An Efficient Process for Recycling Nd-Fe-B Sludge as High-Performance Sintered Magnets
2020-05-22XiowenYinMingYueQingmeiLuMinLiuFengWngYubingQiuWeiqingLiuTieyongZuoShnshunZhXulingLiXiofeiYi
Xiowen Yin, Ming Yue,b,c,*, Qingmei Lu,c, Min Liu,c, Feng Wng, Yubing Qiu, Weiqing Liu,b,c,Tieyong Zuo,c, Shnshun Zh, Xuling Li, Xiofei Yi
a College of Materials Science and Engineering, Beijing University of Technology, Beijing 100124, China
b State Key Laboratory of Rare Earth Permanent Magnetic Materials, Hefei 231500, China
c Key Laboratory of Advanced Functional Materials, Ministry of Education, Beijing University of Technology, Beijing 100124, China
d Earth-Panda Advanced Magnetic Materials Co., Ltd., Hefei 231500, China
Keywords:
A B S T R A C T Given the increasing concern regarding the global decline in rare earth reserves and the environmental burden from current wet-process recycling techniques,it is urgent to develop an efficient recycling technique for leftover sludge from the manufacturing process of neodymium-iron-boron(Nd-Fe-B)sintered magnets. In the present study,centerless grinding sludge from the Nd-Fe-B sintered magnet machining process was selected as the starting material.The sludge was subjected to a reduction-diffusion(RD)process in order to synthesize recycled neodymium magnet(Nd2Fe14B)powder;during this process,most of the valuable elements, including neodymium (Nd), praseodymium (Pr), gadolinium (Gd), dysprosium(Dy), holmium (Ho), and cobalt (Co), were recovered simultaneously. Calcium chloride (CaCl2) powder with a lower melting point was introduced into the RD process to reduce recycling cost and improve recycling efficiency. The mechanism of the reactions was investigated systematically by adjusting the reaction temperature and calcium/sludge weight ratio. It was found that single-phase Nd2Fe14B particles with good crystallinity were obtained when the calcium weight ratio(calcium/sludge)and reaction temperature were 40 wt% and 1050 °C, respectively. The recovered Nd2Fe14B particles were blended with 37.7 wt% Nd4Fe14B powder to fabricate Nd-Fe-B sintered magnets with a remanence of 12.1 kG (1 G =1 × 10-4 T), and a coercivity of 14.6 kOe (1 Oe = 79.6 A·m-1), resulting in an energy product of 34.5 MGOe. This recycling route promises a great advantage in recycling efficiency as well as in cost.
1. Introduction
Green energy is playing an increasingly important role in modern society. Some forms of green energy require strong support from permanent magnet (PM) materials, such as ferrite, alnico,samarium cobalt, and neodymium-iron-boron (Nd-Fe-B) [1,2].Nd-Fe-B sintered magnets are the most popular choice, as they possess the highest energy product of all commercial PM materials[3,4]. With the development of clean energy equipment such as wind turbines and hybrid electric vehicles, the consumption of Nd-Fe-B sintered magnets is growing yearly,and reached a global level of 132.8 kt in 2016 [5]. To obtain final products of a desirable size, Nd-Fe-B sintered magnets are subjected to various machining processes,including wire-electrode cutting,slicing,and centerless grinding. During these manufacturing processes, about 25 wt%of scraps are generated[6].More than 30 wt%of rare earth elements (REEs) are contained in the Nd-Fe-B scraps, including neodymium (Nd), praseodymium (Pr), gadolinium (Gd), dysprosium(Dy),terbium(Tb),and holmium(Ho),which are all essential elements in various industrial applications. The recycling of Nd-Fe-B scraps is thus an important part of maintaining a sustainable supply chain for REEs.
Nd-Fe-B scraps usually contain irregular bulk scraps and sludge. Hydrogen decrepitation (HD) has been conducted on irregular bulk scraps to obtain recycled Nd-Fe-B sintered magnets,and has proven to be a potential route for commercial-scale application [7,8]. However, Nd-Fe-B sludge has mainly been used as a valuable secondary resource for the recovery of REEs.The selective extraction of REEs is the major aim in recycling Nd-Fe-B sludge.Comprehensive reviews have summarized the approaches taken thus far to recover REEs from sludge [9,10]. Wet chemical processes are generally preferred; these processes usually consist of complete dissolution of Nd-Fe-B sludge in an acid, followed by selective precipitation of the REEs as sulfates or fluorides. Such recycling processes consume a large amount of chemicals such as hydrochloric acid (HCl), sulphuric acid (H2SO4), hydrofluoric acid(HF), and sodium hydroxide (NaOH), and generate considerable volumes of waste water[11-13],thereby creating a heavy environmental burden [14]. On the other hand, pyrometallurgical processes such as selective chlorination, vacuum induction melting,thermal isolation, and roasting can selectively and efficiently extract REEs from Nd-Fe-B scraps [15,16]. However, these recycling processes are always operated at a high temperature above 950 °C and are thus quite energy intensive and expensive.
