1,2,4-trichlorobenzene decomposition using non-thermal plasma technology
2020-04-24TaoZHU竹涛XingZHANG张星MingfengMA马名烽LifengWANG王礼锋ZeyuXUE薛泽宇YimingHOU侯益铭ZefuYE叶泽甫andTongshenLIU刘桐珅
Tao ZHU (竹涛),Xing ZHANG (张星),Mingfeng MA (马名烽),Lifeng WANG (王礼锋),Zeyu XUE (薛泽宇),Yiming HOU (侯益铭),Zefu YE (叶泽甫) and Tongshen LIU (刘桐珅)
1 Institute of Atmospheric Environmental Management and Pollution Control,China University of Mining&Technology (Beijing),Beijing 100083,People’s Republic of China
2 Shaanxi Key Laboratory of Lacustrine Shale Gas Accumulation and Exploitation(under planning),Xi’an 710075,People’s Republic of China
3 Gemeng International Co.,Ltd,Taiyuan 030002,People’s Republic of China
4 Beijing Municipal Research Institute of Environmental Protection,Beijing 100037,People’s Republic of China
5 Authors to whom any correspondence should be addressed.
Abstract
Keywords:non-thermal plasma,pulse discharge,influence parameter,1,2,4-trichlorobenzene decomposition,removal efficiency
1.Introduction
Non-thermal plasma(NTP)technology has the advantages of an efficient pollutant removal process,saving space,lower investment and operating costs,operating at atmospheric pressure and room temperature,and no secondary pollution compared to conventional technologies.Meanwhile,NTP technology shows good performance on desulfurization,denitration,and the removal of elemental mercury due to its capability of producing abundant reactive species by the collisions of high-energy electrons with working gas molecules.Therefore,NTP has been widely used in the field of flue gas purification in recent years[1–3].In addition,the ability of NTP to degrade organic pollutants has been confirmed.Many research studies have shown that chemical reactions are prone to occur in the NTP system,which are difficult to be realized under normal conditions.NTP is considered to be one of the most promising methods for the removal of a variety of pollutants based on the advantages above,and it has extensive research and application value in environmental protection [4,5].
Dioxins are a general term for chlorinated polycyclic aromatic compounds with similar structures and properties,including polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans(PCDFs),which are dangerous to human health and even life.The research of dioxin decomposition is very important considering its environmental toxicity,bioaccumulation,and persistence.The waste incineration industry is recognized as a major source of dioxin emission,and activated carbon injection coupled with a bag filter is currently the predominant technology for the reduction of dioxin emission.However,dioxins do not decompose completely,and they only transfer dioxins from flue gas to the solid residues.Developing effective technologies to decompose dioxin-like compounds has become an important issue in the fields of waste incineration and so on [6–10].However,dioxin decomposition by NTP is relatively rare because of the limitations of the detection method and experimental platform.Chlorobenzene is an important precursor to the formation of dioxins and it is a symbiotic source during the incineration of waste,which can cause dizziness and neurasthenia,increase the risk of cancer after exposure,and lead to environmental problems even at very low concentrations.Therefore,chlorobenzene can be used as a dioxin analog [11–13].Wang et al compared the decomposition of dichlorobenzene,pentachlorobenzene,and hexachlorobenzene using sliding arc plasma,and they proposed the dechlorination and oxidation mechanism of chlorobenzenes in an NTP system [14].Cheng et al found that there is a strong correlation between the decomposition of hexachlorobenzene and dioxins in the NTP system [15].Xu et al studied the decomposition of polycyclic aromatic hydrocarbons (PAHs) and dioxins in fly ash by pulsed discharge at a peak voltage of 40 kV,and they concluded that the efficiency of the removal of PAHs and dioxins could reach 80% and 50%,respectively [16].The above the research studies suggest that NTP had high efficiency for dioxin-like compound decomposition,and the influence of the parameters of 1,2,4-trichlorobenzene (TCB) decomposition by NTP are discussed in this study.Meanwhile it is necessary to investigate the influence of the parameters such as voltage,frequency,water content,initial concentration,flow rate,and oxygen content to provide basic research data for industrial application.
