Hepatic Hemangioendothelioma: An update
2020-05-16MayurVirarkarMohammedSalehRadwanDiabMelissaTaggartPeeyushBhargavaPriyaBhosale
Mayur Virarkar, Mohammed Saleh, Radwan Diab, Melissa Taggart, Peeyush Bhargava, Priya Bhosale
Mayur Virarkar, Mohammed Saleh, Radwan Diab, Priya Bhosale, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States
Melissa Taggart, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States
Peeyush Bhargava, Department of Radiology, The University of Medical Branch, Galveston,TX 77555, United States
Abstract
Key words: Epithelioid hemangioendotheliomas; Halo sign; Lollipop sign; Angiosarcoma;Cholangiocarcinomas; Hepatocellular carcinoma
INTRODUCTION
Epithelioid hemangioendotheliomas (EH) are vascular tumors that may affect the liver, lungs, mediastinum, and multiple other sites. However, the most commonly involved organ is the liver[1]. Primary epithelioid hemangioendotheliomas of the liver(EHL) are rare tumors with an incidence rate of less than 0.1 per 100000 population.Due to the low incidence rate, not a lot is understood regarding the pathogenesis of this tumor. However, it has been shown that EHL have a predilection for females,with a female-to-male ratio of 3:2, and affects the right lobe of the liver more than the left lobe. These tumors may be asymptomatic (24.8%), or symptomatic, with right upper quadrant pain being the most common presenting symptom (48.6%)[2]. The lungs, regional lymph nodes, peritoneum, bone, spleen, and diaphragm are the most common sites of extrahepatic involvement[2,3].
GENETICS
The molecular background of EH is still under investigation. To date, many genes involved in cell cycle control, signaling pathways, epigenetic modification, and DNA repair have been implicated in the pathogenesis of EH. A study by Erraniet al[4]sought to determine the genetic alterations of EH irrespective of the site of the tumor.They found that a WWTR1-CAMTA1 was a recurrent mutation in EH. WWTR1 is involved in transcriptional co-activation and is usually inhibited by the Hippo pathway, which causes the protein to translocate from the nucleus to the cytoplasm[5].Mutations that cause WWTR1 to be retained in the nucleus by transcriptions factors such as TEAD 1-4, are thought to cause the oncogenesis[6]. On the other hand,CAMTA1 is a transcription activator that belongs to the calmodulin-binding protein family[7]. The downstream targets are yet to be determined. CAMTA1 seems to behave as a tumor suppressor gene, as its deletion also results in oncogenesis[8,9]. However,the conserved regions in the fusion protein function in transcription and binding calmodulin, which might help promote tumorigenesis[7,10]. Additionally, the WWTR1-CAMPTA1 fusion protein seems to be specific to EH, whereby Erraniet al[4]demonstrated that it is not detected in tumors that mimic EH, such as epithelioid hemangioma and epithelioid angiosarcoma. As such, the authors recommended that WWTR1-CAMPTA1 may function as a tumor marker allowing physicians to identify EH when the diagnosis is unclear.
PATHOLOGY
Macroscopically, EHL seems to have two growth patterns, nodular and diffuse. The patterns represent different stages of the disease. Early disease presents as a nodular growth, and can be seen in 11.1% of patients. The nodular lesions are usually multiple(66.5%) and affect both lobes of the liver (82.2%)[11-13]. Later stages present as diffuse lesions due to nodular tumors growing in size and coalescing together. As the tumor infiltrates the surrounding structures, portal hypertension may develop[11,14].
The World Health Organization describe this tumor as malignant tumor with metastatic potential and variable clinical course (indolent to progressive)[15,16].Microscopically, EH can have three cell types: intermediate, dendritic, and epithelioid cells. Epithelioid cells are present in all cases, are of endothelial origin, and may present with vacuoles causing it to resemble signet cell morphology (Figure 1).Dendritic cells are stellate in shape and have multiple processes. The intermediate cells share features of both cell types[17].

Figure 1 Epithelioid hemangioendothelioma microscopic features.
Due to its endothelial origin, these tumors usually express the FLI-1 protein, which has been shown to be sensitive in identifying vascular tumors. In a single study, FLI-1 allowed for the identification of 100% of endometrioid hemangioendotheliomas with an 85% specificity. This marker showed to have a higher sensitivity than other endothelial markers such as CD31 and 34. Additionally, CD34 is not a specific marker for EH since it is expressed by most vascular tumors (90%). In the liver, the expression of podoplanin proved to be specific to EHL, allowing for accurate identification[18].