In addition to the rare earths, other valuable elements are contained in the Nd-Fe-B sludge,such as cobalt(Co),gallium(Ga),zirconium(Zr),and niobium(Nb).However,Nd-Fe-B sludge is usually severely contaminated by the cutting fluid and other impurities during the machining processes,and thus exhibits high oxygen content.Calcium(Ca)metal has a strong thermodynamic affinity with oxygen, and has been applied to synthesize neodymium magnet(Nd2Fe14B) powder through the reduction-diffusion (RD) process[17]. This process provides a potential route for the recycling of the sludge. An effective method was proposed in Refs. [18-21] to recycle Nd-Fe-B sludge as bonded magnets without acid; this method involves removing carbon by heating the sludge at a high temperature in air, and subsequently reducing oxygen through a calcium RD process.Based on this method,our group obtained recycled Nd-Fe-B sintered magnets from the sludge. The sludge was pretreated by distillation to remove organic impurities and then used to synthesize Nd2Fe14B powder via the RD process.After doping with rare earth hydride, recycled Nd-Fe-B sintered magnets with good magnetic properties were prepared through sintering.The best magnetic properties of the recycled Nd-Fe-B sintered magnets—namely, a remanence of 12.2 kG (1 G = 1 × 10-4T),coercivity of 10.3 kOe (1 Oe = 79.6 A·m-1), and energy product of 35.2 MGOe—were recovered from the sludge through doping with 10 wt%of hydroneodymium compound(NdHx)particles.It was also found that the RD process has advantages when recycling Nd-Fe-B sludge in comparison with the conventional recycling process:It is more environmentally friendly,energy saving,and high efficiency,as we have reported in detail[22,23].
Although the Nd-Fe-B sludge can be successfully recovered as magnets by the RD process,the mechanism of the RD process,recycling efficiency, and cost have not been further discussed. In this study, we have designed an efficient recycling route for Nd-Fe-B sludge in order to address these points. Centerless grinding Nd-Fe-B sludge was selected as the starting material from a variety of sludges in order to improve the recycling efficiency. Calcium chloride (CaCl2) powder was introduced to assist the chemical reactions and reduce the usage of calcium granules,thereby reducing the recycling cost. In order to systematically investigate the mechanism of the RD process, the reaction temperature and calcium/sludge ratio were adjusted in this study.Moreover,Nd4Fe14B powder was doped into the recycled powder as a rare-earth-rich alloy during the sintering process in order to achieve a high magnetic performance, which can also improve the utilization efficiency of rare earths and reduce the recycling cost. Furthermore,the application of a rare-earth-rich alloy in the sintered process increases the feasibility of industrial applications.
2. Materials and methods
The Nd-Fe-B sludge was obtained from Anhui Earth-Panda Advance Magnetic Material Co. Ltd., and was stored in a plastic sealed barrel with ethyl alcohol. Fig. 1 shows the recycling route of the Nd-Fe-B sludge in this study. Before the RD process, the sludge was dried at 60 °C for 2 h in air. The dried sludge, Ca granules, and CaCl2powder were mixed and loaded in a stainless-steel crucible under an argon (Ar) atmosphere. CaCl2powder was added as a fluxing agent to ensure full reduction.Reaction mixtures with a calcium/sludge weight ratio of 20 wt%,30 wt%, 40 wt%, and 50 wt%, respectively, were prepared and mixed with 30 wt% of CaCl2powder. The calcium/sludge weight ratios were decided by the oxygen content of the sludge, as mentioned below.To study the reaction mechanism of the RD process,reaction temperatures of 600, 850, 950, and 1050 °C, respectively,were conducted for 2 h with the optimal calcium/sludge weight ratio. After the RD process, the samples were washed with ice water under a nitrogen atmosphere for 1 h to remove calcium oxide (CaO), calcium chloride hydroxide (CaClOH), and residual Ca, and then dried in a vacuum.
To obtain recycled Nd-Fe-B sintered magnets, Nd2Fe14B powder recovered from the sludge was blended with Nd4Fe14B powder for sintering.Appropriate size distribution of the recycled Nd2Fe14B powder was achieved through 90 min of ball milling with a ball-topowder ratio of 10:1.The Nd4Fe14B powder was prepared through smelting,HD,and a jet-milling process.Because the rare earth content of the recovered Nd2Fe14B powder decreased after the RD process, the milled powder was mixed with different amounts of Nd4Fe14B powder, ranging from 33.3 wt% to 45.8 wt%, and then pressed together under a magnetic field of 2 T.The green compacts were sintered at 1090 °C for 3 h under a vacuum of 1 × 10-3Pa;they were subsequently annealed at 900 °C for 3 h and then at 480 °C for 2 h.