2.Experimental
2.1.Experimental system
The experimental platform is shown in figure 1,and includes a gas generating system,discharge reaction system,and sampling analysis system.The gas generating system was divided into two types,including a carrier gas and balance gas.In the carrier gas,one part of nitrogen entered the TCB gasification chamber and the other part entered the water gasification chamber.The balance gas composition was simulated in accordance with the actual atmosphere of waste incineration,in which the nitrogen was used as background supplemental gas.The carrier gas and balance gas entered the plasma reactor after they were mixed thoroughly in a mixing bottle.The TCB solution and liquid water were injected into the gasification chamber by a microscale digital injector,and the temperature of the gasification chamber was maintained at 260°C and 100°C,respectively.In order to prevent condensation from appearing in the experiment,the tropic cable was wrapped around the plasma reactor and the temperature was maintained at 245°C.The flow rate of each part of the gas was controlled by a mass flow meter in a gas generating system.In the discharge reaction system,the TCB decomposition was tested with a dielectric barrier discharge plasma reactor,which is one of the typical systems for producing NTP.The concentration of TCB before and after treatment with the plasma reactor was analyzed by a gas chromatography-mass spectrometer (Agilent GC-MS 7890B-5977A).The by-products of TCB were discharged after absorption treatment,in which the 2,2,4-trimethylpentane solution was used in exhaust gas absorption.
The self-designed plasma reactor used in the experiment is shown in figure 2.It has a coaxial line structure and the effective length of the plasma reactor was 400 mm.The grounding electrode adopted steel mesh with a length of 380 mm and a tungsten wire with a diameter of 1.25 mm was designed as the internal electrode.The sealing gaskets at both ends were made of polytetrafluoroethylene,which was an excellent insulator.The gas sampling ports were arranged in the front and the back of the plasma reactor.
A Shandong Blue Corfu 25 kV DC pulse power supply was used in the discharge reaction system.The rising edge of the pulse was less than 100 ns and the pulse width was 250 ns.The output waveform of the pulse power supply was measured by a Tektronix-TBS1102 oscilloscope,which was equipped with a P6015A high voltage probe and current loop.The discharge reaction system was stable in the range of 10–17 kV and the discharge voltage waveform is shown in figure 3.
2.2.Experimental method
In this experiment,the by-products of TCB were sampled by bubbling a 2,2,4-trimethylpentane solution using a ZK-3S atmospheric sampler.Through the penetration experiment of 2,2,4-trimethylpentane absorbing TCB,it was determined that the sampling solution was 20 ml.The sampling rate was set to 0.1 l min−1and the sampling time was 20 min.According to the relevant reference and detection standard,the parameters of the GC-MS were as follows:the instrument column was HP-5 MS and it was designed to raise the temperature programmatically.The initial temperature of 50°C was maintained for 1 min and then raised to 170°C at a heating rate of 15°C min−1.The temperatures of the front inlet and gasification chamber were 170°C and 280°C,respectively.The electron ionization EI source was 70 eV and the temperature of the ion source was 230°C [17,18].In order to ensure the accuracy of the test,all concentration values were determined at steady state and the experiments were done in triplicate.If the experimental data fluctuated greatly,it was discarded.If the fluctuation of the experimental data was less than 5%,it was averaged.
