烟草青枯病抗性突变体486-K和117-K的遗传分析
2020-05-25邹文莉牛文利杨华应巫升鑫周应兵余文张兴伟吴新儒丁安明代常波刘贯山孙玉合王卫锋
邹文莉 牛文利 杨华应 巫升鑫 周应兵 余文 张兴伟 吴新儒 丁安明 代常波 刘贯山 孙玉合 王卫锋
摘 要:烟草青枯病是一种典型的维管束细菌性病害,严重影响我国烟叶生产。为了解烟草青枯病抗性突变体的遗传规律和开发抗性相关分子标记,本研究选用EMS诱变烤烟品种翠碧一號获得的烟草青枯病抗性突变体486-K和117-K为研究对象,以翠碧一号和2个突变体为亲本,构建了两个不同的杂交组合,采用卡方检验和植物数量性状“主基因+多基因”混合遗传模型分析方法,进行群体遗传效应分析。结果表明,卡方检验显示突变体486-K和117-K的F2代各病级株数呈正态分布,存在一定性状分离。“主基因+多基因”混合模型分析发现突变体117-K的最优抗性遗传模型为2MG-A,即2对主基因为加性效应控制遗传,无显性效应和上位性效应,主基因的遗传效率为78.57%;突变体486-K的最优抗性遗传模型为2MG-ADI,即2对加性-显性-上位性主基因模型,上位性效应中以显性×显性互作和显性×加性互作效应较大,主基因遗传效率为88.34%。表明烟草青枯病抗性突变体的遗传方式以主基因效应为主,受环境影响较小。
关键词:烟草;青枯病;突变体;主基因+多基因;遗传分析
Genetic Analysis of Bacterial Wilt Resistance Mutants 486-K and 117-K
in Tobacco
ZOU Wenli1,2, NIU Wenli1,2, YANG Huaying3, WU Shengxin4, ZHOU Yingbing3, YU Wen4, ZHANG Xingwei1, WU Xinru1, DING Anming1, DAI Changbo1, LIU Guanshan1, SUN Yuhe1*, WANG Weifeng1*
(1. Tobacco Research Institute, Chinese Academy of Agricultural Sciences, Qingdao 266101, China; 2. Graduate School of Chinese Academy of Agricultural Sciences, Beijing 100081, China; 3. Tobacco Research Institute, Anhui Academy of Agricultural Sciences, Hefei 230001, China; 4. Tobacco Science Research Institute, Fujian Tobacco Monopoly Administration, Fuzhou 350024, China)
Abstract: Tobacco bacterial wilt is a typical vascular bundle bacterial disease that seriously affects tobacco production in China. In order to understand the genetic basis of tobacco bacterial wilt resistance mutants and develop molecular markers related to resistance, and to provide theoretical basis for breeding high quality resistant varieties, in this study, tobacco bacterial wilt resistance mutants 486-K and 117-K obtained by EMS mutagenesis of flue-cured tobacco variety Cuibi 1 were selected as research objects, and Cubi 1 and the two mutants were used as parents to construct two different hybrid combinations. The analysis method of population genetic effect was carried out by using the analysis method of Chi-square test and "major-gene + polygene" mixed genetic model of plant quantitative traits. The results showed that, based on the Chi-square test, the number of disease-level strains of F2 generation of mutants 486-K and 117-K showed a normal distribution, and showed some trait separation. Analysis of the "major-gene + polygene" mixed model showed that 2MG-A was the optimal genetic model for resistance in mutant 117-K, with two pairs of major genes showing additive effects without dominant or epistatic effect, and the genetic efficiency of major genes being 78.57%. The optimal resistance genetic model for mutant 486-K was 2MG-ADI, or the two pairs of additive-dominant-epigenetic master gene model. Among the epistatic effects, the dominant × dominant interaction and dominant × additive interaction effects were larger, and the genetic efficiency of the main genes was 88.34%. It was indicated that the inheritance of tobacco bacterial wilt resistance mutants was dominated by main gene effect and less affected by the environment.
