Impact of preoperative chemoradiotherapy using concurrent S-1 and CPT-11 on long-term clinical outcomes in locally advanced rectal cancer
2020-05-16KeiKimuraNaohitoBeppuHiroshiDoiKozoKataokaTomokiYamanoMotoiUchinoMasatakaIkedaHirokiIkeuchiNaohiroTomita
Kei Kimura, Naohito Beppu, Hiroshi Doi, Kozo Kataoka, Tomoki Yamano, Motoi Uchino, Masataka Ikeda,Hiroki Ikeuchi, Naohiro Tomita
Kei Kimura, Naohito Beppu, Kozo Kataoka, Tomoki Yamano, Masataka Ikeda, Naohiro Tomita,Division of Lower Gastrointestinal Surgery, Department of Surgery, Hyogo College of Medicine, Nishinomiya, Hyogo 663-8501, Japan
Hiroshi Doi, Department of Radiology, Hyogo College of Medicine, Nishinomiya, Hyogo 663-8501, Japan
Hiroshi Doi, Department of Radiation Oncology, Kindai University Faculty of Medicine,Sayama, Osaka 589-8511, Japan
Motoi Uchino, Hiroki Ikeuchi, Department of Inflammatory Bowel Disease, Division of Surgery,Hyogo College of Medicine, Nishinomiya, Hyogo 663-8501, Japan
Abstract
Key words: Preoperative chemoradiotherapy; Rectal cancer; Irinotecan;Tegafur/gimeracil/oteracil; Neoadjuvant chemoradiotherapy; Radiation therapy
INTRODUCTION
In the 2000s, numerous studies were planned to investigate the optimal preoperative treatment strategies for advanced rectal cancer. The National Comprehensive Cancer Network and European Society for Medical Oncology consensus guidelines consider preoperative 5-fluorouracil (5-FU)-based chemoradiotherapy (CRT), 45–50.4 Gy, as standard treatment[1,2]. However, the local recurrence rate remains about 10%, and risk factors for local recurrence include T4 stage, mesorectal fascia invasion (MFI),extramural vascular invasion (EMVI) and lateral lymph node (LLN) swelling[3-6].Multidisciplinary treatments were planned to overcome this issue, such as extended surgery, higher radiation doses, and concurrent use of second drugs, such as oxaliplatin or irinotecan (CPT-11)[7-11]. With regard to the concurrent use of second drugs, six prospective studies failed to confirm any additional benefit of oxaliplatin,and there was a significant increase in severe toxicity and an insufficient response rate[12-17]. However, several Phase II trials have demonstrated the feasibility, safety and effectiveness of CPT-11 as a second drug, with higher pathological complete response(pCR) rates[7,18-25].UGT1A1polymorphisms that can be used to predict the probability of severe toxicity would be of interest for proper therapeutic management using CPT-11[26]. Therefore, the purpose of this study was to investigate the clinical outcomes of 82 patients with locally advanced rectal cancer, located 8 cm from the anal verge,treated with preoperative CRT using tegafur/gimeracil/oteracil (S-1) plus CPT-11.
MATERIALS AND METHODS
Patients
We included 82 patients with T3-4, N0-2, M0 rectal cancer located within 8 cm of the anal verge who were treated with preoperative CRT using S-1 plus CPT-11 between 2009 and 2016. Prior to preoperative therapy, all patients underwent staging work-ups that included digital rectal examination, measurement of tumor marker levels(carcinoembryonic antigen and carbohydrate antigen 19-9), chest X-ray, abdominal and pelvic computed tomography (CT) and magnetic resonance imaging (MRI). MRI was performed on two occasions, as part of initial staging and following preoperative therapy. Testing forUGT1A1*6 and *28 polymorphisms under national insurance was finally given approval in November 2008 in Japan, and it became measurable at our institution in March 2009.UGT1A1polymorphisms are assessed only in cases in which consent is obtained after consultation with a specialist in hereditary diseases[27].The protocol for the present study was based on the SAMRAI-1 trial[28].