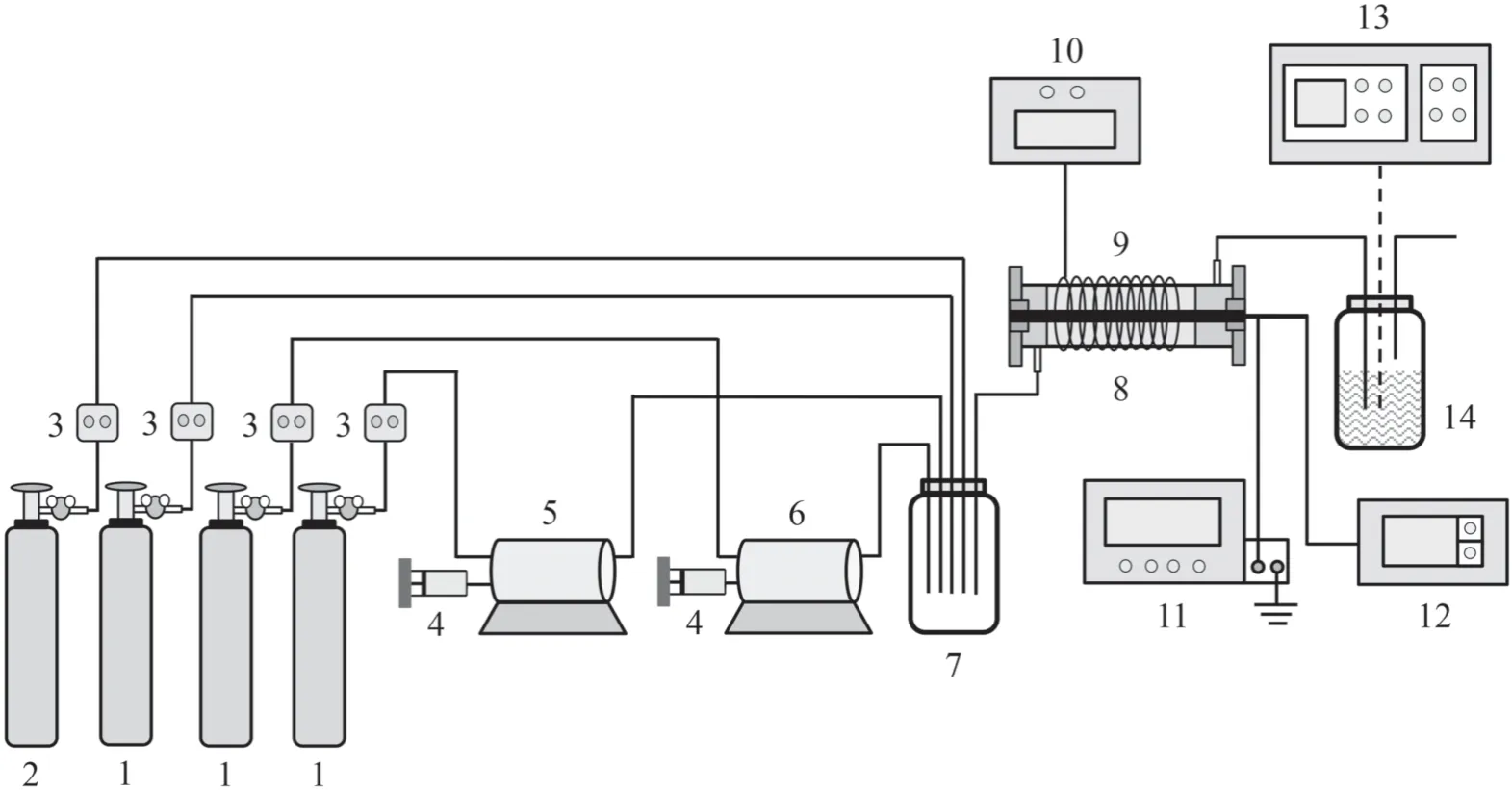
Figure 1.Schematic diagram of the experimental platform:1 nitrogen cylinder,2 oxygen cylinder,3 mass flow meter,4 microscale digital injector,5 TCB gasification chamber,6 water gasification chamber,7 mixing bottle,8 plasma reactor,9 tropic cable,10 temperature controller,11 pulse power supply,12 oscilloscope,13 GC-MS,and 14 exhaust gas absorption.
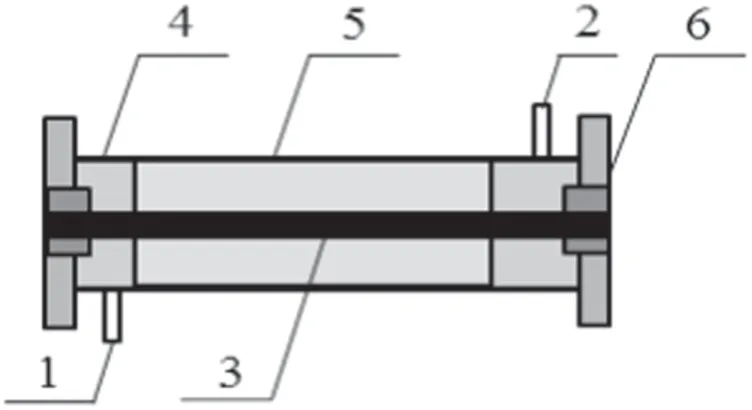
Figure 2.Schematic diagram of the structure of the plasma reactor:1 inlet,2 outlet,3 internal electrode,4 dielectric tube,5 grounding electrode,and 6 sealing gasket.