Keywords: tobacco; bacterial wilt; mutant; major-gene+polygene; genetic analysis
烟草青枯病作为一种细菌性病害,是由青枯雷尔式菌(Ralstonia solanancearum)引起的土传病害,在烟草苗期和大田期均可发生。该菌寄主范围广,可侵染54个科近450余种作物,如花生、番茄、辣椒等,严重影响了许多重要经济作物的生长,对农业生产造成严重经济损失[1-2]。在我国,烟草青枯病主要发生在长江以南地区,近年来逐步向北方地区蔓延。目前烟草青枯病的防治方法有农业防治、化学防治、生物防治等,这些方法仍存在较多问题,不能有效抑制青枯病[3-5]。培育抗病品種是防治青枯病最根本、有效的途径。而培育抗病品种,首先需要选择高抗青枯病的材料作抗源,同时掌握抗性基因的遗传规律。
近年来关于烟草青枯病抗性遗传的研究较多,但在研究材料、环境、方法等方面存在差异,因此青枯病的抗性遗传机理还没有统一的定论。高加明等[6]对香料烟品种Xanthi进行青枯病抗性遗传分析,发现该品种的抗性是受2对加性-显性-上位主基因+加性-显性多基因控制;而MATSUDA等[7]认为Xanthi中存在的抗性是受到局部显性基因Rxa影响。QIAN等[8]根据次要基因座对青枯病发生率和指数的累加效应,认为云烟85、DB101等品种的抗性以加性效应遗传为主。张振臣等[9-10]利用“主基因+多基因”混合遗传模型联合分析方法对广东地方晒烟品种GDSY-1和“大叶密合”进行遗传分析,发现GDSY-1抗性遗传的显性程度高,符合2对加性-显性-上位性主基因+加性-显性-上位性多基因模型,主基因遗传率高;而另一晒烟品种“大叶密合”为部分隐性遗传,符合2对加性-显性-上位性主基因+加性-显性多基因模型。由于前人研究发现不同品种的抗性遗传规律存在较大差异,不利于抗性品种的选育,因此烟草青枯病的优质抗源有待深入研究和挖掘。
本研究选取的烟草青枯病抗性突变体486-K和117-K在田间表现为抗性较高,可探究其抗性基因的遗传规律。利用翠碧一号×突变体为亲本(组合),构建F1和F2群体,根据各世代在田间的抗病性表现进行抗性基因的遗传规律分析,为培育烟草抗青枯病新品种提供试验基础。
1 材料与方法
1.1 试验材料
甲基磺酸乙酯(EMS)诱变处理烤烟品种翠碧一号(CB)获得的抗青枯病突变体:486-K、117-K(中国农业科学院烟草所突变体库提供)。
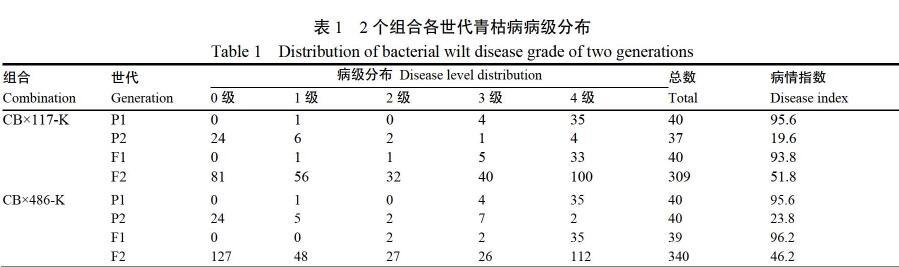
以烤烟品种翠碧一号为母本P1,突变体486-K和117-K为父本P2,形成CB×486-K和CB×117-K组合,杂交获得F1代,F1代自交收种获得F2群体,最终形成P1、P2、F1、F2世代。
1.2 试验设计
试验于2018年在安徽省宣城市寒亭镇(N30°55′3.12″,E118°32′58.29″)进行。田间种植行距为1.2 m,株距为0.5 m。P1、P2、F1代采用随机区组设计,3次重复,每次重复分别种植15株。F2代不设置重复,CB×117-K F2代调查植株309株,CB×486-K F2代调查植株340株。
1.3 病情调查
按照烟草病害分级及调查方法YC/T 39—1996行业标准规定的调查方法,以株为单位详细调查发病情况,并计算病情指数。
病情指数=[Σ(各级病株或叶数×该病级值)/(调查总株数或叶数×最高级值)]×100
1.4 数据分析