The patients were divided into two groups in accordance with the European Society for Medical Oncology guidelines to confirm the outcomes for these subgroups[29]: (1) “bad” rectal cancer [T3(b)c/T4 with peritoneal or vaginal involvement only, N1–2, MFI negative]; and (2) “ugly” rectal cancer (T4 with overgrowth to adjacent organs, pelvic side walls or sacrum, LLN positive, MFI positive).
Preoperative CRT protocol
Preoperative CRT consisted of S-1 (Days 1-5, 8-12, 22-26 and 29-33; 80 mg/m2/d),CPT-11 (Days 1, 8, 22 and 29; 60 mg/m2/d), and radiation (total 45 Gy, 1.8 Gy/d, 5 d per week for 5 wk). Six to eight weeks after completion of preoperative CRT, the patients were scheduled to undergo radical surgery.
Surgical procedure and pathological assessments
All patients underwent total mesorectal excision or extended total mesorectal excision(total mesorectal excision with adjacent visceral resection) to achieve R0 resection. The surgical procedure included low anterior resection, intersphincteric resection and abdominoperineal resection. Intersphincteric resection was recommended in accordance with tumor stage and location, patient age, and preoperative anal function, and patients who did not meet those criteria were selected for abdominoperineal resection. Diverting ileostomy was routinely constructed for all patients with intestinal continuity. LLN dissection was performed when pretreatment MRI showed that the LLNs had a short-axis diameter > 7 mm. Postoperative complications were assessed according to the Clavien-Dindo classification[30].Pathological response to CRT was evaluated according to the Japanese Classification of Colorectal Carcinoma of the Japanese Society for Cancer of the Colon and Rectum(8thedition). Grade 0 was defined as no evidence of a therapeutic effect and Grade 3 was pCR[31]. We defined a good response as Grade 2 or 3 and poor response as Grade 0 or 1a/1b.
Toxicity or relative dose intensity of chemotherapy
Hematological and nonhematological toxicity caused by preoperative CRT was evaluated according to the Common Terminology Criteria for Adverse Events,version 4.0[32]. Relative dose intensity was calculated as the ratio of the actual dose to the scheduled dose; S-1 (1600 mg/m2), CPT-11 (240 mg/m2) and full irradiation dose(45 Gy). Dose reductions of CPT-11 were not applied to the group of patients withUGT1A1mutation.
Patient follow-up
Median follow-up was 51 mo (range, 17-116 mo). Postoperative adjuvant chemotherapy using 5-FU-based chemotherapy was recommended for all patients except those with ypT0/1 stage, high age, comorbidity, postoperative complications,and social factors. Patient surveillance was subsequently performed as follows:chest–abdominal CT every 6 mo, colonoscopy annually, and blood tests (including measurement of carcinoembryonic antigen and carbohydrate antigen 19-9 levels) at 3-mo intervals. Local recurrence was defined as the detection of a recurrent tumor within the pelvis, and recurrence was defined as the presence of recurrent disease outside the pelvis.
Statistical analysis
Local recurrence-free survival (LFS), relapse-free survival (RFS) and overall survival(OS) were estimated using the Kaplan–Meier method and compared using the logrank test. Theχ2test was also used to evaluate associations betweenUGT1A1polymorphisms and toxicity and feasibility of treatment. We further evaluated clinical factors associated with LFS and RFS to determine the optimal clinical criteria of this regimen by Cox proportional hazard regression model. Independent variables withP< 0.1 in univariate analysis were entered into a multivariate analysis andP< 0.05 was considered statistically significant. Statistical analyses were performed using JMP version 12.0 software (SAS Japan Inc., Tokyo, Japan).
RESULTS
Clinical characteristics
The patients’ clinical characteristics are shown in Table 1. Clinical T4 stage was diagnosed in 10 patients (12.2%). Clinical N stage was deemed positive in 46 patients(56.1%). MRI revealed tumor involvement of the MF in 29 patients (35.4%). EMVI was observed in 36 patients (43.9%). According to the risk category of rectal cancer, 50 patients (61.0%) were divided into the bad group and 32 (39.0%) into the ugly group.