The chemical composition and crystal structure of the Nd-Fe-B sludge, RD-treated Nd2Fe14B powders, and final recycled magnets were determined with an inductively coupled plasma optical emission spectrometer (ICP-OES), a C-H-O elemental analyzer, and Xray diffraction (XRD) with Cu-Kα radiation. The morphology of the sludge powder, RD-treated powders, ball-milled powder, and Nd4Fe14B powder and the microstructure of the recycled Nd-Fe-B sintered magnets were observed using scanning electron microscopy(SEM).The particle sizes of the ball-milled powder and the Nd4Fe14B powder were examined with a laser particle size analyzer(LPSA).The magnetic properties of the recycled magnets were measured with a B-H loop tracer,and the densities of the magnets were determined by the Archimedes method.

Fig. 1. Schematics of the recycling process of Nd-Fe-B sludge.
3. Results and discussion
3.1. Characterization of Nd-Fe-B centerless grinding sludge
To obtain a product with a desirable size,numerous machining processes are necessary in the preparation of sintered magnets.These processes include grinding, slicing, centerless grinding,trepanning,and wire-electrode cutting.During the machining process, some processes require high temperatures and use organic cooling liquid, such as the wire-electrode cutting process. Therefore, the sludge obtained from the wire-electrode cutting process is severely contaminated and oxidized. The raw material selected in this study was Nd-Fe-B centerless grinding sludge, which is generated from the centerless grinding process with water cooling.To reduce the surface roughness of the cylindrical product,the centerless grinding process is conducted on the surface of the magnets.
Fig. 2 shows the morphology, XRD pattern, and chemical composition of Nd-Fe-B centerless grinding sludge that was dried at 60°C for 2 h in air.As shown in Fig.2(a),the particles do not have sharp edges, and needle-like and hexagonal flaky particles appear on the surface of the sludge powder. The sludge powder is composed of hydrogen neodymium oxide (Nd(OH)3) and ferroferric oxide (Fe3O4), as determined by the XRD scan shown in Fig. 2(b).The core of the large sludge particles is composed of Nd2Fe14B hydride(Nd2Fe14BHx).Since the cooling liquid is water,less impurity remains in the centerless grinding sludge, aside from oxygen and hydrogen, as shown in Fig. 2(c). Due to various grades of Nd-Fe-B sintered magnets being processed in the same machine,the chemical composition of the sludge is complex, and contains the rare earths Nd, Pr, Gd, Dy, and Ho; the substituted elements Co,aluminum(Al),copper(Cu),Ga,and Zr;and the basic elements iron (Fe) and boron (B). Machine oil is responsible for the carbon and hydrogen content in the centerless grinding sludge, which can be reduced in the RD process,as described in the next section.Although the centerless sludge has a high oxygen content, it is an ideal starting material for recycling into Nd-Fe-B sintered magnets through the RD process.
3.2. Calcium RD process
The calcium RD mechanism has been studied previously with commercial REEs oxide as the reactant [24-26]. However, to the best of our knowledge, use of the RD mechanism with Nd-Fe-B sludge as the starting material has not been reported.In this study,a range of reaction temperatures(600,850,950,and 1050°C) was studied in order to determine the reaction kinetics during the RD process. Fig. 3 shows the XRD patterns, before and after washing,of the RD powders for different reaction temperatures, and Fig. 4 shows the SEM images of the RD powders,after washing,for different reaction temperatures.For the reaction temperature of 600°C,a series of peaks can be identified as alpha-iron(α-Fe),neodymium oxygen chloride (NdOCl), and calcium chloride tetrahydrate(CaCl2·4H2O) (Fig. 3(a), part (i)) before washing; however, no Nd2Fe14B phase can be detected, which indicates that the tetragonal structure of Nd2Fe14B was destroyed and the NdOCl phase was formed. After washing, the peaks of α-Fe and NdOCl appear distinctly in part(i)of Fig.3(b).On the other hand,the Fe3O4particles contained in the Nd-Fe-B sludge were reduced to Fe at 600°C.The SEM image in Fig. 4(a) shows that numerous small particles have adhered to the larger particles, which differ from the sludge particles shown in Fig. 2(a). Since the temperature is lower than the melting point of calcium (839 °C), the calcium granules are not involved in the chemical reaction. In this stage, the CaCl2powder has already reacted with the sludge;this finding differs from what has previously been reported [27].
