Decomposition of dioxin-like components in a DBD reactor combined with Hg/Ar electrodeless ultraviolet
2020-04-24WeixuanZHAO赵玮璇LipingLIAN连莉萍YanpeiWU吴妍婄YanghaichaoLIU刘杨海超RenxiZHANG张仁熙GangLUO罗刚andHuiqiHOU侯惠奇
Weixuan ZHAO(赵玮璇),Liping LIAN(连莉萍),Yanpei WU(吴妍婄),Yanghaichao LIU (刘杨海超),Renxi ZHANG (张仁熙),3,Gang LUO (罗刚),3 and Huiqi HOU (侯惠奇)
1 Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention (LAP3),Institute of Environmental Science,Fudan University,Shanghai 200433,People’s Republic of China
2 National Engineering Laboratory for VOCs Pollution Control Material &Technology,University of Chinese Academy of Sciences,Beijing 100049,People’s Republic of China
3 Authors to whom any correspondence should be addressed.
Abstract
Keywords:dielectric barrier discharge (DBD),dioxin-like,3,4-dichlorodiphenyl ether,EDUV photolysis
1.Introduction
Dioxin-like components,which are the persistent organic pollutants (POPs),have been verified not only to induce malformation,cancer and mutagenesis,but also to have the ability of long-distance migration and biological accumulation [1–3].More importantly,dioxin-like components have strong lipophilic properties,which signified that dioxin-like components might accumulate in the human body and lead to a serious threat to human health [4–6].Although,control of pollution sources and restrictions on the use of halogenated chemical products could suppress the total amount of dioxinlike components,the risk of global environment contamination by dioxin-like components is still not neglected [7–9].Therefore,it is necessary to explore the practical removal method of dioxin-like components for preventing its potential danger.
Recently,researches on abatement of dioxin-like components have attracted more and more attention because of their wonderful potential for improving reducing emission and excellent referential significance on dioxins removal[10–15].The technologies to remove dioxin-like components mainly include active carbon absorption [16–19],pyrolysis[20–22]and catalysis oxidation [23–25].Compared with these traditional methods,dielectric barrier discharge (DBD)technology has attracted special attention in the field of abatement of dioxin-like components [26,27]because of its high activity and low energy consumption.However,there are still some unavoidable drawbacks in single DBD system,such as limited product selectivity,removal efficiency and working stability [28,29].Therefore,the combined DBD with other technologies,especially photolysis,has attracted considerable attention for its wonderful superiority in pollution control [30,31].Further,previous studies show that dioxinlike components could also be decomposed by absorbing UV radiation[32].Considering that dioxin-like components could be removed by plasma and photolysis,combining plasma and UV radiation in one reactor might achieve synthetical activity in the removal of dioxin-like components.Interestingly,the electrodeless ultraviolet (EDUV) radiation could be steadily activated by DBD during discharge [33,34].Therefore,we designed a new combined reactor,which could simultaneously produce EDUV radiation and plasma.In this paper,we called this combined process as DBD-EDUV system.This new combined reactor has been verified to achieve effective removal of several pollutions such as H2S [35,36],CS2[37]and butyl acetate [38].However,as far as we know,the research on abatement of dioxin-like components by DBDEDUV process is limited.
In this paper,the combined reactor with Hg/Ar EDUV photolysis activated by DBD was applied on removal of 3,4-dichlorodiphenyl ether.The performance of the DBDEDUV system was studied through investigating the effects of specific input energy(SIE)and feeding gas components on 3,4-dichlorodiphenyl ether abatement.The involved chemical mechanism was also discussed through products analysis by GC-MS and the emission spectra.
2.Experimental section
2.1.Experimental procedure and setup
The schematic process of the DBD-EDUV hybrid system was shown in figure 1.The feeding gas consisted of O2or Ar with N2as the balance gas.The flow rate of feeding gas was 3 l min−1and each feeding gas was controlled independently by Horiba Stec-4400 mass flow controller.3,4-dichlorodiphenyl ether (64.5 g l−1,10 ml) dissolved in decane was injected into the gap between the outer electrode and inner electrode.The feeding gas was driven into 3,4-dichlorodiphenyl ether reaction liquid to produce bubbles.
