Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells
2020-05-16ZhuLinLiZhenJunWangGuangHuiWeiYongYangXiaoWanWang
Zhu-Lin Li, Zhen-Jun Wang, Guang-Hui Wei, Yong Yang, Xiao-Wan Wang
Zhu-Lin Li, Zhen-Jun Wang, Guang-Hui Wei, Yong Yang, Department of General Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
Xiao-Wan Wang, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences,Beijing 100005, China
Abstract
Key words: Colorectal cancer; Extracellular matrix; MMP; Proliferation; Collagen; TIMP
INTRODUCTION
With the development of the “soil-seed” theory, the effect of the tumor microenvironment on tumor cells has received more attention[1]. The tumor microenvironment is a complex system consisting of the extracellular matrix (ECM),many types of cells, and bioactive factors, which control the complex interactions between tumor cells and stromal cells and between cells and the ECM[2]. During tumorigenesis, the ECM plays a role of “double-edged sword” in the process of tumor proliferation and invasion[3]. On the one hand, the ECM controls the proliferation,differentiation, and metastasis of tumor cells, and acts as a natural barrier. On the other hand, the remodeled ECM provides a loose “soil” for tumor cells to promote the occurrence and development of tumors[4,5]. This process of remodeling occurs at the same time with tumor formation, as shown by changes in the molecular composition,amount, and structure of the ECM[6].
The research on cell function is either in two-dimensional (2D) environment or in three-dimensional (3D) environment. Studies have shown that there are differences in cell proliferation, gene expression, and cell migration in 2Dvs3D cultures[6-8]. The 3Din vitroexperiments were better than 2D cultures in imitating tumor cell microenvironmentin vivo. Thein vitro3D culture refers to tumor cells cultured in collagen, Matrigel, or fibrin[9]. However, none of them can capture the complexity of the native matrix. The tumor ECM obtained by decellularization technology can contain almost all the proteins and their ratios in the natural tissue, which can more realistically simulate the tumor environment in which tumor cells live.
Previous studies have demonstrated that the composition of the ECM changes during cancer formation[10,11]. However, the relationship between the stages of colorectal cancer (CRC) and the changes in the ECM and the proliferation of cancer cells is unclear. Therefore, we obtained the tumor ECM by decellularization and aimed to observe the changes in the ECM during different stages of CRC and the effects of these changes on the proliferation of cancer cells.
MATERIALS AND METHODS
Patients and tissue samples
Tissues were removed from 60 patients with CRC during surgery at Beijing Chaoyang Hospital, Capital Medical University. All procedures in this study were approved by the Medical Ethics Committee of Beijing Chaoyang Hospital, and all patients provided written informed consent. Tumor stage was classified according to the 8thedition of the American Joint Committee on Cancer TNM staging system for CRC.The patients included 34 males and 26 females and none had received preoperative chemoradiotherapy. Of these patients, 10 were classified with stage I disease, 22 with stage II, 19 with stage III, and 9 with stage IV. Ten cases with a normal colon were selected as a control group. All samples were put into the digestive solution composed of 0.25% trypsin and 0.02% EDTA and were continuously oscillated at 37°C for 24 h at 130 rounds per minute. After that, the tissues were put into 0.5% Triton X-100 buffer and continuously oscillated at 150 rounds per minute for 24 h. Then, the ECM was obtained. After freeze-drying, the ECM samples were sterilized by Co-60 radiation and stored at -20°C.
Western blot analysis
Western blot assays were performed to detect the protein level. The protein concentrations were tested with a BCA Protein Assay Kit (Pierce, United States).Equal amounts of protein (20 μg) were loaded. Type I collagen, type IV collagen,MMP-2, MMP-9, and TIMP-3 antibodies were purchased from Beijing Biosynthesis Biotechnology Co, LTD. All primary antibodies were used at a 1:1000 dilution. The enhanced chemiluminescence reaction was used to detect the protein bands.
Co-culture of cells and the extracellular matrix
The sterilized ECM was cut into 3 mm × 3 mm × 3 mm pieces under aseptic conditions and placed in a 96-well culture plate. One hundred microliter of colon cancer HT29 cells at a density of 1×106cells/mL (1×105cells) was slowly added vertically into the ECM, and then cultured in an incubator containing 5% CO2.
