脉冲强光诱变对黑曲霉产果胶酶活力的影响
2020-04-09张佰清葛新宇马凤鸣马艺超张士豪谢加豪
张佰清,葛新宇,马凤鸣,马艺超,张士豪,谢加豪
脉冲强光诱变对黑曲霉产果胶酶活力的影响
张佰清,葛新宇,马凤鸣,马艺超,张士豪,谢加豪
(沈阳农业大学食品学院,沈阳 110866)
脉冲强光技术可应用于微生物诱变育种以及获得高产菌株。该文采用响应面法确定脉冲强光诱变黑曲霉高产果胶酶的最佳条件,同时探究突变菌株高产果胶酶的酶学性质。结果表明:脉冲强光诱变条件为脉冲电压2 075 V,脉冲次数36次,脉冲距离5.4 cm。以此工艺参数进行诱变,得到了高产果胶酶的突变菌株L9,与原始菌株相比,其果胶酶活力提高了82.22%±0.18%。突变菌株L9遗传性能良好,所产果胶酶具有更高的pH稳定性和热稳定性。因此,脉冲强光可用于黑曲霉诱变,得到稳定性良好的高产果胶酶的突变菌株。
酶;农产品;电场;脉冲强光;诱变;黑曲霉;果胶酶;响应面法
0 引 言
果胶酶[1]是一种能将果胶裂解,使其变成多聚半乳糖醛酸的复合酶,主要用于提高果蔬汁出汁率和果酒澄清处理[2],增加产品风味。目前,食品加工用黑曲霉生产果胶酶,虽然其果胶酶组分较为齐全,但产量较低[3]。人工诱变筛选的黑曲霉能够提高果胶酶产量,因此,探索新型物理方法,将其应用于黑曲霉诱变育种,以获得具有稳定遗传性状的高产菌株是食品工业的重要研究热点[4]。
脉冲强光是一种利用瞬间放电以脉冲光形式诱变微生物的新型冷处理技术[5]。目前,脉冲强光逐渐应用于优良菌株的诱变选育,具有处理时间短、耗能低[6]、效率高、污染小[7]等优点。孟宪军等[8]利用脉冲强光诱变纳塔尔链霉菌得到高产纳他霉素突变菌株,较原始菌株提高1.6倍。孙栏梦等[9]以啤酒酵母为出发株,通过脉冲强光抗性筛选得到产双乙酰含量高、凝聚性强、发酵速率快的优良突变株。张佰清等[10-11]通过脉冲强光诱变乳酸乳球菌和保加利亚乳杆菌,分别得到Nisin高产突变株和高产多糖菌株,提高了菌株发酵性能。Orcajo等[12-13]研究表明脉冲强光通过改变菌体细胞的核酸结构来引起细胞变异,但DNA修复机制不发生作用,从而诱导微生物突变[14]。
本试验以产果胶酶的黑曲霉为研究对象,进行脉冲强光诱变,通过响应面优化法确定最优诱变条件,初步探究诱变菌株果胶酶的酶学性质,考察脉冲强光作为一种新型诱变技术,应用于黑曲霉诱变的可行性。为脉冲强光作为一种新的诱变技术应用于优良菌株诱变提供理论参考。
1 材料与方法
1.1 材料
1.1.1 供试菌株
黑曲霉(CICC40493),沈阳农业大学生物学院提供。
1.1.2 主要设备
脉冲强光装置波长范围在200~1 100 nm,其中紫外光部分占15%~20%,脉冲宽度20s,最大输入能量644 J,沈阳农业大学食品学院自制;高压蒸汽灭菌锅上海三申器械有限公司;PHS-3C型酸度计;DK-S22恒温水浴锅,常州国华科技有限公司;超净工作台,上海博讯有限公司;恒温培养箱,上海一恒科学仪器有限公司;离心机,Sigma 公司。