The structures of DBD reactor and DBD-EDUV reactor were shown in figure 2.The DBD reactor was coaxial cylinders-type with 4 mm gap and consisted of an inner electrode(copper,diameter 2 mm)covered by the inner quartz tube (4 mm outer diameter and 150 mm length) and outer electrode(aluminum foil,thickness 0.2 mm)packaging on an outer quartz tube(12 mm inner diameter and 150 mm length).The gap between the inner electrode and outer electrode of DBD reactor was 6 mm (quartz tube thickness 1 mm).The DBD was driven by AC power supply,which could provide a sinusoidal alternating SIE varying from 0 to 2500 J l−1at frequencies of 15–25 kHz.The SIE represented the energy density of DBD reactor,which was equal to the applied power divided by flow rate of feeding gas.The applied power was detected by a 200 MHz digital phosphor oscilloscope (Tektronix,TDS2024B,USA) connected to a 1000:1 HV probe(Tektronix,P6015A,USA).
On the basis of DBD reactor,the DBD-EDUV reactor added an airtight cavity filled with Hg/Ar (1.5 mm width,60 mm length) around the inner quartz tube,which could be activated by plasma to generate UV radiation during.
2.2.Analytical measurements
The concentration of 3,4-dichlorodiphenyl ether was quantified by an 8-channel UV spectrophotometer (Scinco,S-3100,Korea,0.2 nm),consisting of a DH-2000-BAL light source and six 1 cm pathlength transflectance dip probes.Wavelength scans (<1 nm resolution) were performed on samples at 25°C from 200 nm to 800 nm.The products of DBDEDUV process were analyzed via gas chromatography-mass spectrometer (Thermo Fisher,Focus DSQ II,USA).The emission spectrum was measured by fiber optic spectrometer(Avantes,AvaSpec-Rackmount,Netherlands).The conversion of 3,4-dichlorodiphenyl ether was assessed by following equation:

Here,ρuntreatedandρtreatedrepresent the concentration(g l–1)of 3,4-dichlorodiphenyl ether under the condition of untreated and after treated by DBD-EDUV system.
3.Results and discussion
3.1.Performance of DBD-EDUV hybrid system
Figure 3 presented the effect of SIE on 3,4-dichloro-diphenyl ether conversion in single DBD,single EDUV and DBDEDUV systems.As shown in figure 3,although the conversion of 3,4-dichloro-diphenyl ether in DBD,EDUV and DBDEDUV systems all increased as increasing of SIE,DBD-EDUV system performed better activity on 3,4-dichloro-diphenyl ether removal.When the SIE was 2500 J l−1and O2content was 10%,the 3,4-dichloro-diphenyl ether conversion in DBDEDUV system could reach 72.9% but the value was only 20.7% and 51.2% in single EDUV and DBD systems,respectively.Plasma could produce high energetic electrons to induce electron avalanche and generate a large number of high energy intermediate particles such as radicals,ions and excited molecules,which contribute to the decomposition of 3,4-dichlorodiphenyl ether.Further,apart from the single DBD,the UV radiation generated from EDUV activated by plasma could also perform photolysis activity to remove 3,4-dichlorodiphenyl ether.Therefore,the DBD-EDUV system performed preeminent synthesis effect and best activity for 3,4-dichlorodiphenyl ether removal.