Methyl thiazolyl tetrazolium assay
The culture medium in the 96-well plate was removed and treated with methyl thiazolyl tetrazolium (MTT) solution for 4 h. After removing the supernatant, 150 μL of DMSO was added to dissolve the tetrazolium salt and measure the optical density using a Multiskan Spectrum Microplate Reader (Thermo Labsystems, Milan, Italy) at 570 nm. The experiment was repeated three times.
Animal experiments
Six-week-old male nude BALB/c mice were randomly divided into five groups with 10 mice in each group. The density of colon cancer HT29 cells was adjusted to 1 × 106cells/mL. The ECM from each group was cut into 3 mm × 3 mm × 3 mm pieces under aseptic conditions and placed in a 96-well plate. In each well, 50 μL of the above cell suspension (5×104cells) was slowly added and allowed to stand for 1 h. Abdominal anesthesia was performed with 10% chloral hydrate (0.01 g/mL), and the right forearm underarm skin in each mouse was cut under aseptic conditions, and the ECM and cancer cell complex were embedded subcutaneously. The animals were killed on the 30thday, and the long diameter (a) and short diameter (b) of the tumor were measured with a Vernier caliper. Approximate tumor volume was obtained using the following equation: V = a × b × b/2.
All animals were housed under a 12/12 h light/dark cycle at 22 °C and 40%-60%relative humidity conditions. They were given free access to water and food. All animal experimental protocols were done according to the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China.
Statistical analysis
RESULTS
Preparation of extracellular matrix
An overview of ECM preparation is provided in Figure 1. The normal tissue ECM and CRC ECM were obtained by decellularization. From outward appearance, there was no obvious difference in normal tissue ECM and CRC ECM. All of them presented milk white, sticky surface and soft texture.
High expression of type I collagen, MMP-2, and MMP-9 and low expression of type IV collagen and TIMP-3 in extracellular matrix of colorectal cancer
We used proteomics to analyze the differential expression of proteins in normal colorectal tissue and colorectal cancer tissue, and some of them were selected for analysis in each stage of colorectal cancer (Table 1). The expression of type I collagen was highest in stage III and stage IV and lowest in normal tissue and stage I.Spearman correlation analysis showed that the expression of type I collagen was positively correlated with the stage of CRC (Figure 2). The expression of MMP-2 was higher in the colorectal cancer tissues and it increased with the increased tumor stage.The expression of MMP-9 was higher in the colorectal cancer tissue, but it was highest in the stage III CRC (Figure 3). However, the expression of type IV collagen and TIMP-3 gradually decreased with increased CRC stage. Spearman correlation analysis showed that type IV collagen was negatively correlated with the stage of CRC(Figures 2 and 3).
Extracellular matrix of colorectal cancer is conducive to the proliferation of tumor cells in vitro and in vivo
To study the growth of cancer cells in each group, we co-cultured cancer cells and ECMin vivoandin vitro. The proliferation of cancer cells was determinedin vitroby the MTT assay. We found that cancer cells co-cultured with CRC ECM grew significantly better than cancer cells with normal tissue ECM (Figure 4).In vivotumor volume in each group was larger and was greatest in stage IV CRC ECM. Compared to the normal tissue ECM, the CRC ECM was more conducive to the proliferation of cancer cells (Figure 5).
DISCUSSION
The occurrence of malignant tumors is a complex process of interactions between cancer cells and the tumor microenvironment[12,13]. Paget described the relationship between cancer cells and the tumor microenvironment as the seed and soil, indicating that the tumor microenvironment is very important for tumorigenesis and tumor progression[14]. The tumor microenvironment is a unique environment that emerges during the course of tumor progression[12,15]. The tumor microenvironment is composed of ECM, cells, and interstitial fluids, and the ECM is the major component[16]. During cancer progression, the structure and composition of the ECM become disorganized, and this change can promote cellular transformation and metastasis[4,16,17].
Collagen is an important component of the ECM and is considered a structural barrier against tumor invasion[18,19]. Paradoxically, increased expression of collagen is associated with an elevated incidence of proliferation and invasion[20,21]. Abnormal expression of collagens and pathological collagen crosslinking ultimately resulted in increased tissue stiffness and altered tissue homeostasis[6]. The stiff ECM affects many aspects of the cell, such as motility, proliferation, and chemotherapeutic drug efficiency[22,23]. In our study, we found increased expression of type I collagen and decreased expression of type IV collagen in the ECM of CRC. The imbalance of ECM composition could result in an increase in ECM stiffness, which provides enough traction for cell proliferation and migration[24].