When a higher temperature was used (850 °C), some peaks of the Nd2Fe14B phase were present (Fig. 3(a) part (ii) and (b) part(ii)), suggesting that the NdOCl phase was reduced and subsequently formed the Nd2Fe14B phase because the reaction temperature reached the reactivity of calcium.At this stage,aggregation of small spherical particles on the surface of a large particle was clearly observed, as shown in Fig. 4(b). This is the beginning of the nucleation of the Nd2Fe14B grains, which implies that the Nd and B atoms have diffused into the matrix of α-Fe particles to form Nd2Fe14B grains. This finding aligns with the results reported by Chen et al. [28]. Despite the formation of the Nd2Fe14B phase at 850 °C, distinct peaks from α-Fe can be seen in Fig. 3(b) part (ii),implying that a higher reaction energy is needed to improve the nucleation driving force. It is also worth mentioning that peaks indicating CaClOH rather than CaCl2appear in Fig. 3(a) part(ii), which indicates that the CaCl2powder was involved in the chemical reactions. When the reaction temperature reached 950°C,Nd2Fe14B was obtained as the main phase,but a weak peak indicating the α-Fe phase remained in the XRD pattern, as shown in Fig.3(b)part(iii).Nevertheless,more Nd2Fe14B grains were present on the surface of a large particle, which did not have clear edges due to the low driving force of nucleation. Therefore,clean-cut and smooth-faced Nd2Fe14B particles were clearly observed when the reaction temperature reached 1050 °C, as shown in Fig. 4(d).
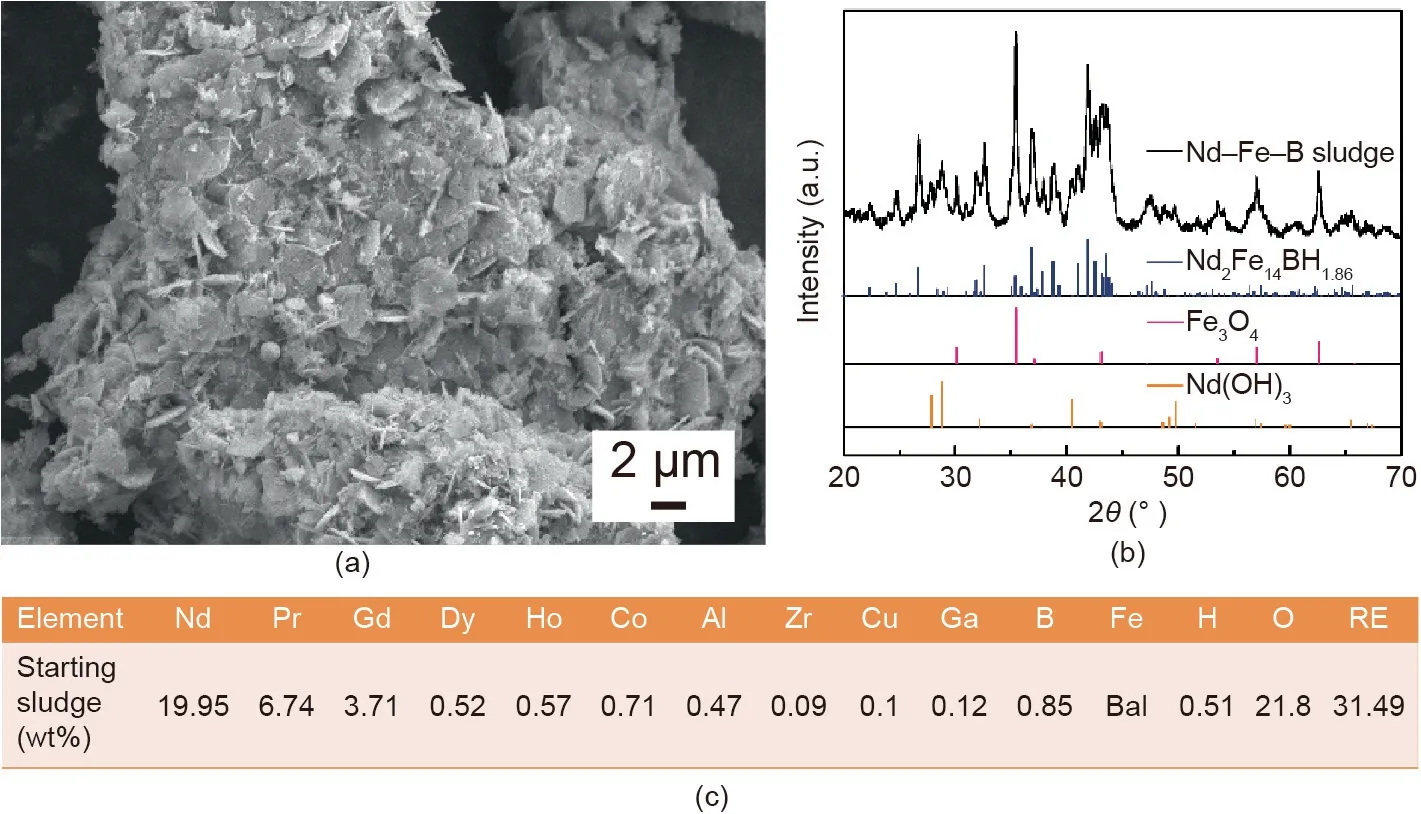
Fig.2. Nd-Fe-B centerless grinding sludge dried at 60°C for 2 h in air.(a)SEM secondary electronic image;(b)XRD pattern;(c)chemical composition of the sludge.RE:rare earth; Bal: balance.