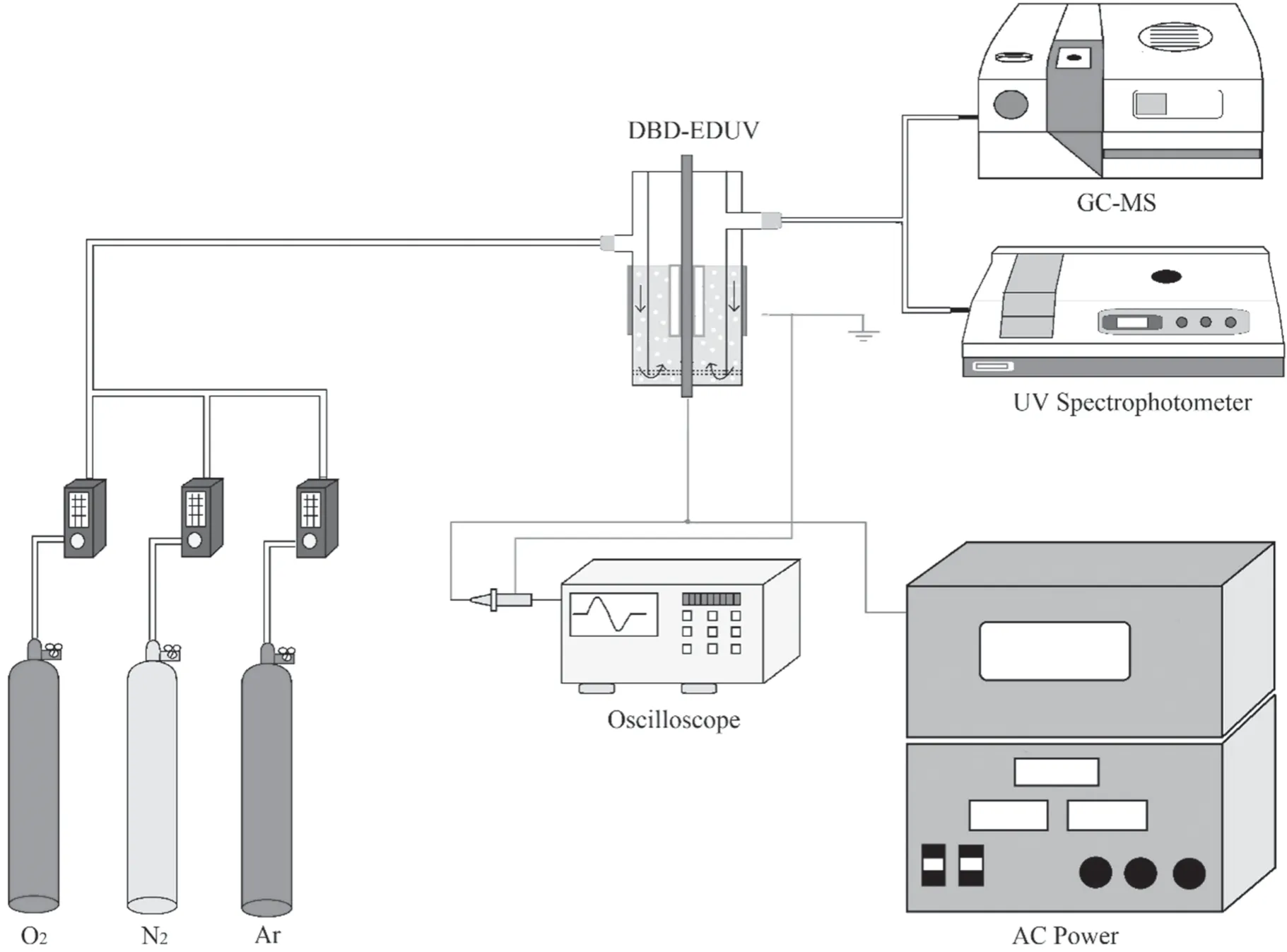
Figure 1.Schematic of the DBD-EDUV hybrid system.

Figure 2.Structure of DBD reactor and DBD-EDUV reactor.
The effect of different feeding gas on 3,4-dichlorodiphenyl ether abatement in DBD-EDUV system was shown in figure 4.According to this figure,when the feeding gas only contained N2or 10% Ar in N2,the conversion of 3,4-dichlorodiphenyl ether was lower than 7%.However,when there was 10% O2in feeding gas,the conversion of 3,4-dichlorodiphenyl ether could reach 72.9%,and moreover,the activity of this hybrid system increased with increasing O2content in feeding gas.Previous researches [39]showed that O2could be excited by plasma to generate excited oxygen and OH radical,which had superb oxidative activity and contributed to removal pollution.Further,chlorobenzene and polychlorinated biphenyls (PCBs) could be oxidized by the excited O and OH radical[23,24].Therefore,O2had obvious positive effects on dioxin-like abatement in DBD-EDUV system.In addition,for DBD-EDUV system,when there were 10%O2and 10%Ar in feeding gas,the 3,4-dichlorodiphenyl ether conversion was slightly better than when there was only 10%O2in feeding gas.This is because that O2is more easily excited by plasma in Ar than in N2[40].In other words,mingling Ar in feeding gas could enhance the excitation of O2and further improve the decomposition of 3,4-dichlorodiphenyl ether.