1.1.3 培养基
斜面培养基:PDA培养基[15]
初筛培养基:果胶5 g/L、NaNO33 g/L、NaCl 1 g/L、MgSO41 g/L、K2HPO4·3H2O 1 g/L、KH2PO40.5 g/L、溴酚蓝0.1 g/L、琼脂粉20 g/L、pH值5.5
发酵培养基:桔皮粉20 g/L、蛋白胨5 g/L、NaCl 1 g/L、MgSO41 g/L、K2HPO4·3H2O 1 g/L、KH2PO40.5 g/L。
1.2 试验方法
1.2.1 脉冲强光诱变条件的确定
斜面活化好菌株后,将制备的孢子悬液转移到直径为9 cm的灭菌培养皿中,放入脉冲强光处理室氙灯正下方,进行脉冲强光照射处理。考察最佳脉冲电压、脉冲次数、脉冲距离和菌液量[16]对菌株致死率和突变菌株胞外果胶酶活力的影响。文献报道,致死率在70%~80%时,菌株突变性可以保持在较稳定的水平[17]。前期单因素试验结果得到脉冲电压2 000 V、脉冲次数30次、脉冲距离5 cm为最佳处理条件,菌液量对脉冲处理结果无显著影响。进而通过最陡爬坡试验以快速逼近最佳产酶区域。
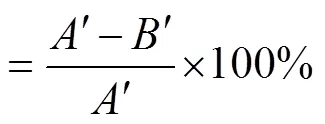
式中为对照组平皿上的菌落数;为诱变处理后平皿上的菌落数。
1.2.2 响应面试验设计
以最陡爬坡试验的最高响应值点为中心点进行中心组合设计,运用Design-Expert软件对试验数据进行回归拟合[18-19],试验因素与水平如表1。
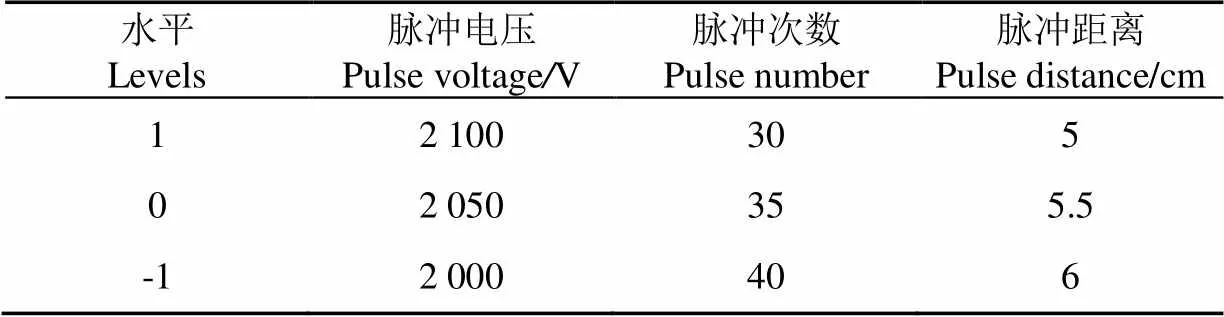
表1 响应面试验自变量因素与水平
1.2.3 高产果胶酶突变菌株的筛选
1)初筛
将适当浓度孢子悬液[20]进行照射处理,取100L孢子悬液涂布于筛选培养基上。30 ℃锡箔纸避光培养3 d,用未处理的孢子液涂布筛选培养基进行对照[21],每组做3个平行。观察透明圈与菌落直径的(/)比值,选择(/)值较大的菌株进行复筛[22]。
2)复筛
将初筛菌株进行液体发酵,对粗酶液进行果胶酶活力测定[23],选取果胶酶活力高的菌株。
1.2.4 酶活的测定
采用3,5-二硝基水杨酸比色(DNS法)测定[24]。底物为0.4%果胶溶液。酶活力单位(U):在pH值为4.0,45 ℃条件下,每分钟催化果胶水解生成1mol半乳糖醛酸所需酶量为1个酶活力单位。
1.2.5 突变菌株遗传稳定性实验
将突变菌株连续传代6代,同时进行发酵培养[25],测定每一代菌株产酶活性,比较其产酶活性变化。
1.2.6 果胶酶酶学性质研究
试验将菌株发酵液经离心取上清液为粗酶液,加入(NH4)2SO4饱和度为60%,经冷冻离心取沉淀,溶于缓冲液,4 ℃透析12 h。通过Cellulose DE-52阴离子交换柱层析,收集酶活部分。再经SepHadex G-100凝胶过滤柱层析,二次纯化,收集酶活部分。采用SDS-PAGE电泳进行纯化结果的验证。
1)果胶酶最适作用pH值