采用Microsoft Excel和SPSS 20.0软件进行数据处理与统计分析。采用卡方检验和植物数量性状“主基因+多基因”混合遗传模型分析方法[11-12]进行遗传分析。根据本研究中的田间烟草青枯病抗性调查数据,计算2个组合的极大似然函数值和AIC(Akaike's Information Criterion)值。通过IECM算法[12-13]得到5类24种模型,依据AIC值最小的准则选择最佳候选模型,同时进行5个统计量、、、Smirnov(nW2)和Kolmogorov(Dn)适合性检验,根据检验结果选择最优遗传模型。采用最小二乘法从最优遗传模型的各成分分布参数估计各基因效应值,分析各组合的遗传效应。
2 结 果
2.1 病圃青枯病发病情况统计
2个组合各世代青枯病单株病级分布情况如表1,2个组合中P2代的病级大多集中在0级和1级,其中CB×117-K P2代的病情指数为19.6,CB×486-K P2代的病情指数为23.8,均表现为抗病。P1代的病级分布主要集中在3级和4级,2个组合中P1代的病情指数均为95.6,表现为高感青枯病。CB×117-K F1和CB×486-K F1的病情指数分别为93.8和96.2,均接近P1代的病情指数,且无0级
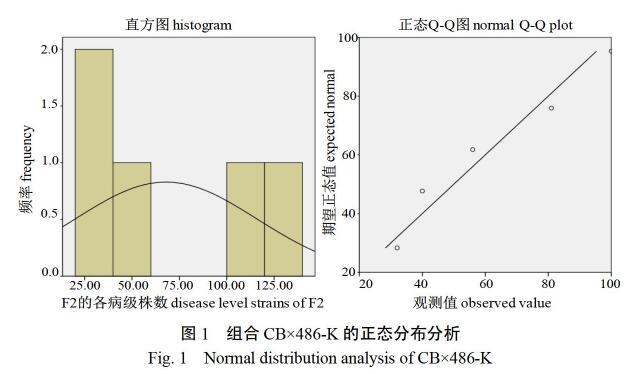
完全抗病植株存在,说明F1代表现为感病。2个组合F2代病情指数分别为51.8和46.2,以CB×486-K组合为例(图1),通过SPSS 20.0软件对F2代各病级株数进行正态分布分析(卡方检验),发现直方图中频数分布有明显的波峰,且偏度≈0,说明F2代各病级株数呈正态分布;Q-Q图检验看出各频数基本分布在直线附近,则进一步验证服从正态分布。CB×117-K F2代的卡方检验结果同CB×486-K。该结果表明2个组合的F2代均存在一定性状分离,可以进行下一步遗传规律分析。
2.2 “主基因+多基因”混合模型遗传分析
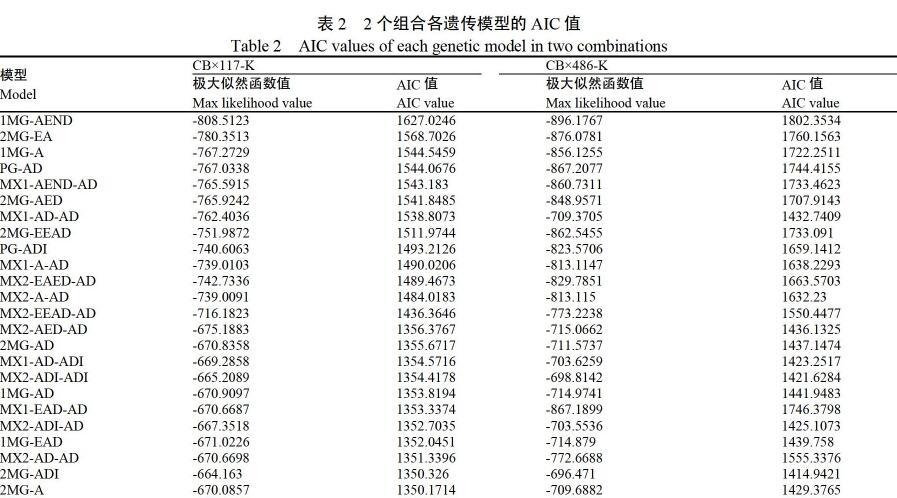
利用“主基因+多基因”混合模型[13]分别对2个组合的单株病级数据进行分析,得到各个组合5类24种模型的AIC值(表2)。根据AIC值较小原则选择最佳模型。组合CB×117-K中,AIC值较低的有MX2-AD-AD、2MG-ADI和2MG-A模型;组合CB×486-K中,AIC值较低的有MX1-AD-ADI、MX2-ADI-ADI、MX2-ADI-AD和2MG-ADI模型。将以上模型分别作为各抗源抗性的备选模型。
参考文献
[1]刘勇,范江,李永平. 烟草抗青枯病育种研究进展[J]. 中国烟草学报,2012,18(6):93-99.
LIU Y, FAN J, LI Y P. Research progress in breeding of tobacco resistant to bacterial wilt[J]. Chinese Tobacco Journal, 2012, 18(6): 93-99.
[2]徐树德,尚志强,秦西云. 烟草青枯病研究进展[J]. 天津农业科学,2010,16(4):49-53.
XU S D, SHANG Z Q, QIN X Y. Research progress of tobacco Ralstonia solanaeearum[J]. Tianjin Agricultural Sciences, 2010, 16(4): 49-53.