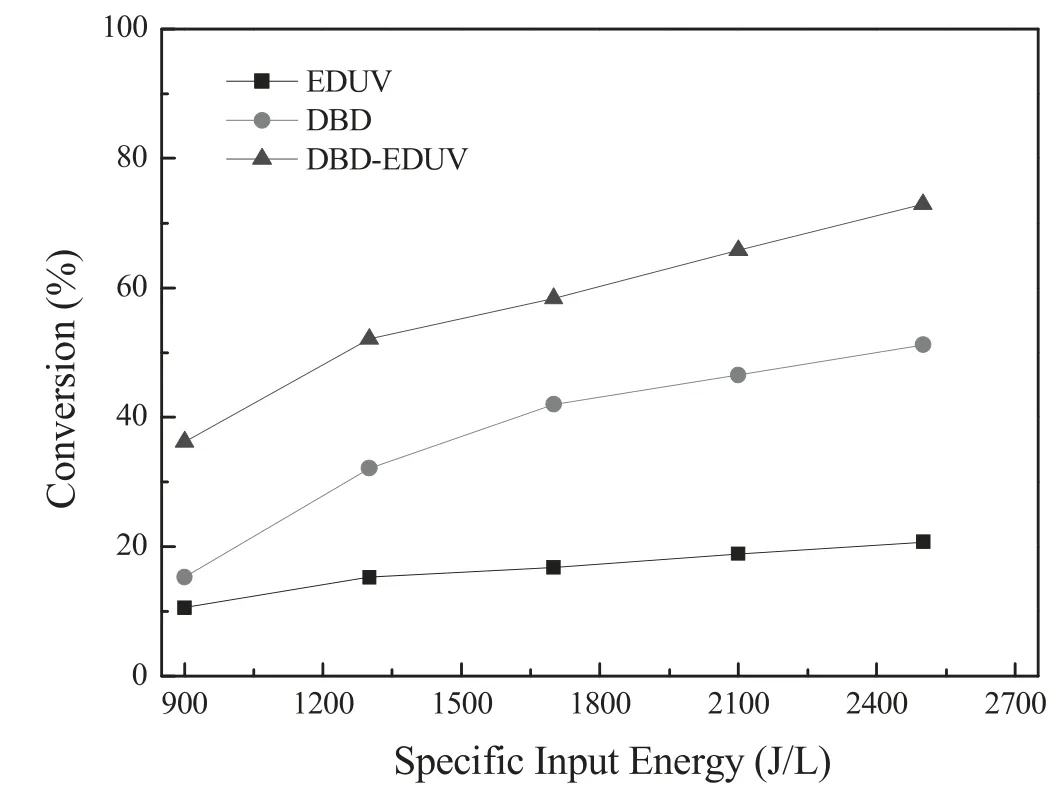
Figure 3.Effect of SIE on the 3,4-dichloro-diphenyl ether conversion in DBD,EDUV and Hg/Ar-DBD systems.Conditions:10% O2,balance N2 and flow rate of 3 l·min−1.64.5 g l−1 3,4-dichloro-diphenyl ether,the SIE was changed from 900 J l−1 to 2500 J l−1,treatment duration was 2 min (1) DBD:single DBD system.(2) EDUV:single EDUV system.(3) DBD-EDUV:DBD system with Hg/Ar EDUV.
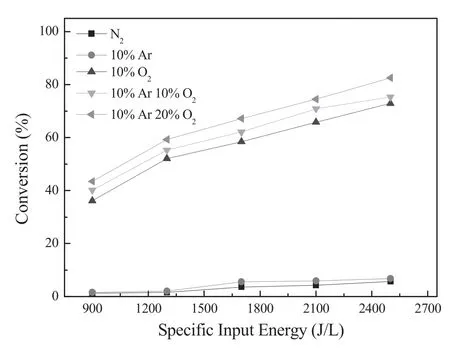
Figure 4.Effect of different feed gas on the 3,4-dichloro-diphenyl ether conversion in Hg/Ar-DBD system.Conditions:0%–20% O2,0%–10% Ar,balance N2 and flow rate of 3 l·min−1.64.5 g l−1 3,4-dichloro-diphenyl ether,the SIE was changed from 900 J l−1 to 2500 J l−1,treatment duration was 2 min.