Pathologically, EH can be misdiagnosed in up to 80% of cases[2]. Most commonly,EH is misdiagnosed with angiosarcoma, cholangiocarcinomas (CC), metastatic carcinoma, and hepatocellular carcinoma (HCC) (sclerosing variant)[2,17,19,20]. Therefore,awareness of pathological differences is necessary to accurately diagnose these tumors.
EHL and angiosarcoma have similar presentations on immunohistochemistry profiling and hematoxylin-eosin staining, however differentiating features may still be found. On high power fields, angiosarcoma shows greater atypia, mitotic activity,and nuclear pleomorphism. Additionally, relative to angiosarcomas, EHL demonstrates greater sclerosis but less parenchymal destruction on low power fields[17]. Although both tumors may stain for CD34, factor VIII, and CD31, some markers such as D2-40 are more common in EHL[21].
Unlike EHL, intrahepatic cholangiocarcinomas (ICC), metastatic carcinomas, and HCCs do not usually express CD34, factor VIII, or CD 31, but are more likely to express cytokeratins. Finally, unlike EHL, HCC stains for CD10, arginase and HepPar-1 with polyclonal carcinoembryonic antigen and CD10 showing canalicular pattern.Some HCC’s may contain vessels that express CD34 due to capillarization of sinusoids, however, this stain is not positive n the neoplastic cells proper and is only expressed along the affected sinusoids[21,22]. On the other hand, the neoplastic cell composing EHL expresses CD34 diffusely.
IMAGING
Imaging is an indispensable component in initial tumor staging, treatment response evaluation, and recurrence identification. Lung or multiorgan involvement, disease progression, the presence of ascites, age more than 55 years, and male gender were found to be associated with a worse prognosis[23]. Extrahepatic tumor extension beyond portal lymph nodes is a negative prognostic factor[1].
There are two different types of EHL with different stages. The early stages of the disease present as the nodular type, while advanced stages appear as diffuse disease with coalescence of different lesions that may invade hepatic vasculature. The discrete nodules of EHL range in size from 0.5 cm to 12 cm and may progress to complex and confluent masses[2,24-26]. They are located peripherally and extend up to the liver capsule. Fibrosis and compensatory hypertrophy of the unaffected liver segments causes flattening or retraction of the liver capsule[27]. Because of the tumors metastatic potential and risk of recurrence after surgical approaches, imaging is pivotal for identifying the prognostic markers that can guide treatment. Imaging findings should include the exact number, dimensions, location, vascular or ductal involvement,locoregional lymphadenopathy and extrahepatic extension, as they are important factors for surgical decision on resectability[1].
ULTRASOUND
The appearance of EHL on ultrasound is important to recognize, since ultrasound might be the first modality used to assess right upper quadrant pain, which is the most common presenting symptom of EHL[28]. On ultrasound (US), EHL usually appear as hypoechoic lesions that may demonstrate heterogeneous echotexture(Figure 2). The presence of capsular retraction, calcifications, and multifocal lesions may further support the diagnosis[29].
EHL shows intratumoral vascularity and better visualized on color Doppler.Multifocal disease may cause intrahepatic congestion which will decrease the portal vein flow and result in portal hypertension with splenomegaly and hepatomegaly[30].
Another beneficial form of US is contrast enhanced US (CEUS). In a single study,EHL mostly depicts rim enhancement in the arterial phase on CEUS, but some masses may display heterogeneous hyperenhancement. Additionally, in one study all the lesions expressed early wash-out of the contrast agent on late and portal phases,secondary to the absence of portal veins in the tumor[29]. Though this is not specific for EHL, the presence of early contrast wash-out on late and portal phases signifies that the lesion is not benign and requires further investigation, be it biopsy or other imaging modalities[28].
COMPUTED TOMOGRAPHY
On non-contrast enhanced computed tomography (CT) scans, most lesions appear hypodense relative to the surrounding liver parenchyma[31]. Occasionally, a hyperdense rim may be evident due to the hypercellularity of the periphery. Relative to contrast enhanced CT scans, non-contrast enhanced CT scans can better visualize the bulk of the tumor, since they can additionally detect capsular retraction and coarse/nodular intratumoral calcifications, which can be present in up to 25% of cases[32-34]. The capsular retraction is due to hypertrophy of normal tissue in response to fibrosis of diseased tissue[35-37]. However, though capsular retraction is suggestive of EHL, it is not specific to it and other pathologies such as metastatic lesions and cholangiocarcinomas may cause capsular retraction[35,38,39].