Fig.3. XRD patterns of Nd-Fe-B sludge treated by the RD process with a calcium/sludge weight ratio of 50 wt%(a)before and(b)after removing CaO,for different reaction temperatures: (i) 600 °C; (ii) 850 °C; (iii) 950 °C; (iv) 1050 °C. Calcium granules were removed from the sample after annealing at 600 °C.

Fig.4. SEM images of Nd-Fe-B sludge treated by the RD process with a calcium/sludge weight ratio of 50 wt%at different temperatures: (a) 600 °C; (b) 850 °C; (c) 950 °C;(d) 1050 °C.
Meanwhile,sharpened peaks can be seen in the XRD pattern of Fig.3(b)part(iv),which indicate the single-phase of Nd2Fe14B.It is clear that reaction temperature is a crucial parameter when synthesizing Nd2Fe14B particles from Nd-Fe-B sludge through the RD process. The sequence of each reaction during the RD process can be stated as follows:
Step 1:
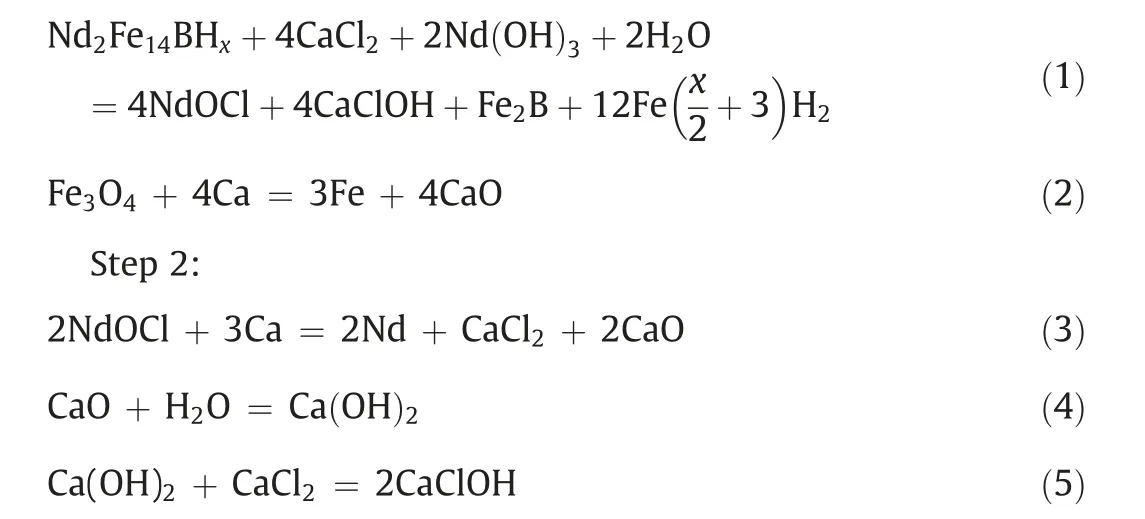

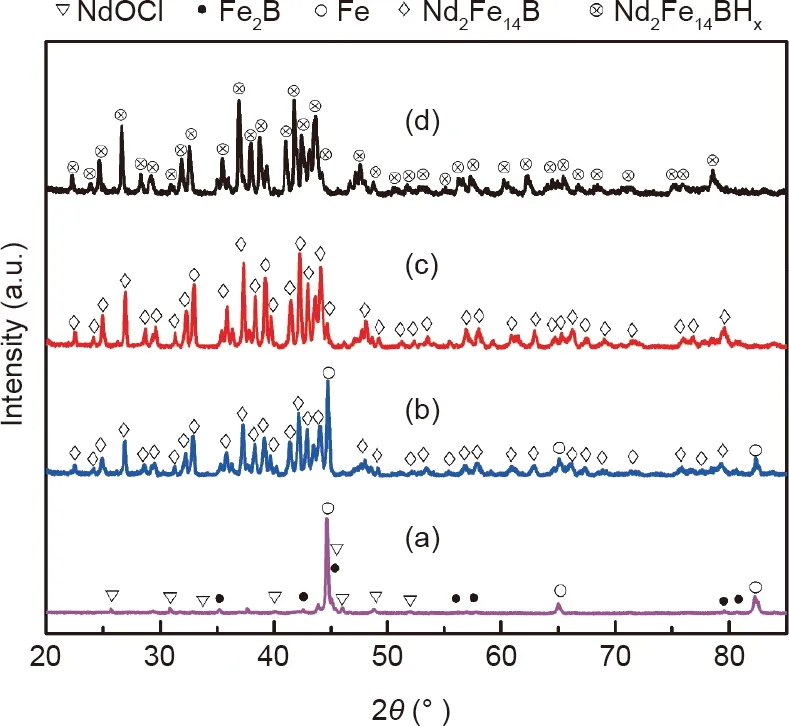
Fig. 5. XRD patterns of Nd-Fe-B powder treated by the RD process with different calcium/sludge weight ratios: (a) 20 wt%; (b) 30 wt%; (c) 40 wt%; (d) 50 wt%.