MMP-2 and MMP-9 are members of the MMP family, and they play an important role in the degradation and remodeling of the ECM[25]. TIMP-3 exists only in the ECM and could inactivate the MMPs by binding to MMPs[26]. Thus, reaching a balance between TIMPs and MMPs is conducive to the stability of the ECM. The remodeled ECM affects the motility and proliferation of cancer cells[22]. In our study, we found that the expression of MMP-2 and MMP-9 was higher in CRC tissue than in the normal tissue. The expression of MMP-2 increased with increased tumor stage. The expression of MMP9 was highest in stage III and we speculated that this is associated with the deactivation of MMP9.

Figure 1 Overview of extracellular matrix preparation.
In conclusion, our study showed that the expression of type I collagen, MMP-2, and MMP-9 increases in CRC while the expression of type IV collagen and TIMP-3 decreases in this malignancy. The changes in the composition of the ECM are conducive to cell proliferation. Thus, these findings will provide a new platform for the future design of anticancer drugs based on the biophysical properties of the tumor microenvironment.
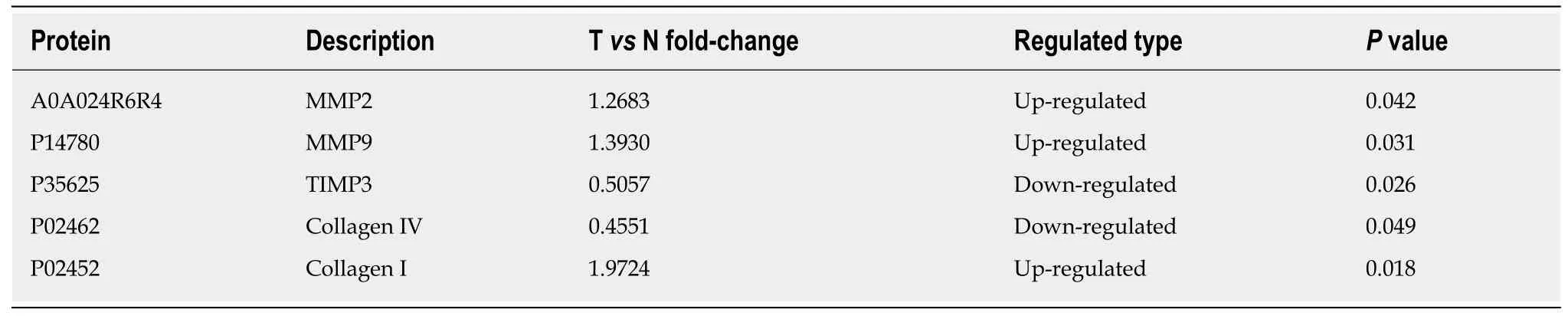
Table 1 Analysis of differential expression of proteins in normal colorectal tissues and colorectal cancer tissues
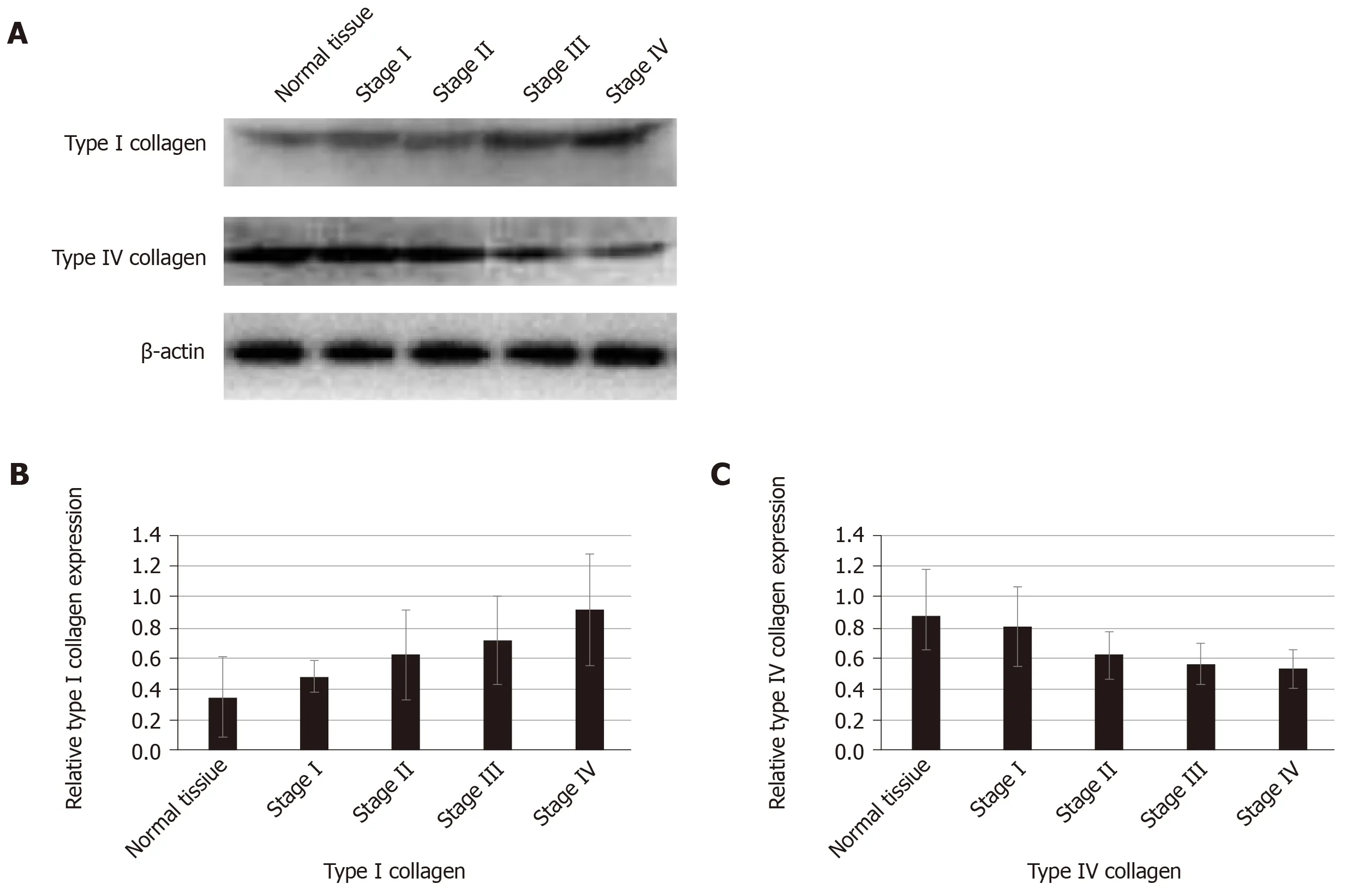
Figure 2 Expression of type I collagen and type IV collagen in normal tissue and colorectal cancer.
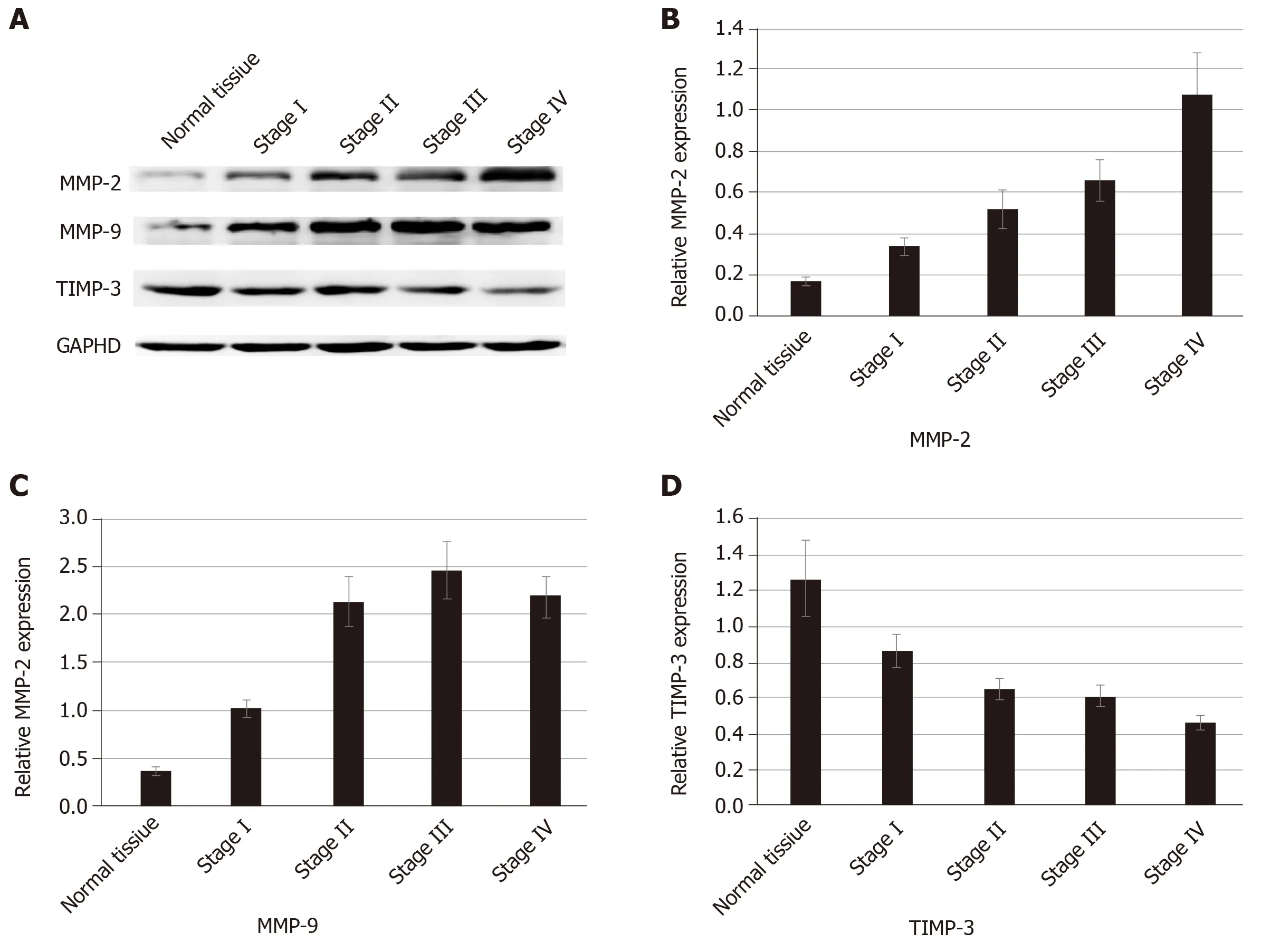
Figure 3 Expression of MMP-2, MMP-9, and TIMP-3 in normal tissue and colorectal cancer.
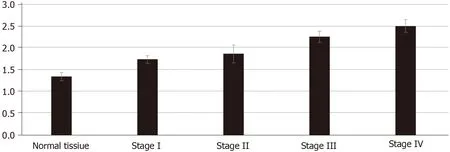
Figure 4 Cancer cells co-cultured with colorectal cancer extracellular matrix and normal tissue extracellular matrix in vitro.