On contrasted enhanced studies, three patterns of enhancement have been described. In the arterial phase, some tumors demonstrate mild homogeneous enhancement that does not increase in the delayed or portal vein phases. Other tumors develop a ring like enhancement during the arterial phase, with central filling on the delayed and portal phases, which is termed a “halo sign”. Lastly, some tumors demonstrate a heterogeneous pattern that progresses during the delayed and portal phases. The type of enhancement expressed seems to depend on the size of the tumor,as tumors more than 3 cm exhibited delayed heterogeneous enhancement, tumors 2 to 3 cm exhibited ring like enhancement, and tumors less than 2 cm exhibited homogenous enhancement (Figure 2)[40]. An imaging finding on contrast enhanced CT/MRI that is rather specific to EHL was first described by Alomari, which he termed the “lollipop sign” (Figure 3). EHL infiltrate sinusoids, venules, and veins,leading to narrowing or obstruction of these structures. Radiologically, this histological feature causes tapering and termination of the portal and hepatic vein and their branches as they approach the lesion. These occluded vessels are likened to the stick of the lollipop, while the hypodense well-defined tumor itself is likened to the candy, giving rise to the lollipop appearance. In its original description, only vessels that terminate within or at the edge of the rim meet the criteria of the lollipop sign.Additionally, lesions that enhance irregularly, are hyperdense, and/or have a central scar are excluded[41]. Relative to the other imaging characteristics, the lollipop sign is the least likely to be seen in other malignant or benign liver malignancies, making this imaging finding the most characteristic for EHL[40].

Figure 2 Hepatic hemangioepithelioma imaging feature.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is preferred over CT for the diagnosis of EHL due to its ability to detect smaller subcapsular lesions[11,12,42,43]. On non-contrast enhanced images, EHL typically demonstrates a “halo sign”, consisting of three layers of varying signal. These tumors usually demonstrate a hypointense core with a hyperintense rim on T1-Weighted Imaging (T1WI), while the tumor core is hyperintense on T2-Weighted Imaging (T2WI) with heterogeneous signal intensity with a hypointense rim[40]. This is secondary to a hypocellular center that may exhibit necrosis, prior hemorrhage, thrombosis, and/or calcification[44]. With contrast administration, EHL demonstrate findings similar to contrast enhanced CT, with three patterns of enhancement. In the arterial phase, tumors may demonstrate mild homogeneous enhancement, ring like enhancement, or a heterogeneous pattern depending on the size of the lesion (Figure 2 and 3).
Similar to T1WI and T2WI, most lesions (60%) exhibit a “target sign” on diffusion weighted imaging (DWI) with a hyperintense external rim and core. On higher bvalues, the periphery’s signal increases, signifying restricted diffusion. On the other hand, the core exhibits a lower signal, which once again highlights the hypocellular core and hypercellular periphery[28,34]. Likewise, on ADC map, tumors typically demonstrate high signal intensity centrally and low signal intensity peripherally. The high ADC values centrally help differentiate EHL from other tumors, as other tumors in the liver rarely exhibit high ADC values[34].

Figure 3 Hepatic hemangioepithelioma imaging feature.
POSITRON EMISSION TOMOGRAPHY/ COMPUTED TOMOGRAPHY
The healthy hepatocytes express high levels of glucose-6-phosphatase, which generally leads to rapid dissolution of the FDG avid signal relative to malignant tissue[45]. However, since EHL may exhibit variable levels of glucose-6-phosphatase,uptake and excretion of FDG is unpredictable, which limits evaluation of EHL in the early phase. Secondary to the unpredictability of FDG uptake, it has been recommended to review positron emission tomography/computed tomography(PET/CT) studies of EHL at two different time points to improve detection (Figures 4,5, 6 and 7)[45-47].
Similar to MRI and CT, EHL tumors demonstrate different patterns of FDG uptake.The lesions in the same patient may demonstrate different behavior on PET/CT, as well as appear at different time interval following FDG administration. Most EHL lesions demonstrate increased uptake relative to the surrounding liver parenchyma,however, up to one third of EHLs may demonstrate similar FDG uptake to the surround tissue. Additionally, the hypercellular periphery may cause higher uptake near the edge of the tumor which appears as a hypermetabolic rim[40,48]. Hence, the performance of PET/CT in the evaluation of metastatic disease and recurrence requires further assessment.