Compliance and toxicity
The relative dose intensity was 90.1% for S-1, 92.9% for CPT-11 and 97.6% for RT.Toxicity data are shown in Table 2. Grade 3 or 4 hematological toxicity consisted of leukopenia (n= 15; 18.3%), neutropenia (n= 16; 19.5%) and febrile neutropenia (n= 3;3.6%). Grade 3 or 4 nonhematological toxicity consisted of diarrhea (n= 22; 26.8%).For Grade 3 or 4 hematological toxicity, four of 16 neutropenia patients (25.0%) whose neutrophil count was reduced to < 500 cells/μL received granulocyte colonystimulating factor. For Grade 3 or 4 nonhematological toxicity, four of 22 diarrhea patients (18.2%) were prescribed loperamide. All patients recovered after these conservative treatments.
UGT1A1 genotype distribution and its association with toxicity profiles
Associations between toxicity/feasibility andUGT1A1polymorphisms were investigated (Table 3). Forty-eight of 82 patients (58.5%) were assessed forUGT1A1polymorphism, and 25 (52.1%) were wild type and 23 (47.9%) were mutant type.Patients with the mutant type had more Grade 3 or 4 hematological toxicity than those with the wild type had (P< 0.05). However, there was no significant difference in the incidence of nonhematological toxicity, including diarrhea, in either genotype(P= 0.65). There was no significant difference in CPT-11 dose intensity according toUGT1A1polymorphisms despite the significant differences observed in hematological toxicity (P= 0.26).
Operative findings and postoperative complications
Thirty-one patients (37.8%) underwent low anterior resection, 43 (52.4%)intersphincteric resection and eight (9.8%) abdominoperineal resection. Five patients(6.1%) underwent combined adjacent organ resection and eight (9.8%) LLN dissection.
The postoperative complications are shown in Table 4. Grade 3 pelvic infection was confirmed in nine patients (11.0%) and five (6.1%) developed Grade 3 ileus. Among the patients undergoing sphincter-preserving surgery, seven (9.5%) had Grade 3 anastomosis leakage. During follow-up, six patients could not undergo stoma takedown because of pelvic infection with anastomotic leakage (n= 4) and local recurrence (n= 2).
Pathological findings
Pathological findings are listed in Table 5. Thirteen patients (15.9%) achieved complete tumor regression with tumor regression grade 3 (pCR). T downstaging was seen in 41 patients (50.0%) and N downstaging in 36 (43.9%). R0 resection was performed in 79 of 82 patients (96.3%) and R1 resection in three (3.7%), with microscopic residual tumor in the anus levator muscle (n= 2) and pelvic plexus on the pelvic sidewall (n= 1). No patient had R2 resection. Patients withUGT1A1mutations showed a significantly better response to CRT (including CPT-11) than those without mutations (Table 3).
Recurrence and survival
Twenty-six patients (31.7%) received 5-FU-based adjuvant chemotherapy: UFT plus leucovorin (n= 19), mFOLFOX6 (n= 4), S-1 (n= 2) and capecitabine (n= 1). The reasons for not receiving adjuvant chemotherapy were: ypT0/1 stage (n= 18), high age (n= 11), comorbidity (n= 2), postoperative complications (n= 12), social factors (n= 6), and others (n= 7).
After a median follow-up of 51 mo, 5-year LFS, 5-year RFS and 5-year OS rates were 90.1%, 72.5% and 91.3%, respectively (Figure 1). Local recurrence was seen in sixpatients: LLNs (n= 4) and other sites (n= 2). Distant recurrence was detected in 20 patients: lung (n= 15), liver (n= 6), para-aortic region (n= 2), inguinal region (n= 1)and bone (n= 1). Some patients had overlapping metastases. LFS did not differ significantly between the bad and ugly groups (96.0%vs76.2%;P= 0.10); however,RFS was significantly poorer in the ugly group (38.5%vs87.8% in bad group;P<0.01).