The mathematical expression for the concentration of TCB is as follows:

In this equation,C represents the concentration of TCB in mg m−3,α stands for the concentration of absorbent in mg ml−1,μ stands for the volume of the absorbent in milliliters,υ is the sampling rate in m3s−1,and t is the sampling in seconds.
The TCB removal efficiency was used as an index for the degradation effect of NTP.The mathematical expression for the TCB removal efficiency is given below:

In this equation,η represents the TCB removal efficiency in percent,Cinis the concentration of TCB at the reactor inlet in mg m−3,and Coutis the concentration of TCB at the reactor outlet in mg m−3.

Figure 3.Discharge voltage waveform.
3.Results and discussion
3.1.Influence of the electrical parameters on the TCB removal efficiency
The matching of the power supply and the plasma reactor is at the core of the discharge reaction system,and the efficiency of the removal of pollutants efficiency is related to the NTP system energy closely.Therefore,the voltage and frequency of the NTP were investigated,and were the basis of the operation of the discharge reaction system.
3.1.1.Influence of voltage on the TCB removal efficiency.In this experiment,there was no obvious discharge phenomenon when the voltage was less than 10 kV.However,the discharge was unstable and electric breakdown easily occurred when the voltage was more than 17 kV.So the range of the variation of the voltage was selected to be between 10 and 17 kV.
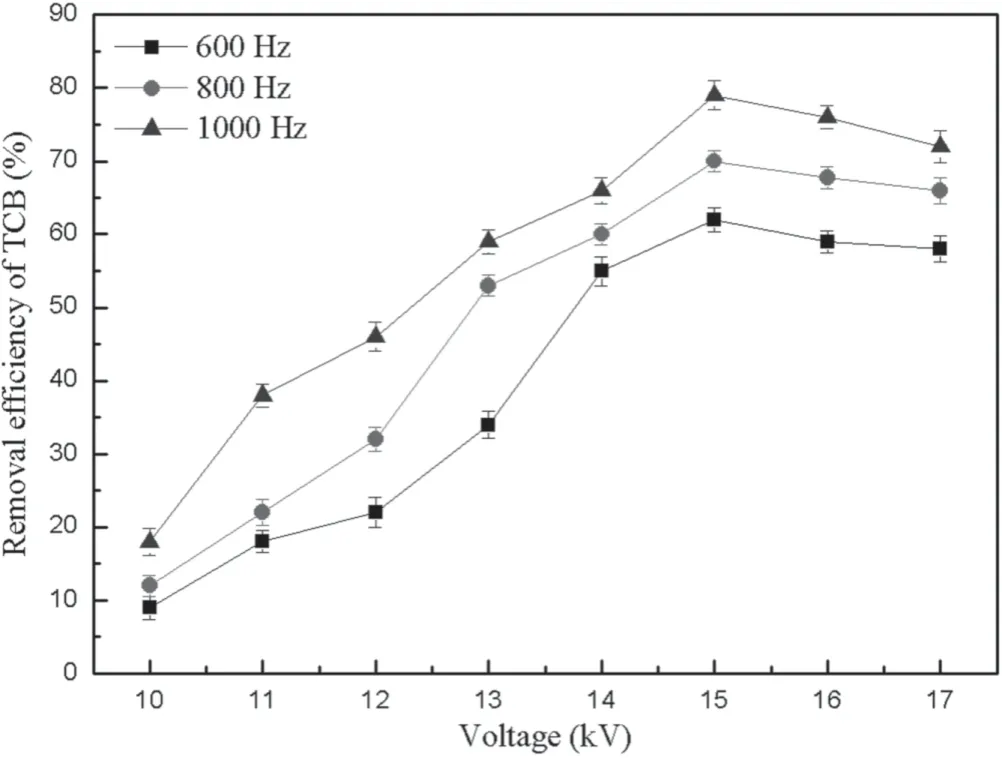
Figure 4.Influence of voltage on the TCB removal efficiency.