In order to analyze the effect of EDUV,the emission spectra of DBD and DBD-EDUV systems were shown in figure 5.Compared with single DBD system,significantly UV radiation of 253.7 nm (105 μW cm−2) was detected in the DBD-EDUV system.According to the UV absorption spectrum of 3,4-dichlorodiphenyl ether inserted in figures 5,253.7 nm UV radiation could be easily absorbed by 3,4-dichlorodiphenyl ether.Further,previous researches showed that the direct UV photolysis of PCBs has two potential reaction pathways including photodechlorination(C–Cl bond,3.41 eV) and C–O bond (3.38 eV) photodissociation [41].It means that the 253.7 nm UV radiation(4.89 eV)absorbed by 3,4-dichlorodiphenyl ether could break its C–Cl bond and C–O bond to achieve effectively photodissociation.Therefore,the UV radiation produced by EDUV played a role in removal of 3,4-dichlorodiphenyl ether and contributed to synthesis activity of DBD-EDUV system,which further supported the conclusion of figure 3.

Figure 5.N2 emission spectrum of DBD and DBD-EDUV systems.Conditions:N2 as balance gas and SIE was 1700 J l−1.
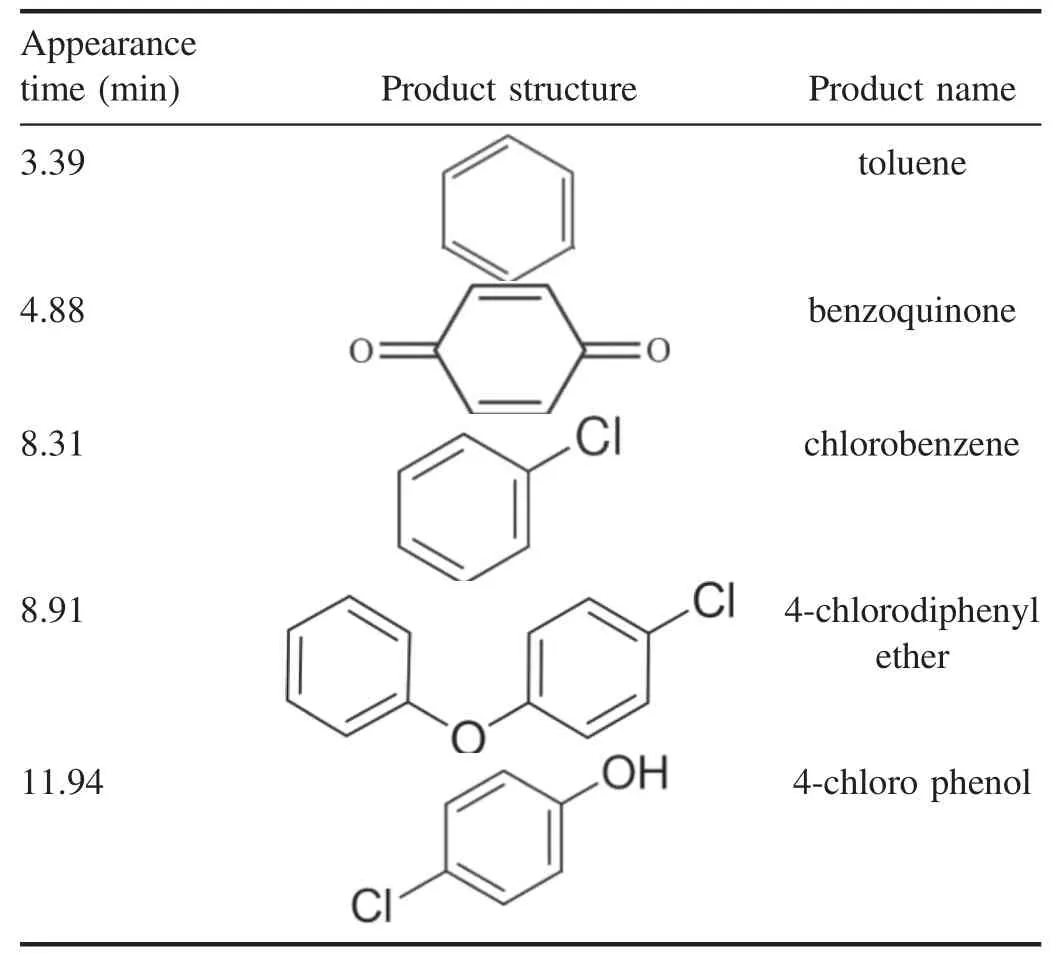
Table 1.Products of 3,4-dichlorodiphenyl ether abatement in DBDEDUV system.