合理设置教学情境 学习是一件苦差事,知识不是在幸福快乐中就能被完全掌握的。微课的学习时间主要集中在碎片化时间段,要提高受众的接受能力,不仅要调动受众的学习欲望,还要让课程内容对其产生吸引力。

Table 1 Patient characteristics
Risk factors for LFS and RFS
We investigated the risk factors for LFS and RFS (Table 6). Multivariate analysis showed that no risk factors for LFS were detected, including previously described risk factors such as T4 stage, MFI, EMVI and LLN swelling. However, MFI and EMVI were associated with poor RFS for locally advanced rectal cancer (OR: 5.82, 95%CI:1.68-20.2,P< 0.01; OR: 3.42, 95%CI: 1.02-11.5,P= 0.04).
DISCUSSION
We reported the safety, effectiveness and long-term outcomes of concomitant use of CPT-11 with 5-FU-based CRT for locally advanced rectal cancer. S-1 is an oral anticancer agent containing tegafur (a prodrug of 5-FU) with two modulators,gimeracil and oteracil potassium, which markedly increase the radiosensitivity of cancer cells[33]. CPT-11 augments inhibition of thymidylate synthase – the target enzyme of 5-FU[34]. In addition, 5-FU induces topoisomerase I, and cancer cells overexpressing topoisomerase I show increased chemosensitivity to CPT-11[35]. Suchin vitromechanisms are effective in combination with 5-FU as a radiosensitizer for preoperative CRT[7]. Furthermore,UGT1A1polymorphisms that can predict the probability of developing potentially severe toxicity during treatment with CPT-11-based regimens could be clinical factors in the proper management of treatment[26].The purpose of this study was to investigate the clinical outcomes of patients with locally advanced rectal cancer treated with preoperative CRT using S-1 plus CPT-11.

Table 2 Acute toxicity according to Common Terminology Criteria for Adverse Events 4.0, on patients receiving chemoradiotherapy, n (%)
Current standard CRT regimens include only 5-FU. However, several clinical trials incorporating a second active systemic agent into conventional CRT regimens have been performed to examine the ability of the regimens to increase pCR rate and improve resectability and locoregional control[6,10]. Two such second drugs, oxaliplatin and CPT-11, have been investigated in clinical trials.
With regard to oxaliplatin, six randomized Phase III studies have compared oxaliplatin-based with 5-FU-based regimens[12-17]. Among these, the STAR-01 (16%both groups), ACCORD 12/0405 (19%vs14%), NSABP R-04 (21%vs19%) and PETACC-6 (15%vs13%) studies reported that there were no substantial improvements in pCR rates, and significantly increased intolerable Grade 3 or 4 toxicity. For this reason, the concomitant use of oxaliplatin in 5-FU-based CRT has not been permitted (Supplementary Table 1). No Phase III studies using CPT-11 have been documented; however, nine Phase II studies (2 randomized controlled trials and 7 single-arm studies) have assessed the usefulness of CPT-11 as a radiosensitizer[7,18-25].These studies indicated that this CPT-based regimen was promising in terms of pCR rate (range 13.7%-37%). Grade 3 or 4 toxicity was mild and led to good relative dose intensity with on-schedule treatment without dose reduction (Supplementary Table 2).
The most frequent severe toxicity was neutropenia (2.1%-12%) and diarrhea (2.1%-22%). Generally, toxicity was correlated with the dose of chemotherapy. Junget al[25],who used 40 mg/m2CPT-11, demonstrated that the rate of Grade 3 or 4 hematological toxicity was 1.4% and the rate of Grade 3 or 4 nonhematological toxicity was 5.7%.Satoet al[7], who used 80 mg/m2CPT-11, demonstrated that the rate of Grade 3 or 4 hematological toxicity was 6% and the rate of Grade 3 or 4 nonhematological toxicity was 4.5%. These results suggest that concurrent use of second drugs, such as CPT-11 as a radiosensitizer, is well tolerated in terms of toxicity.
