[J].Sleep Breath,2017,21(2):535-541.
[10] Kainulainen S,T?yr?s J,Oksenberg A,et al.Severity of desaturations reflects OSA-related daytime sleepiness better than AHI[J].Journal of Clinical Sleep Medicine,2019,15(8):1135-1142.
[11] Imayama I,Prasad B.Role of leptin in obstructive sleep apnea[J].Annals of the American Thoracic Society,2017,14(11):1607-1621.
[12] Iannella G,Vicini C,Colizza A,et al.Aging effect on sleepiness and apneas severity in patients with obstructive sleep apnea syndrome:a meta-analysis study[J].European Archives of Oto-Rhino-Laryngology,2019,276(12):3549-3556.
[13] Bassi M,Furuya W I,Zoccal D B,et al.Control of respiratory and cardiovascular functions by leptin[J].Life Sciences,2015,125(15):25-31.
[14] Lecube A,Romero O,Sampol G,et al.Sleep biosignature of type 2 diabetes: a case-control study[J].Diabetic Medicine,2017,34(1):79-85.
[15] Stolarczyk E.Adipose tissue inflammation in obesity:a metabolic or immune response?[J].Current Opinion in Pharmacology,2017,37(11):35-40.
[16] Bingol Z,Karaayvaz E B,Telci A,et al.Leptin and adiponectin levels in obstructive sleep apnea phenotypes[J].Biomarkers in Medicine,2019,13(10):865-874.
(收稿日期:2019-12-25) (本文編辑:李盈)