[3]LI Y, FENG J, LIU H L, et al. Genetic diversity and pathogenicity of Ralstonia solanacearum causing tobacco bacterial wilt in China[J]. Plant Disease, 2016, 100(7): 1288-1296.
[4]MA L, ZHANG H Y, ZHOU X K, et al. Biological control tobacco bacterial wilt and black shank and root colonization by bio-organic fertilizer containing bacterium Pseudomonas aeruginosa NXHG29[J]. Applied Soil Ecology, 2018, 129(5): 136-134.
[5]YI Y N, LIU R S, YIN H Q, et al. Isolation, identification and field control efficacy of an endophytic strain against tobacco bacterial wilt (Ralstonia solanacarum)[J]. Chinese Journal of Applied Ecology, 2007, 18(3): 554-558.
[6]高加明,王志德,张兴伟,等. 香料烟青枯病抗性基因的遗传分析[J]. 中国烟草科学,2010,31(1):1-4.
GAO J M, WANG Z D, ZHANG X W, et al. Genetic analysis on resistance to bacterial wilt in oriental tobacco[J]. Chinese Tobacco Science, 2010, 31(1): 1-4.
[7]MATSUDA T, OHASH I Y. Inheritance of resistance to bacterial wilt resistant varieties in tobacco[J]. Jap Jour Breeding, 1973, 23: 175-180.
[8]QIAN Y L, CHEN J, DONG J J, et al. Genetic analysis of the major and minor locus groups of bacterial wilt resistance in tobacco using a diallel cross design[J]. Genetics and Molecular Research, 2016, 15(1): 255-262.
[9]张振臣,袁清华,马柱文,等. 烟草品种GDSY-1的青枯病抗性与遗传分析[J]. 中国烟草科学,2017,38(4):9-16.
ZHANG Z C, YUAN Q H, MA Z W, et al. Inheritance of resistance to bacterial wilt in Chinese domestic tobacco cultivar GDSY-1[J]. Chinese Tobacco Science, 2017, 38(4): 9-16.
[10]张振臣,吕永华,马柱文,等. 烟草品种“大叶密合”青枯病抗性遗传分析[J]. 中国烟草学报,2015,21(3):57-64.
ZHANG Z C, LYU Y H, MA Z W, et al. Genetic analysis of resistance to bacterial wilt in tobacco cultivar dayemihe[J]. Acta Tabacaria Sinica, 2015, 21(3): 57-64.
[11]THANO P, AKARAPISAN A. Phylotype and sequevar of Ralstonia solanacearum which causes bacterial wilt in Curcuma alismatifolia gagnep[J]. Letters in Applied Microbiology, 2018, 66(5): 384-393.
[12]王建康,蓋钧镒. 利用杂种F2世代鉴定数量性状主基因-多基因混合遗传模型并估计其遗传效应[J]. 遗传学报,1997(5):432-440.
WANG J K, GAI J Y. Identification of major-gene-polygene hybrid genetic models of quantitative traits using hybrid F2 generation and estimating their genetic effects[J]. Acta Genetica Sinica, 1997(5): 432-440.
[13]章元明,盖钧镒. 数量性状分离分析中分布参数估计的IECM算法[J]. 作物学报,2000(6):699-706.
ZHANG Y M, GAI J Y. The IECM algorithm for estimation of component distribution parameters in segregating analysis of quantitative traits[J]. Acta Agronomica Sinica, 2000(6): 699-706.
[14]程亚增,马冰,蒋彩虹,等. 烤烟不同黄瓜花叶病毒病(CMV)抗源的抗性遗传分析[J]. 烟草科技,2016,49(6):8-14.
CHENG Y Z, MA B, JIANG C H, et al. Genetic analysis of CMV resistance in different flue-cured tobacco germplasms[J]. Tobacco Science & Technology, 2016, 49(6): 8-14.
[15]代帅帅,任民,蒋彩虹,等. 烟草骨干亲本主要病毒病抗性鉴定及遗传多样性分析[J]. 中国农业科学,2015,48(6):1228-1239.
DAI S S, REN M, JIANG C H, et al. Identification of resistance to main virus diseases and genetic diversity study of tobacco foundation parents[J]. Scientia Angricultura Sinica, 2015, 48(6): 1228-1239.
[16]冯莹,蒋彩虹,程立锐,等. 两个烟草赤星病抗源的遗传分析[J]. 中国烟草科学,2015,36(5):1-7.