UGT1A1polymorphisms have been confirmed as predictive markers of severe toxicity of CPT-11 in a metastatic setting[26]. Our previous study demonstrated the effectiveness ofUGT1A1polymorphism in predicting the toxicity of preoperative CRT using CPT-11, although it was only a small retrospective study[36]. Thus, to provide patients with the full benefit of CRT, good tolerance of CPT-11-based regimens for patients withUGT1A1mutant type, as well as the prevention and early treatment of severe toxicity, is important. This suggests that drawing definitive conclusions about the role ofUGT1A1polymorphisms requires a randomized trial, to assess whether genotype-adjusted dose of CPT-11 would help establish a well-tolerated, effective dose for tumor response in patients with wild-type and mutantUGT1A1.
The present study included patients with highly advanced rectal cancer: 29 (35.4%)with T4 or T3 with MFI, 36 (43.9%) with EMVI, 24 (29.8%) with N2, and 32 (39.0%)with ugly rectal cancer. Even such highly advanced rectal cancer demonstrated favorable local control. With respect to systemic recurrence, highly advanced rectal cancer has a high recurrence rate, with poor prognosis; therefore, combined use of systemic treatment, mainly including chemotherapy, is important for prolonging survival benefit[37]. Further studies are warranted to examine the additional effect ofCPT-11 on those tumors.

Table 3 Associations between toxicity, feasibility and treatment effect and UGT1A1 polymorphisms, n (%)
Our study had several limitations. First, it was a small retrospective study performed in a single institution. Second, we excluded atypical rectal cancer, such as mucinous carcinoma caused by anal fistula, which is associated with a poorer response to CRT, because we chose surgery without radiation. Third, we excluded patients with performance status 3/4 or those aged > 80 years who cannot tolerate this regimen owing to comorbidity and old age. Such patients (n= 3) were treated with stoma creation alone. Fourth, the follow-up time was not sufficient to evaluate OS, LFS and RFS. Fifth,UGT1A1polymorphism analysis was not performed for all patients receiving preoperative CRT. Finally, we did not study toxicity-based dosefinding methods for S-1 plus CPT-11 preoperative CRT in a Phase I study.Nevertheless, this study demonstrated the safety, effectiveness and long-term oncological outcomes of locally advanced rectal cancer treated with concomitant CPT-11 and 5-FU-based CRT.
In conclusion, our single-center retrospective study confirmed good compliance,favorable tumor regression and feasible oncological outcomes of preoperative CRT using S-1 plus CPT-11, and favorable local control of highly advanced rectal cancer by this regimen.
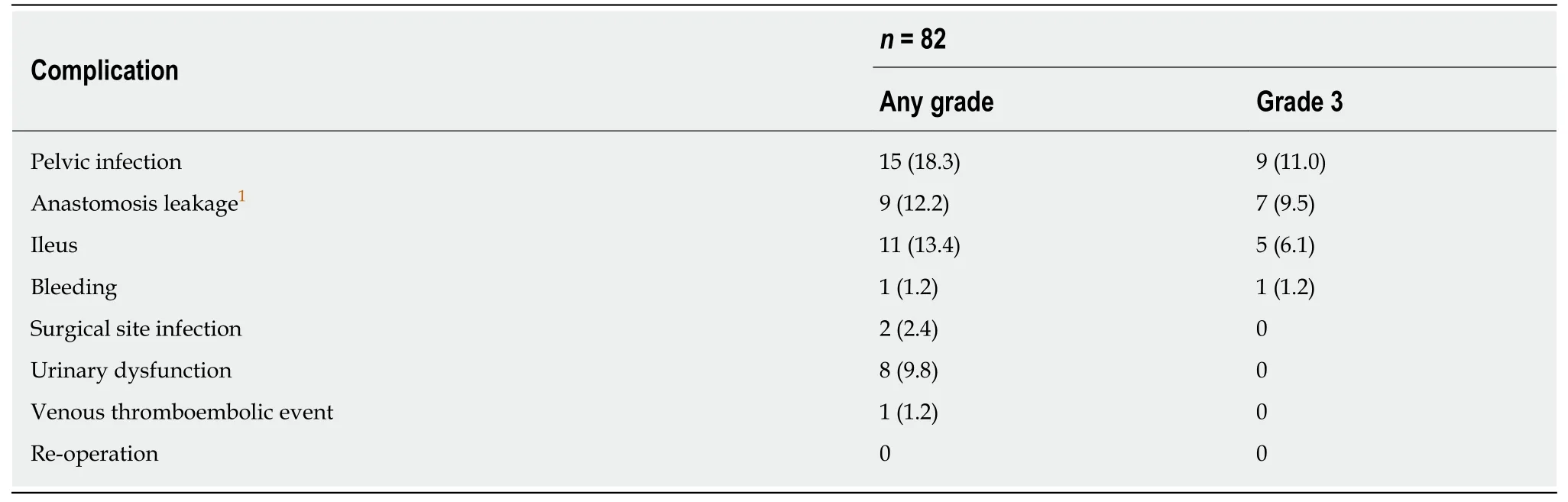
Table 4 Postoperative surgical complications, n (%)

Table 5 Pathological tumor characteristics, n (%)