To minimize the amount of calcium granules used in the RD process, a range of calcium/sludge weight ratios (20 wt%, 30 wt%,40 wt%,and 50 wt%)were studied.Figs.5 and 6 show the XRD patterns and SEM images of the washed samples after the RD process for different weight ratios of calcium/sludge.In the case of 20 wt%,the presence of the α-Fe phase can clearly be observed in Fig.5(a),indicating that this amount of calcium is insufficient to reduce the total oxide contained in the starting materials.The irregular particles with good crystallinity shown in Fig.6(a)were identified as α-Fe particles that had formed due to the high reaction temperature of 1050 °C. Nd2Fe14B peaks appeared in the sample at 30 wt% of calcium; however, the presence of α-Fe peaks suggested that the sludge was not fully reduced by the calcium granules.In this sample, spherical particles of Nd2Fe14B and irregular particles of α-Fe were concomitant, as shown in the SEM image (Fig. 6(b)). When the amount of calcium reached 40 wt%, only the Nd2Fe14B phase was observed in the XRD pattern(Fig.5(c));furthermore,spherical particles of Nd2Fe14B were observed(Fig.6(c)),indicating that this amount of calcium was sufficient to remove the oxygen contained in the sludge. However, the Nd2Fe14BHx(x = 1-5) phase appeared instead of Nd2Fe14B (Fig. 5(d)) when the calcium/sludge weight ratio reached 50 wt%, indicating that this amount of calcium was excessive. The residual calcium granules generated hydrogen (H2)gas vigorously during the washing process with water (H2O), and the hydrogen diffused interstitially into the lattice of Nd2Fe14B to form Nd2Fe14BHxaccording to Eqs.(4) and (6).This finding is consistent with the results reported by Claude et al.[29].Nevertheless,the obtained recycled particles maintained regular shapes, as shown in Fig. 6(d).
According to Fig.2(c),the oxygen content in the sludge powder was 21.8 wt%, which—in theory—would consume the calcium granules at 54.5 wt%. However, this study showed that fewer calcium granules can fully reduce the sludge powder and form single-phase Nd2Fe14B, indicating that CaCl2plays an important role during the RD process. Due to the presence of oxygen and chlorine, oxychloride can easily be formed at high temperatures,and contributes to consuming oxygen. Furthermore, the melting point of CaCl2is lower than that of the calcium granules,so it provides a liquid system for the chemical reactions. Hence, the presence of CaCl2is beneficial for decreasing the overall calcium usage and improving the efficiency of the RD process in order to recoup the cost of recycling.In addition,the formation of Nd2Fe14-BHxis more stable than that of Nd2Fe14B,which reduces corrosion[30]. As shown in Table 1, the carbon, hydrogen, and oxygen contents in the final product were 0.08 wt%, 0.22 wt%, and 0.42 wt%,respectively, indicating that the carbon content is reduced after the RD process. On the other hand, the most valuable elements contained in the sludge were efficiently recovered as recycled powders, as shown in Table 1, which is a merit of the calcium RD process with CaCl2.