MANAGEMENT
There are no standardized guidelines for treating EHL. The treatment options are broad with inconsistent results and include chemotherapy, ablation, surgery and liver transplantation[49-53]. In fact, one study reported that there was no significant difference in 5-year survival rates among the different treatments[54]. Consequently, few studies have been conducted on EHL and thus natural history of the disease is unpredictable ranging from localized and indolent to aggressive and metastatic.
Furthermore, observation alone can yield favorable outcomes including spontaneous regression or disease stabilization if the tumor is non-aggressive. For example, a retrospective study on pediatric cases reported spontaneous remission without treatment in 60% of patients with focal disease (n= 10) and 42% of patients with multifocal disease (n= 12)[55]. Consequently, another study also demonstrated that overall survival does not significantly differ between local and metastatic or unilateral and bilateral disease[54]. These findings reinforce the unpredictability of tumor behavior, thus complicating the treatment. However, the general consensus is to begin with observation to assess tumor behavior before intervention, if applicable at all.

Figure 4 Imaging of hepatic hemangioepithelioma.
MEDICATIONS
Prednisone and propranolol are described in literature for medical management of EHL with variable results (Table 1). In a group of nine children with focal, multifocal,and diffuse disease assigned to propranolol (n= 3) or a combination of propranolol/prednisolone (n= 6), 100% of them in each treatment achieved tumor regression. Interestingly, three out of six of all patients with diffuse disease and five out of twelve patients with multifocal disease achieved tumor regression after receiving propranolol or propranolol/prednisolone[55]. Due to EHL’s association with hypothyroidism, L-thyroxine might be needed for patients with severely low T3 and T4 levels[55,56]. Emadet al[55]concludes that treatment for EHL should be escalated gradually according to disease’s response to treatment beginning with close observation for focal disease, then medical therapy and followed by chemotherapy.
CHEMOTHERAPY
Chemotherapy such as interferon, vincristine, or cyclophosphamide have variable results and should be added in patients not responsive to medical therapy such as propranolol or prednisolone (Table 2). Furthermore, patients presenting with early and aggressive disease tend to respond poorly to the medical management[55]. A study reported that regardless of disease severity and initial diagnosed stage, chemotherapy consistently and significantly decreased overall survival (OS) compared to those without chemotherapy[54]. However, some studies have advocated that chemotherapy should be used to reduce tumor burden and slow disease progression, and hepatic transplantation adopted as the optimal management due to favorable prognosis[57-59].Thalidomide has been suggested as a front-runner for tackling metastatic disease due to its anti-angiogenic properties[60]. In addition, sorafenib and intra-arterial 5-fluorouracil have demonstrated encouraging results for overall survival[14,61,62]. Due to its ability to block vascular endothelial growth factor (VEGF), a signaling protein highly expressed in EHL, Bevacizumab has been utilized for the management of EHL[19,63,64].
INTERVENTIONAL
Alternative treatment modalities include radiotherapy and radiofrequency(RFA)/microwave ablation (Figure 8). A case report described significant remission of EHL lesions two months after performing selective internal radiotherapy (SIRT), with a single dose 1.8 GBq 90-yttrium (48.6 mCi)[65]. On the other hand, RFA has proved to be a safe and efficient intervention for EHL lesions up to three centimeters large with up to 1% and 7% mortality and complication rates, respectively. RFA could eventually be proposed as an alternative to hepatic resection[66,67].
SURGERY
The hepatic resection is reserved for single, intrahepatic and resectable lesions (Table 3), while liver transplant is performed for patients with multiple bilobar hepatic lesions (Table 4). The surgical intervention for EHL includes hepatic resection, hepatic transplantation, and hepatic artery ligation (HAL)[68]. Due to the lack of established guidelines for EHL management, there has been a debate on the most effective surgical intervention. Rodriguezet al[69]argued that liver transplants should be adopted at higher rates due to EHL’s ability to metastasize and difficulty to resect,and to avoid liver failure in complicated intrahepatic disease. Some studies provided conflicting recommendations for extrahepatic disease. For example, two studies suggested extrahepatic spread as an acceptable criteria for liver transplants, since its resection did not necessarily correlate with survival[2], while a study considered it as a surgical contraindication[57,70]. Therefore, clear criteria for surgical procedures are yet to be established.