In order to simulate the actual atmosphere of a waste incineration,the experimental conditions were as follows.The gas compositions of nitrogen and oxygen were 75% and 25%,respectively.The flow rate of the nitrogen in the carrier gas was 0.5 l min−1,and was used to carry out TCB vapor into the discharge reaction system.The flow rates of nitrogen and oxygen in the balance gas were 1.0 and 0.5 l min−1,respectively.The total flow rate was 2.0 l min−1and the corresponding residence time was 10.89 s in the plasma reactor.The TCB initial concentration was 20 mg m−3.The TCB removal efficiency was investigated under different voltages at frequencies of 600,800,and 1000 Hz,respectively.
The relationship between TCB removal efficiency and voltage is presented in figure 4.The TCB removal efficiency increases with the increasing voltage at frequencies of 600,800,and 1000 Hz,and reaches the maximum at 15 kV.The energy of the NTP system may be dissipated by exotherm instead of degrading TCB,so the TCB removal efficiency shows a downward trend at high voltage.Du et al found that more energy was injected into the NTP system and a lot of high-energy electrons,active particles,and free radicals were generated in the NTP system with the increase of voltage,and the decomposition mechanism of organic pollutants by NTP was complicated [19].On one hand,high-energy electrons collide with pollutant molecules directly and break their chemical bonds.On the other hand,high-energy electrons collide with O2and H2O in the air to generate active particles and free radicals,such as hydroxyl radical (·OH),oxygen radical (·O),hydrogen radical (·H),and hydroperoxyl radical(HO2·),which react with pollutant molecules to form new substances that are easily removed.In this experiment,this improves the probability of TCB reacting with high-energy electrons and free radicals with the increase of voltage.Therefore,the TCB removal efficiency shows an upward trend at frequencies of 600,800,and 1000 Hz.
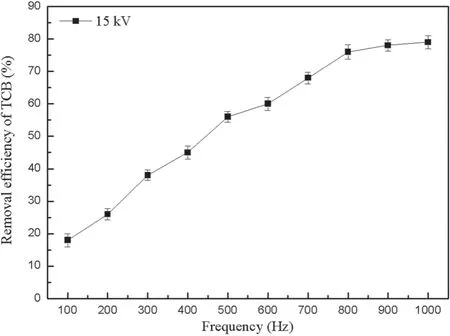
Figure 5.Influence of frequency on the TCB removal efficiency.
3.1.2.Influence of frequency on the TCB removal efficiency.According to the above research,the conditions of the gas generating system and initial concentration of TCB remained constant.The TCB removal efficiency was investigated under different frequencies at a voltage of 15 kV.
Figure 5 shows that the effect of frequency correlates positively with the TCB removal efficiency in the range of 100–1000 Hz,which indicates that frequency is the main factor affecting the TCB removal efficiency.Increasing frequency can improve the system’s energy at the same voltage.The function relation isIn this experiment,the efficiency of the removal of TCB increases with the frequency increasing.This is because the probability of TCB reacting with high-energy electrons and free radicals is improved.However,the TCB removal efficiency changes slowly at high frequency,which suggest that the energy utilization efficiency in the NTP system is lowered compared to at low frequency.Jiang et al found that the efficiency of the removal of pollutants could not be improved even if the frequency was increased when the reaction reached equilibrium in an NTP system [20].
3.2.Influence of water content on the TCB removal efficiency
The conditions of the gas generating system and the initial concentration of TCB remained constant,and the water content of the discharge reaction system was adjusted by controlling the injection speed.The TCB removal efficiency was investigated at different levels of water content under the condition of a voltage of 15 kV and frequencies of 600,800,and 1000 Hz,respectively.
As shown in figure 6,the TCB removal efficiency reaches the maximum at a water content of 4%.However,the TCB removal efficiency decreases when the water content is high.There are two reasons for this phenomenon according to Zheng’s research[21].On the one hand,most of the energy of the NTP system is used to generate free radicals instead of generating high-energy electrons.On the other hand,the water can absorb the high-energy electrons generated in the NTP system due to its electronegativity,which reduces the probability of high-energy electrons colliding with TCB molecules.In addition,the TCB removal efficiency changes slowly at a frequency of 1000 Hz compared to other frequencies,which suggests that water vapor has little effect on the TCB removal efficiency at high frequency due to the higher density of high-energy electrons.In summary,there is an optimum point of water content in the TCB decomposition.