Table 6 Multivariate prognostic analysis for local recurrence-free survival and relapse-free survival

Figure 1 Long-term outcomes of patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy using tegafur/gimeracil/oteracil plus irinotecan.
ARTICLE HIGHLIGHTS
Research background
Prospective studies have investigated the optimal treatment strategies for management of locally advanced rectal cancer, and have concluded that preoperative 5-fluorouracil-based chemoradiotherapy (CRT) at 45–50.4 Gy is a standard treatment. However, local recurrence rate remains about 10%; mainly for highly advanced cases.
Research motivation
Multidisciplinary treatments were planned to overcome highly advanced rectal cancer, such as extended surgery, higher radiation doses, and concurrent use of second drugs, such as oxaliplatin or CPT-11.
Research objectives
The aim of this study was to investigate the safety, therapeutic effect, and outcome of preoperative CRT using S-1 plus irinotecan for locally advanced lower rectal cancer.
Research methods
Between 2009 and 2016, 82 patients underwent total mesorectal excision after preoperative CRT.Preoperative CRT consisted of S-1 (80 mg/m2/d), CPT-11 (60 mg/m2/d), and radiation (total 45 Gy). The median follow-up was 51 months (range: 17-116 mo).
Research results
This regimen was welltolerated in terms of toxicity. Associations between toxicity/feasibility and UGT1A1 polymorphisms were investigated. Compared with patients with wild-type UGT1A1, those with mutant type had more Grade 3 or 4 hematological toxicity (P < 0.05). With regard to oncological outcome, mesorectal fascia invasion and extramural vascular invasion were associated with poor relapse-free survival for locally advanced rectal cancer. However, Cox regression analysis did not detect any risk factors for local recurrence-free survival.
Research conclusions
This regimen had favorable oncological outcomes for highly advanced rectal cancer.
Research perspectives
This was a small retrospective study performed in a single institution. A randomized multicenter study is needed to investigate the influence of dose setting by UGT1A1 polymorphism for preoperative CRT using irinotecan.
猜你喜欢
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Utility of positron emission tomography-computed tomography scan in detecting residual hepatocellular carcinoma post treatment: Series of case reports
- Clinical outcomes of patients with duodenal adenocarcinoma and intestinal-type papilla of Vater adenocarcinoma
- FOLFOXIRI vs FOLFIRINOX as first-line chemotherapy in patients with advanced pancreatic cancer: A population-based cohort study
- Surgical intervention for malignant bowel obstruction caused by gastrointestinal malignancies
- Circulating cytokines and outcome in metastatic colorectal cancer patients treated with regorafenib
- Association between interleukin-21 gene rs907715 polymorphism and gastric precancerous lesions in a Chinese population