Figure 6 Imaging of hepatic hemangioepithelioma.
In a retrospective single center study from 2003-2014, three out of six patients who underwent hepatic resection had disease relapse and all of them survived (Figure 9).On the other hand, one out of two patients who underwent liver transplants died from complications of metastasis[70]. Consequently, there was an overall decrease in disease free survival rates for both surgical interventions. For the hepatic resection group, the disease-free survival rate decreased from 83.3% to 44.4% for one and three years respectively, while only one out of two patients had recurrence after two months (with the other surviving without recurrence) in the liver transplant group.Another case report showed rapid recurrence after only 1-month post liver transplant[71]. Conversely, larger studies have demonstrated that hepatic resection has better OS rates than liver transplants. Mehrabiet al[2]reported that the 5-year survival rate of patients that underwent hepatic resection and liver transplant was 75% and 54.5%, respectively, while Grotzet al[57]reported 86% and 73% 5-year survival rates,respectively. Consequently, disease free survival is higher in hepatic resection compared to liver transplants 62% and 46%, respectively. However, due higher number of hepatic resections compared to liver transplants, large patient cohort studies are required to achieve statistically significant results.
Interestingly, a retrospective study of 149 patients from the European Liver and Transplant Registry proposed an EHL -LT scoring system to predict the risk of posttransplant recurrence[72]. This study recommended liver transplants rather than observation as the main intervention due to better prognosis and concluded that extrahepatic disease was not found to be a significant risk factor. In fact, this study suggested that lymph node metastasis should not necessarily delay liver transplant.Consequently, macrovascular invasion, waiting time of 120 d or less for transplant,and hilar lymph node invasion were all found to be risk factors for post-transplant recurrence in multivariate regression analysis. This can potentially impact management of EHL, since macrovascular invasion can be detected before transplant,but imaging modalities still need further refinement to increase sensitivity and specificity. This scoring system has the potential to guide health care providers on whether or not to pursue liver transplant and to determine the frequency of posttransplant follow up. On the other hand, in order to provide a chance for administering neoadjuvant therapy, a mandatory waiting time from diagnosis to liver transplant is recommended to provide a chance for administering neoadjuvanttherapy. In addition, increasing waiting time could help prevent avoid inappropriate liver transplants for cases misdiagnosed as EHL such as hepatic hemangiosarcoma which is associated with poorer prognosis post-transplant[72,73]. Consequently, a 93.9%5-year survival rate was observed for patients with prognostic score of two or less based on the analysis of the European Liver Transplant Registry- European Liver and Intestinal Transplant Association (ELTR-ELITA) registry, while patients with a prognostic score of six or higher had a significantly lower (P< 0.001) 5-year survival rate of 38.5%[72]. Furthermore, adjuvant therapy could be assigned according to the new prognostic scoring system. Patients with a low score should have the priority for liver transplants, due to lower risk of post-transplant recurrence, while those with a high score should be given low doses of immunosuppression or antineoplastic immunosuppression combined with other neoadjuvant therapy[61,63,64,74,75].

A, B, C and D: Axial contrast enhanced CT images show hepatic hemangioepithelioma nodules (arrows); E:Axial 18F-labeled fluoro-2-deoxyglucose positron emission tomography/computed tomography image shows a heterogeneous uptake by hepatic hemangioepithelioma nodule (arrow), while rest of the nodules showed no fluoro-2-deoxyglucose update.
The left hepatic artery ligation (HAL) has been used in treatment of disseminated disease complicated by severe congestive heart failure (CHF). Bachmannet al[68]described a case of neonatal CHF refractory to digitalis that resolved with uncomplicated left HAL under Doppler ultrasound. According to literature, HAL generates favorable outcomes for EHL related CHF refractory to medical treatment.However, it is discouraged for EHL related CHF associated with other severe symptoms due to higher rates of complication including death. For example, one patient with thrombocytopenia and another with portal hypertension both died from diffuse intravascular coagulation[76]and biliary complications[77], respectively.Therefore, more research is required to define optimum criteria for surgical candidates and prevent ineffective and unnecessary surgery.
CONCLUSION
EHL has a very low incidence rate, and the pathogenesis is not completely known.The imaging characteristic “halo sign” and “lollipop sign” on CT and MRI can aid in diagnosis. The differential diagnosis includes angiosarcoma, cholangiocarcinomas(CC), metastatic carcinoma, and HCC (sclerosing variant). The histological and IHC findings confirms the diagnosis. Currently, there are no standardized guidelines for the management. The treatment options are broad and include chemotherapy,ablation, surgery and liver transplantation, with inconsistent results.