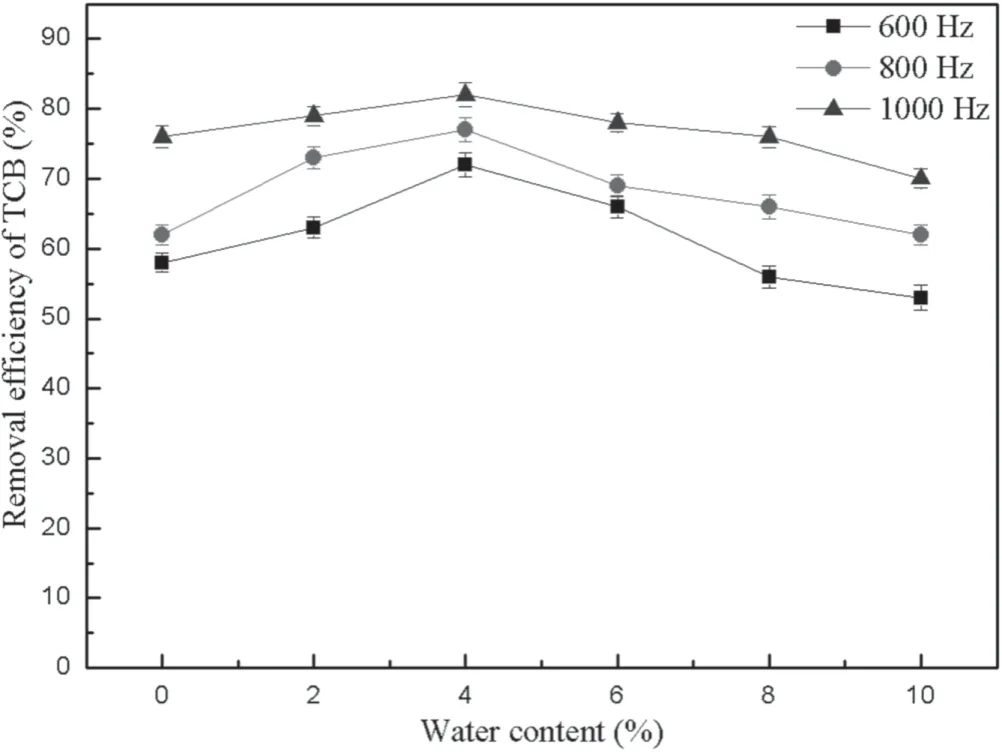
Figure 6.Influence of water content on the TCB removal efficiency.

Figure 7.Influence of the initial concentration on the TCB removal efficiency.
3.3.Influence of the initial concentration on the TCB removal efficiency
The condition of gas in the generating system remained constant.The TCB removal efficiency was investigated at different initial concentrations under the condition of a voltage of 15 kV and frequencies of 600,800,and 1000 Hz,respectively.
Figure 7 shows that the TCB removal efficiency increases firstly and then decreases in the range of 10–90 mg m−3.According to the experiment on the decomposition of VOCs,it was found that the density of high-energy electrons and free radicals had an important impact on the efficiency of the removal VOCs.The efficiency of the removal of VOCs was relatively high due to the higher density of high-energy electrons and free radicals when the initial concentration was low [22,23].In this experiment,the TCB removal efficiency increases with the increase of initial concentration when TCB molecules colliding with high-energy electrons do not exceed the upper limit of full reaction.However,the TCB removal efficiency decreases and then stabilizes gradually when the upper limit of full reaction is exceeded.Generally speaking,the density of high-energy electrons and free radicals is constant under the same condition.The electron energy allocated to pollutant molecules decreases with the TCB concentration increasing.This result is consistent with Yu’s research [24],and the efficiency of the removal of pollutants increases firstly and then decreases within certain concentration ranges.Moreover,Yu found that the efficiency of the removal of naphthalene decreased at high concentration,but the total removal of naphthalene increased in the experiment of naphthalene decomposition.