Table 1 showed the products of 3,4-dichlorodiphenyl ether abatement in DBD-EDUV system,which was measured by GC-MS.According to this table,there were toluene (3.39 min),benzoquinone (4.88 min),chlorobenzene(8.31 min),4-chlorodiphenyl ether (8.91 min) and 4-chloro phenol(11.94 min)in products of DBD-EDUV system.These products indicated that the decomposition process of 3,4-dichlorodiphenyl ether in DBD-EDUV system was mainly accomplished through the dechlorination,C–O bond dissociation and further oxidation process.
Based on above results,for DBD-EDUV system,the plasma would produce a large of high-energetic electrons,radicals (OH radicals) and excited molecules (O),and meanwhile the EDUV would be activated by plasma to generate 253.7 nm UV radiation.The high-energetic electrons could impact the 3,4-dichlorodiphenyl ether to break its chemical bonds.Simultaneously,the effect of EDUV photolysis contributes to the dechlorination and C–O bond dissociation of 3,4-dichlorodiphenyl ether in DBD-EDUV system.More importantly,excited O and OH radicals generated from O2excited by plasma could further oxidize 3,4-dichlorodiphenyl ether and its primary decomposition products.In general,the reaction process of DBD-EDUV system was presented in figure 6.
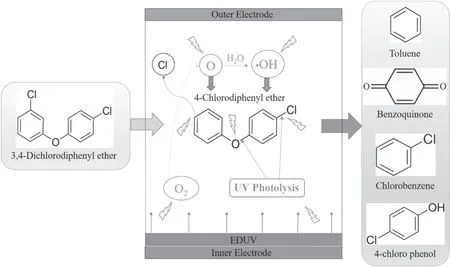
Figure 6.Reaction mechanism of DBD-EDUV system.
4.Conclusion
A new hybrid process with Hg/Ar EDUV photolysis excited by plasma achieved acceptable synergistic effect on 3,4-dichlorodiphenyl ether abatement.Compared with single DBD system,the removal efficiency of 3,4-dichlorodiphenyl ether in DBD-EDUV system could improve about 20%.The O2in feeding gas had significant positive effect on the synthesis activity of DBD-EDUV system.In addition,mangling Ar and O2in feeding gas could improve the excitation of O2and enhance oxidative decomposition of 3,4-dichlorodiphenyl ether.The EDUV excited by plasma in DBDEDUV system produced UV radiation of 253.7 nm,which also played a role in removal of 3,4-dichlorodiphenyl ether.The abatement process of dioxin-like components in DBDEDUV system was accomplished through the dechlorination,C–O bond dissociation and further the oxidation process.
Acknowledgments
This research was funded by National Natural Science Foundation of China (No.21577023),the Special Research Project on Causes and Control Technology of Air Pollution(No.2017YFC0212905) and the science and technology innovation action project supported by the Science and Technology Commission of Shanghai Municipality (No.18DZ1202605).The authors thank Jianyuan Hou and Yanpei Wu for their help in the research work.
ORCID iDs
杂志排行
Plasma Science and Technology的其它文章
- Three dimensional nonlinear shock waves in inhomogeneous plasmas with different size dust grains and external magnetized field
- Study of ionic wind based on dielectric barrier discharge of carbon fiber spiral electrode
- Theoretical research on the transport and ionization rate coefficients in glow discharge dusty plasma
- Study on plasma cleaning of the large-scale first mirror of the charge exchange recombination spectroscopy diagnostic on EAST
- Measurements of plasma parameters in a hollow electrode AC glow discharge in helium
- Investigation of the self-induced magnetic field characteristics in a pulsed plasma thruster with flared electrodes