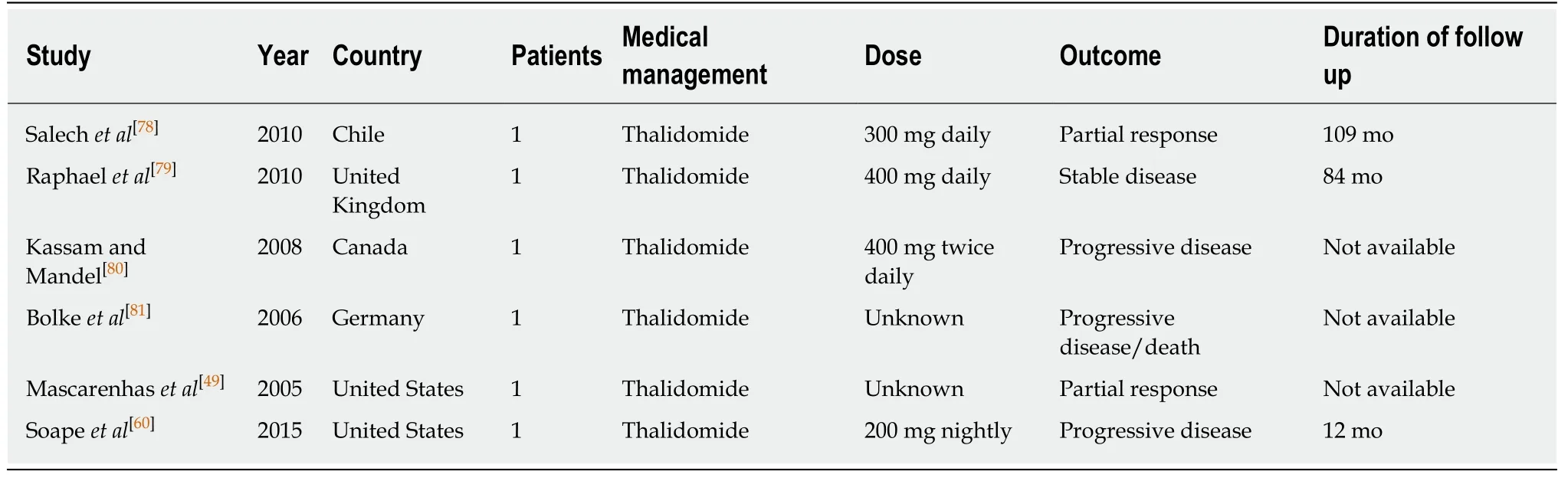
Table 1 Summaries of medical management studies for hepatic hemangioepithelioma

Table 2 Summaries of chemotherapeutics management studies for hepatic hemangioepithelioma
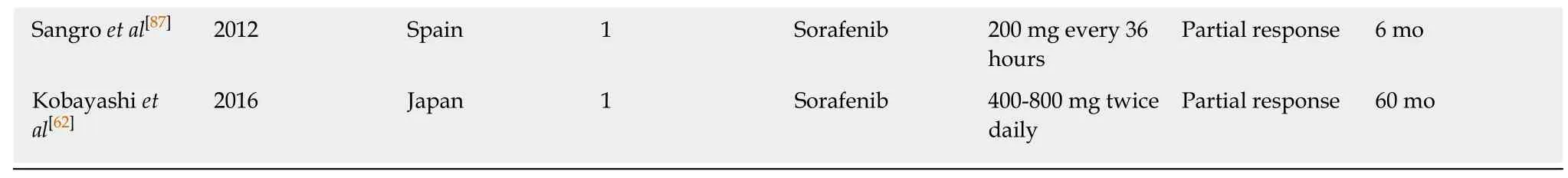
1On prednisolone/interferon treatment, regression was reported in 1 patient and progression in the other patient.

Table 3 Summary of surgical management studies for hepatic hemangioepithelioma

Table 4 Summary of liver transplant studies for hepatic hemangioepithelioma

EHL: Hepatic hemangioepithelioma.