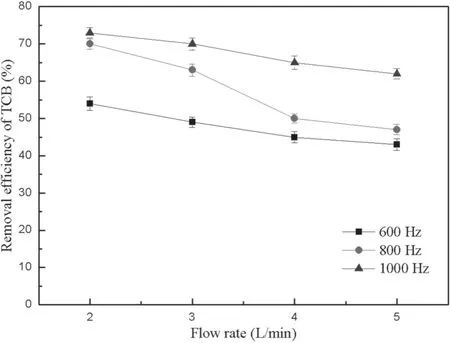
Figure 8.Influence of the flow rate on the TCB removal efficiency.
3.4.Influence of the flow rate on the TCB removal efficiency
The conditions of gas composition and initial concentration of TCB remained constant.The flow rate has negative correlation with residence time in fixed size reactor,which has an important effect on the removal of pollutants.In this experiment,the flow rate was adjusted to 2.0,3.0,4.0,and 5.0 l min−1,so the corresponding residence time was 10.89,8.18,5.45,and 4.36 s,respectively.The TCB removal efficiency was investigated at different flow rates under the condition of voltage of 15 kV and frequency of 600,800,and 1000 Hz,respectively.
As shown in figure 8,the TCB removal efficiency decreases with the increase of the flow rate at a frequency of 600 Hz,and presents a straight line that approximates proportional decrease.It indicates that TCB molecules take time to react with high-energy electrons and free radicals at low frequency.But the TCB removal efficiency changes smoothly at a frequency of 1000 Hz.This is because the system energy is relatively high and TCB molecules react with high-energy electrons to reach equilibrium in a short time.So the flow rate has little effect on the TCB removal efficiency at high frequency.The TCB removal efficiency is unstable when the frequency is 800 Hz.It may be limited by the density of highenergy electrons and residence time in a discharge reaction system,so it is shown that the TCB removal efficiency is irregular.Ren et al proposed that the flow rate had a great impact on the discharge reaction system.If the flow rate increased,the density of high-energy electrons and the efficiency of the removal of pollutants would decrease [25].

Table 1.By-products of TCB decomposition by NTP.
3.5.Influence of the oxygen content on the TCB removal efficiency
The experimental conditions were as follows:the flow rate of nitrogen in carrier gas was 0.5 l min−1.The composition of the balance gas was adjusted by controlling the flow rate of nitrogen and oxygen.The TCB removal efficiency was investigated at different levels pf oxygen content under the condition of a voltage of 15 kV and frequencies of 600,800,and 1000 Hz,respectively.
The relationship between the TCB removal efficiency and oxygen content is presented in figure 9.The TCB removal efficiency decreases with the oxygen content increasing,which shows similar characteristics at different frequencies and this result is consistent with Hung et al’s research [26].Hung et al found that the efficiency of the removal of PCDDs and PCDFs decreased with the increase of oxygen content in the experiment on the decomposition of dioxin-like compounds.The reason for this result was that the discharge power of the NTP system decreased when the oxygen content was increased,so the TCB removal efficiency showed a downward trend.
According to the above research,the optimal reaction condition was at 15 kV,1000 Hz,an initial concentration of 20 mg m−3,a flow rate of 2 l min−1,H2O at 4%,and O2at 0%.As can be seen from figure 9,the TCB removal efficiency reached 92% under that condition.

Figure 9.Influence of the oxygen content on the TCB removal efficiency.
3.6.Analysis of the TCB decomposition mechanism by NTP
In this study,the by-products of TCB were measured by GCMS,and are listed in table 1.The monocyclic benzene series include C6H4Cl,C6H4Cl2,and so on.The organic products consist of a C–OH structure and oxygen-containing groups:ketone group,aldehyde group,and ester group,etc,which mainly concentrates in the range of C4–C6.