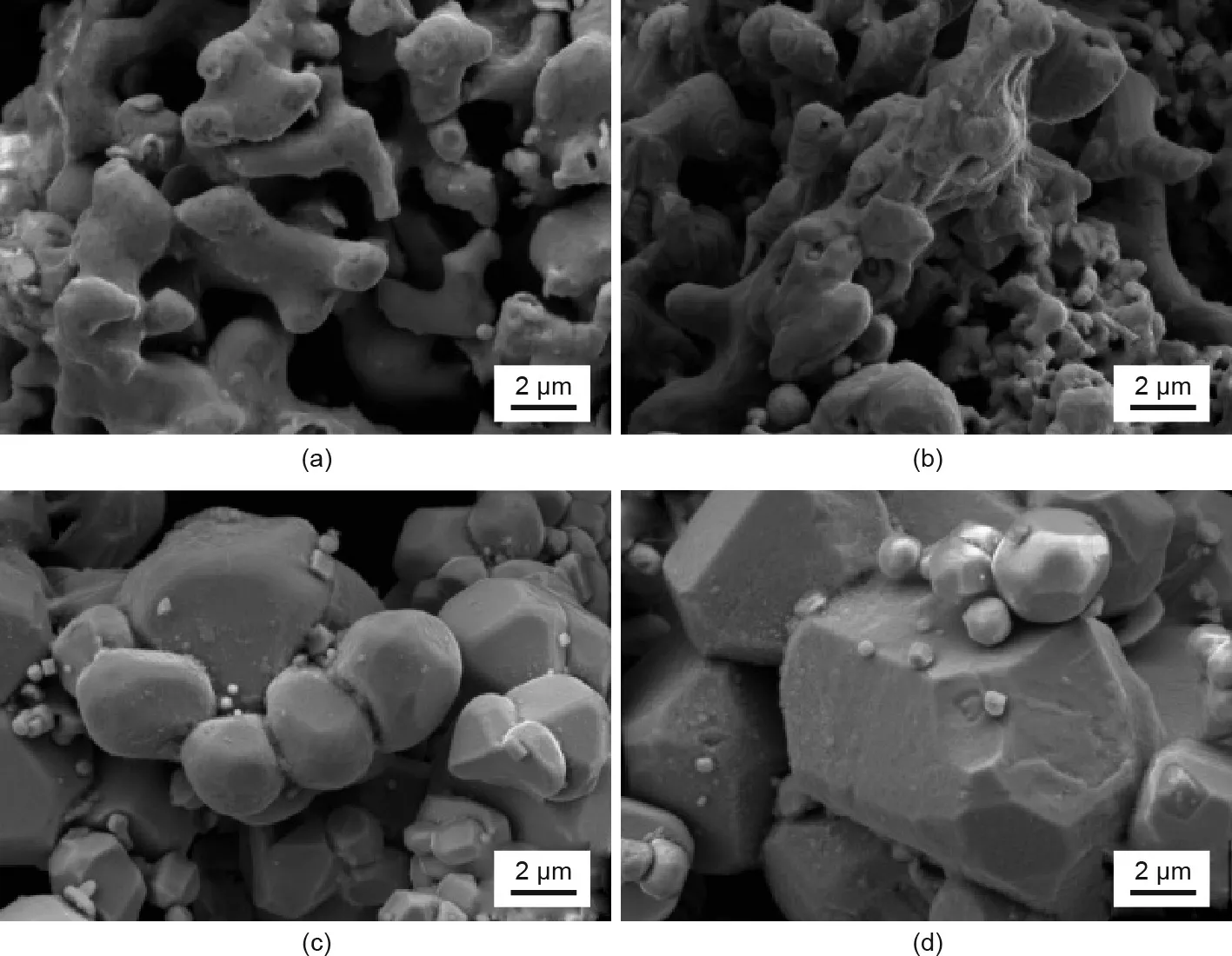
Fig. 6. SEM images of Nd-Fe-B powder treated by the RD process with different calcium/sludge weight ratios: (a) 20 wt%; (b) 30 wt%; (c) 40 wt%; (d) 50 wt%.
3.3. Preparation of recycled magnets and their magnetic properties
Recycled Nd-Fe-B powder was recovered from the Nd-Fe-B centerless grinding sludge through the RD process with 50 wt%calcium and 30 wt%CaCl2at 1050°C for 2 h.Fig.7(a)shows the SEM morphology of the RD powder.It can be observed that most of the particles are sphere-like crystals with diameters of less than 10 μm. However, the size distribution of the particles is rather wide.Further analysis through LPSA indicated that the particle size exhibits a bimodal distribution,with two mean diameters of 4 and 8 μm, as shown in the inset of Fig. 7(a). To prepare recycled magnets,ball milling was applied to the RD powder in order to achieve particles with uniform size distribution. After milling, most of the particles had equiaxed shapes, with an appropriate size distribution and a mean diameter of 3.5 μm,as shown in Fig.7(b).In addition, the recycled powders had a rare earth content of 30.26 wt%and an oxygen content of 0.42 wt%, indicating that additional rare earth needed to be added in order to fabricate fully dense magnets.In previous studies, rare earth hydrides were blended with the powder when fabricating Nd-Fe-B sintered magnets[31,32].However, the hydrides are easily oxidized in the ball-milling process,which reduces the utilization efficiency of the rare earth. In our previous studies, powders prepared from rare-earth-rich alloys proved to be effective additives for the fabrication of Nd-Fe-B sintered magnets with good magnetic properties [7,33]. Therefore,Nd4Fe14B powder was prepared through the previously used method, yielding a good size distribution with a mean size of 3 μm, as shown in Fig. 7(c).