FENG Y, JIANG C H, CHENG L R, et al. Genetic analysis of resistance to brown spot disease in tobacco cultivars Jingyehuang and Beinhart1000-1[J]. Chinese Tobacco Science, 2015, 36(5): 1-7.
[17]郭璇,闫杏杏,蒋彩虹,等. 雪茄烟Beinhart1000-1对黑胫病0号生理小种的抗性遗传分析[J]. 中国烟草科学,2017,38(2):56-62.
GUO X, YAN X X, JIANG C H, et al. Genetic analysis of Beinhart1000-1 resistance to black shank in tobacco[J]. Chinese Tobacco Science, 2017, 38(2): 56-62.
[18]張兴伟,王志德,刘艳华,等. 植物数量性状“主基因+多基因”混合遗传模型及其在烟草上的应用[J]. 中国烟草学报,2013,19(3):41-44.
ZHANG X W, WANG Z D, LIU Y H, et al. “Major-gene + polygene” mixed genetic model in quantitative characters and its application in tobacco[J]. Acta Tabacaria Sinica, 2013, 19(3): 41-44.
[19]范江. 烟草种质青枯病抗性鉴定与分子标记筛选[D]. 长沙:湖南农业大学,2012.
FAN J. Tobacco germplasm bacterial wilt resistance identification and molecular marker screening[D]. Changsha: Hunan Agricultural University, 2012.
[20]杨友才,周清明,朱列书. 烟草青枯病抗性基因的遗传分析及RAPD标记[J]. 中国烟草学报,2006(2):38-42.
YANG Y C, ZHOU Q M, ZHU L S. Heredity and RAPD markers analysis of resistance gene to tobacco bacterial wilt[J]. Acta Tabacaria Sinica, 2006(2): 38-42.
[21]孙学永,王新胜,张丽娜,等. 烟草青枯病抗性的遗传分析[J]. 中国农学通报,2013,29(34):56-60.
SUN X Y, WANG X S, ZHANG L N, et al. Genetic analysis of resistant to tobacco bacterial wilt[J]. Chinese Agricultural Science Bulletin, 2013, 29(34): 56-60.
[22]耿锐梅,程立锐,刘旦,等. 烟草青枯病抗性遗传效应分析[J]. 中国烟草科学,2019,40(4):7-13.
GEN R M, CHENG L R, LIU D, et al. Genetic analysis of resistance to bacterial wilt in tobacco[J]. Chinese Tobacco Science, 2019, 40(4): 7-13.
[23]孙学永,周应兵,杨华应,等. 烟草种质抗青枯病鉴定及其抗性分类[J]. 中国烟草学报,2011,17(3):61-66,88.
SUN X Y, ZHOU Y B, YANG H Y, et al. Identification and classification of tobacco germplasm resistant to bacterial wilt[J]. Acta Tabacaria Sinica, 2011, 17(3): 61-66, 88.
[24]王新,吴新儒,王卫锋,等. 烟草抗青枯病突变体的室内接种鉴定[J]. 分子植物育种,2018,16(19):6468-6475.
WANG X, WU X R, WANG W F, et al. Indoor inoculation identification of tobacco mutant resistant to bacterial wilt[J]. Molecular Plant Breeding, 2018, 16(19): 6468-6475.
[25]臧辉,任卫波. EMS诱变在植物育种中的研究与应用[J]. 分子植物育种,2018,16(17):5782-5788.
ZANG H, REN W B. Research and application of EMS mutation in plant breeding[J]. Molecular Plant Breeding, 2018, 16(17): 5782-5788.
[26]刘晓蓓,吴赓,张芊,等. 烟草突变体库的创建策略及其应用[J]. 中国农业科技导报,2010,12(6):28-35.
LIU X B, WU G, ZHANG Q, et al. Strategy and application for constructing tobacco mutant library[J]. Journal of Agricultural Science and Technology, 2010, 12(6): 28-35.
[27]刘翔. EMS诱变技术在植物育种中的研究进展[J]. 激光生物学报,2014,23(3):197-201.
LIU X. Progresses on EMS mutagenesis in plant breeding[J]. Acta Laser Biology Sinica, 2014, 23(3): 197-201.
基金項目:中国烟草总公司科技重大专项项目“烟草青枯病抗性基因定位及翠碧一号新品系培育”(110201601030);中国农业科学院科技创新工程(ASTIP-TRI02)
作者简介:邹文莉(1995-),女,在读硕士生,主要研究方向为分子遗传育种。E-mail:zouwenli950806@163.com
*通信作者,E-mail:sunyuhe@caas.cn;wangweifeng@caas.cn
收稿日期:2019-12-11 修回日期:2020-03-25