Ono et al measured the electronic ground state ·OH,·O,·H,and HO2· radicals generated in an NTP system by laserinduced fluorescence with a tunable KrF excimer laser,and it was verified that the oxidation capacity of ·OH is stronger than·O,·H,and HO2·[27–29].Brubaker and Hite found that·OH participated in the dechlorination and oxidation reactions due to its strong oxidation,and it reacted with PAH in three steps in the research of the·OH reaction kinetics of PAH[30].Firstly,the benzene ring was attacked by high-energy electrons and·OH reacted with PAH to form an OH-PAH adduct.Secondly,the OH-PAH adduct reacted with ·OH to form a split ring product.Lastly,the split ring product continued to be oxidized by·OH and the final products were CO2and H2O[30].This result is consistent with Ji et al’s research,which found that ·OH as an active free radical played an important role in the bond breaking process and participated in the dechlorination process [31].

Figure 10.A possible decomposition pathway of TCB by NTP.
In this experiment,a large number of high-energy electrons as well as varieties of free radicals and other excited state atoms are generated in the dielectric barrier discharge process.These particles react with TCB molecules,which results in a series of chemical reactions such as excitation,dissociation,ionization,and recombination.The specific reaction process is as follows:the TCB molecules collide with high-energy electrons directly and then form an excited state or are broken inside the molecule to form molecular fragments.Meanwhile,the free radicals ·H,·O,·OH,and HO2·react with the molecular fragments to form an adduct and intermediate product.Lastly,the active particles attack the intermediate product and the benzene ring breaks or forms the benzene ring binding product.In the dechlorination process of TCB,the dissociation energy of the C–Cl bond on the benzene ring in TCB is Cl (2)<Cl (1)<Cl (4),in which the Cl(2)–C bond is relatively unstable.The presence of 1,4-dichlorobenzene suggests the dechlorination process of the TCB decomposition [32–36].The possible decomposition pathway of TCB by NTP is shown in figure 10,which is inferred from the by-products of TCB.
4.Conclusions
Environmental pollution and ecological deterioration caused by dioxins have become a critical problem throughout the world that all countries are trying to find an effective solution to limit their presence in the atmosphere.NTP showed good performance on the decomposition of pollutants and it could serve as an alternative technology for the removal of dioxin-like compounds compared with traditional technologies,such as absorption,catalytic oxidation,and thermal incineration.In the basic research of TCB decomposition using NTP technology generated by a dielectric barrier discharge,the influence of the parameters of voltage,frequency,water content,initial concentration,flow rate,and oxygen content on the TCB removal efficiency were investigated and the experimental results are as follows:
(1) The energy injected into the plasma system has a positive correlation with voltage and frequency,and the TCB removal efficiency increases with the increase of voltage and frequency.
(2) Water content plays an important role in the decomposition of TCB and the water content of 4% has an optimal removal efficiency due to its electronegativity.The TCB removal efficiency is affected by the initial concentration,which increases firstly and then decreases when the TCB initial concentration ranges from 10–90 mg m−3.
(3) The flow rate has a negative correlation to the residence time in the reaction system.The TCB removal efficiency decreases with the increase of the flow rate at low frequency.But at high frequency,the TCB removal efficiency is less affected by the flow rate.
(4) In the plasma system,the TCB removal efficiency decreases with the increase of the oxygen content.According to the decomposition mechanism of NTP,a lot of high-energy electrons as well as varieties of free radicals and other excited state atoms are generated in the barrier discharge process,in which the ·OH participate in the oxidation and dechlorination reactions in the decomposition of TCB.
Acknowledgments
This work was supported by the Major Science and Technology Projects of Shanxi Province (No.20181102017),the Hebei Province Central Guidance Local Science and Technology Development Special (No.19943816G),the Open Foundation of Shaanxi Key Laboratory of Lacustrine Shale Gas Accumulation and Exploitation(under planning),and the Fundamental Research Funds for the Central Universities(No.2009QH03).
杂志排行
Plasma Science and Technology的其它文章
- Three dimensional nonlinear shock waves in inhomogeneous plasmas with different size dust grains and external magnetized field
- Study of ionic wind based on dielectric barrier discharge of carbon fiber spiral electrode
- Theoretical research on the transport and ionization rate coefficients in glow discharge dusty plasma
- Study on plasma cleaning of the large-scale first mirror of the charge exchange recombination spectroscopy diagnostic on EAST
- Decomposition of dioxin-like components in a DBD reactor combined with Hg/Ar electrodeless ultraviolet
- Measurements of plasma parameters in a hollow electrode AC glow discharge in helium