Recycled Nd-Fe-B sintered magnets were obtained by adding different amounts of Nd4Fe14B powder, ranging from 33.3 wt% to 45.8 wt%. Fig. 8 shows the demagnetization curves of the recycled magnets. It can be observed that the curves exhibit good squareness,which is a necessary condition for their practical application.To compare the magnetic properties and density in detail, the obtained parameters are shown in the inset table of Fig. 8. It can be seen that the recycled magnets are fully dense and that their magnetic properties have reached the level of commercial sintered magnets.Moreover,it can be seen that the changes in the densities,remanences,and energy products are negligible when the additive amount of Nd4Fe14B powder is increased linearly, as shown in Fig.8.Furthermore,the coercivity increases slowly when the additive amount ranges from 33.3 wt% to 41.6 wt%, and subsequently decreases when the doping amount is 45.8 wt%.
For clarification,SEM images of recycled Nd-Fe-B magnets with additive ratios of 33.3 wt%and 45.8 wt%,respectively,are shown in Fig.9.It can be observed that the microstructure of both magnets is similar,indicating that the magnets may possess similar magnetic properties. However, the rare earth and oxygen contained in the two magnets are quite different, as shown in Table 1. With an increasing rare earth content, the oxygen content of the recycled magnet increases, which undermines the coercivity and reduces the corrosion resistance of the magnet.In addition,redundant Ndrich particles induce magnetic dilution and deteriorate the microstructure of the recycled magnets.On the other hand,reducing the amount of rare earth added during the recycling process can be part of a green design to cut the recycling cost and promote resource circulation. Nevertheless, minimizing the rare earth additive amount to obtain a recycled magnet with optimized magnetic properties during the recycling process is a goal for future research Fig.9.
4. Conclusions
This paper presented an efficient recycling route for fabricating Nd-Fe-B sintered magnets from centerless grinding sludge,involving the calcium RD process and a rare-earth-rich alloy-dopingtechnique.To reduce the recycling cost and improve efficiency during the recycling process,the mechanism of the RD process and the role of the additive amount of Nd4Fe14B powder were investigated.CaCl2powder played an important role as an assistant agent in promoting the efficiency of the chemical reaction. The Nd-rich alloy Nd4Fe14B made a great contribution to the magnetic hardening of the recycled Nd-Fe-B sintered magnets,thereby making it possible to achieve good magnetic performance.The best magnetic properties, with a remanence of 12.1 kG, a coercivity of 14.6 kOe, and a maximum energy product of 34.5 MGOe,were obtained for a magnet doped with 37.7 wt% Nd4Fe14B powder. The magnetic performance of the obtained magnets reached that of mid-level commercial Nd-Fe-B sintered magnets, indicating that the recycling technique described here provides a potential route for recycling Nd-Fe-B sludge into sintered magnets on a commercial scale.

Table 1 Chemical composition of the recycled powder and magnets for different doping amounts of Nd4Fe14B powder.

Fig.7. SEM images of(a)RD powder,(b)recycled Nd2Fe14B powder milled for 1.5 h,and(c)jet-milled Nd4Fe14B powder.Inset is the particle size distribution of corresponding powders. -d is the diameter of the particles.

Fig.8. Demagnetization curves of recycled Nd-Fe-B magnets doped with Nd4Fe14B powder: (a) 33.3 wt%; (b) 37.7 wt%; (c) 41.6 wt%; (d) 45.8 wt%. The inset table provides the corresponding properties of the magnets. J: magnetic polarization; H:magnetic field; Br: remanence; Hcj: coercivity; (BH)max: magnetic energy product;ρ: density.

Fig. 9. SEM images of recycled magnets doped with different ratios of Nd4Fe14B powder: (a) 33.3 wt%; (b) 45.8 wt%.
Acknowledgements
This work was financially supported by the National High Technology Research and Development Program of China(2012AA063201), the Beijing Municipal Natural Science Foundation(2172012),and the State Key Laboratory of Rare Earth Permanent Magnetic Materials Opening Foundation (SKLREPM17OF02).Ming Yue is indebted to Professor Zaven Altounian of McGill University for very helpful discussions.
Compliance with ethics guidelines
Xiaowen Yin, Ming Yue, Qingmei Lu, Min Liu, Feng Wang,Yubing Qiu, Weiqiang Liu, Tieyong Zuo, Shanshun Zha, Xuliang Li,and Xiaofei Yi declare that they have no conflict of interest or financial conflicts to disclose.
杂志排行
Engineering的其它文章
- Fabrication and Mechanical Testing of Ultralight Folded Lattice-Core Sandwich Cylinders
- Multi-Peptide Adsorption on Uncharged Solid Surfaces:A Coarse-Grained Simulation Study
- Thermal and Mechanical Properties Optimization of ABO4 Type EuNbO4 By the B-Site Substitution of Ta
- Efficient Nanostructuring of Isotropic Gas-Atomized MnAl Powder by Rapid Milling (30 s)
- Tunable In-Plane Anisotropy in Amorphous Sm-Co Films Grown on(011)-Oriented Single-Crystal Substrates
- Neutron Diffraction Investigation of the DyFe11Ti Magnetic Structure and Its Spin Reorientations