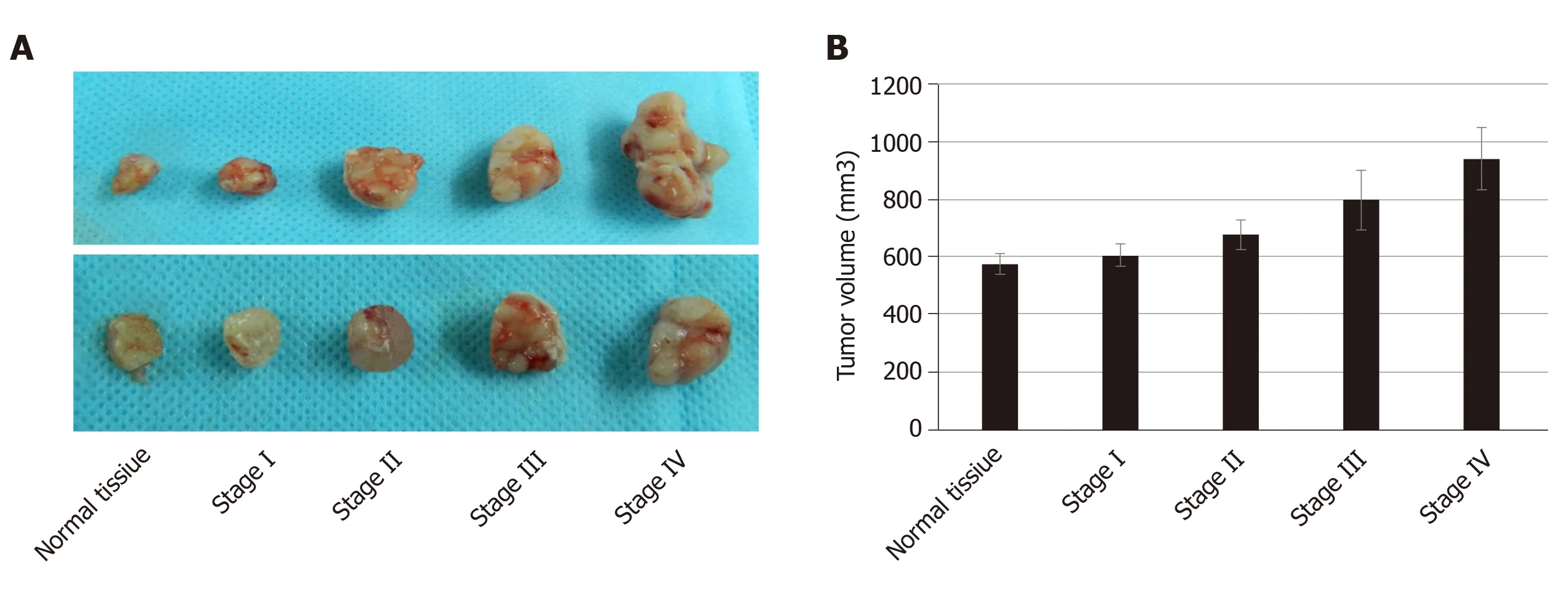
Figure 5 Cancer cells co-cultured with colorectal cancer extracellular matrix and normal tissue extracellular matrix in vivo.
ARTICLE HIGHLIGHTS
Research background
The extracellular matrix is not only the substantial support for tumor cells but also promotes the occurrence and development of tumors.
Research motivation
The extracellular matrix changes in the structure and composition during the process of oncogenesis and development of tumors. However, little is known about the changes of the extracellular matrix in different stages of colorectal cancers and the effect of these changes on the development of colorectal cancer. The answer to this may provide a new platform for the future design of anticancer drugs.
Research objectives
In this study, the authors aimed to study the changes of the extracellular matrix in different stages of colorectal cancer and the relationship between the changes of the extracellular matrix with the proliferation of cancer cells.
Research methods
The extracellular matrix was obtained by acellular technology from 60 colorectal cancer patients.Type I collagen, type IV collagen, MMP-2, MMP-9, and TIMP-3 were analyzed by Western blot.Besides, the extracellular matrix and the cancer cells were co-cultured in vivo and in vitro to study the effect of the extracellular matrix on the cancer cell proliferation.
Research results
The expression of type I collagen, MMP-2, and MMP-9 increased with increased tumor stage.The expression of type IV collagen and TIMP-3 decreased with increased tumor stage. The changed extracellular matrix promotes the cancer cell proliferation.
Research conclusions
This study showed that the extracellular matrix plays an important role in the development of tumor and this provides a certain theoretical basis for anti-tumor therapy
Research perspectives
The tumor microenvironment is a complex system. The extracellular matrix obtained by decellularization provides an ideal tumor model to study the occurrence and development of tumor.
ACKNOWLEDGEMENTS
We gratefully acknowledge Mrs. Gu Bei from the Chinese Academy of Medical Sciences for her technical help during the cell culture. We would also like to thank Mr.Li Lei-Lei for helping us in the data analysis.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Hepatic Hemangioendothelioma: An update
- Increased KIF21B expression is a potential prognostic biomarker in hepatocellular carcinoma
- Association between interleukin-21 gene rs907715 polymorphism and gastric precancerous lesions in a Chinese population
- Circulating cytokines and outcome in metastatic colorectal cancer patients treated with regorafenib
- Impact of preoperative chemoradiotherapy using concurrent S-1 and CPT-11 on long-term clinical outcomes in locally advanced rectal cancer
- Surgical intervention for malignant bowel obstruction caused by gastrointestinal malignancies