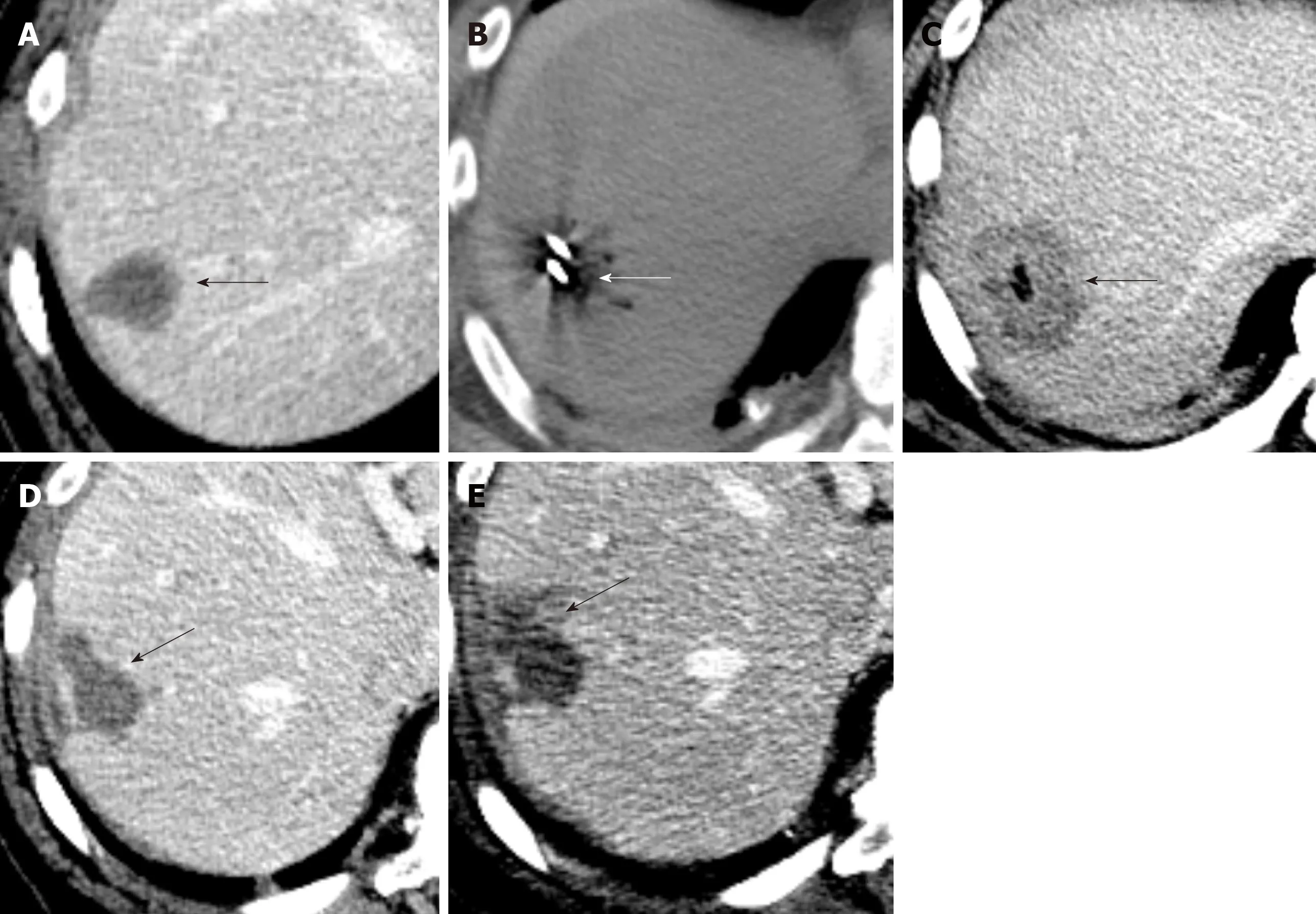
Figure 8 Ablation of hepatic hemangioepithelioma.

Figure 9 Recurrent hepatic hemangioepithelioma.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells
- Increased KIF21B expression is a potential prognostic biomarker in hepatocellular carcinoma
- Association between interleukin-21 gene rs907715 polymorphism and gastric precancerous lesions in a Chinese population
- Circulating cytokines and outcome in metastatic colorectal cancer patients treated with regorafenib
- Impact of preoperative chemoradiotherapy using concurrent S-1 and CPT-11 on long-term clinical outcomes in locally advanced rectal cancer
- Surgical intervention for malignant bowel obstruction caused by gastrointestinal malignancies