用醋酸-醋酸钠缓冲溶液配制pH值分别为3.0、3.5、4.0、4.5、5.0、5.5、6.0和6.5的底物,测定果胶酶在不同pH环境中的酶活。
2)果胶酶最适作用温度
将最适pH环境下的果胶酶活测定体系分别置于30、35、40、45、50、55、60、65 ℃的不同温度环境中,测定果胶酶在不同温度环境中的酶活。
3)果胶酶的pH稳定性
将粗酶液用不同pH值的缓冲液(3.0、3.5、4.0、4.5、5.0、5.5、6.0、6.5)处理1 h后与底物反应,在最适条件下测定其酶活,以未经处理的酶液活性为100%,计算相对酶活,分析果胶酶pH稳定性。
4)果胶酶的热稳定性
将粗酶液置于不同温度(40、50、60、70 ℃)恒温水浴锅中水浴保温,每隔20 min取少量酶液迅速在冷水浴中降温冷却,测定酶活,以未处理的果胶酶活力以100%计。
1.2.7 数据分析
试验数据均平行测定3次,测定结果以平均值±标准差(SD)表示,使用SPSS 17.0软件进行数据分析[26]。
2 结果与分析
2.1 脉冲强光对黑曲霉诱变效果研究
脉冲强光诱变黑曲霉是一个多因素作用的复杂过程,以透明圈与菌落直径的比值(/)为指标,通过统计分析得出脉冲强光诱变黑曲霉的最佳优化条件。前期试验结果表明,菌液量对(/)值无显著性影响,因此将脉冲电压、脉冲次数和脉冲距离作为试验因素,通过最陡爬坡试验以快速逼近最佳产酶区域,最后以最陡爬坡试验的最高点为中心点进行中心组合设计。运用Design-Expert软件对试验数据进行回归拟合,得出最优诱变条件[27-28]。
1)最陡爬坡试验结果
最陡爬坡试验是考察某一因素的变化来确定其在试验中的具体影响,为响应面试验提供一个合理数据范围。试验的设计及结果如表2所示。
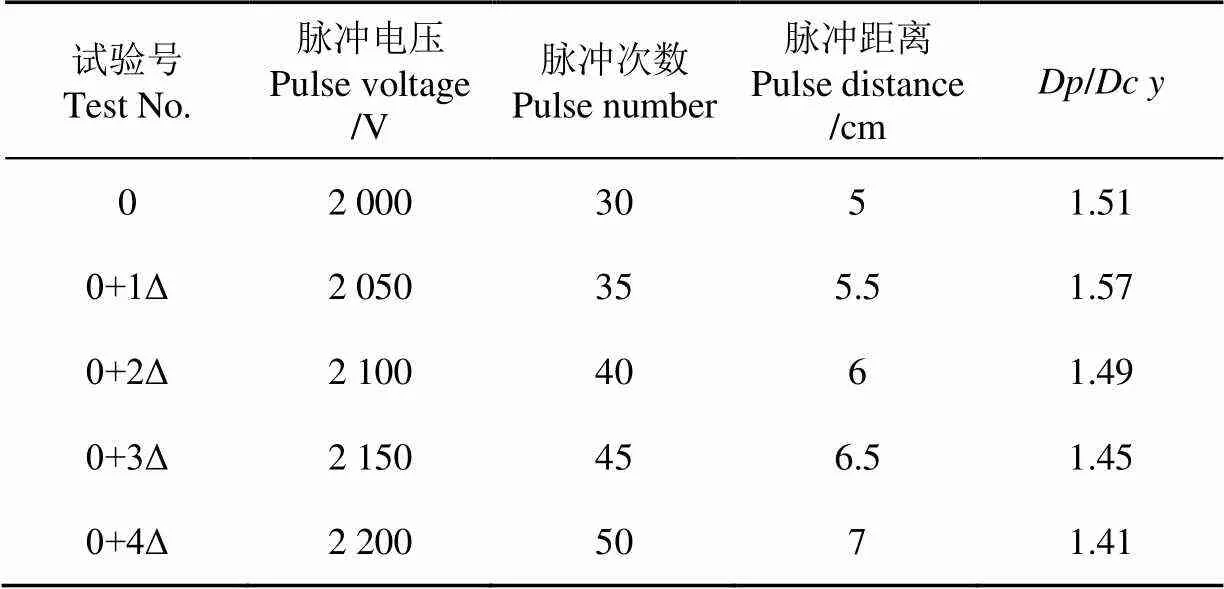
表2 最陡爬坡试验设计及结果
注:/为透明圈与菌落直径的比值,下同。
Note:is the ratio of transparent circle to colony diameter, the same below.
由表2可知,0+1Δ号试验组的比值响应值达到最大1.57,之后开始下降。因此,选取此拐点试验条件作为响应面设计中心点。
2)响应面优化试验结果
利用Design-Expert10软件设计试验,进行3因素3水平的Box-Behnken响应面试验。试验的设计及结果与方差分析分别如表3、表4所示。

表3 响应面试验设计及结果

表4 试验结果方差分析
注:**表示对试验结果有极显著影响(<0.01);*表示对试验结果有显著性影响(<0.05)。
Note: ** means significant influence on the test results (< 0.01);* means significant impact on test results (< 0.05).
由表3、4可知,菌株的水解圈与菌落直径比值()对脉冲电压()、脉冲次数()和脉冲距离()的多元二次回归方程为:
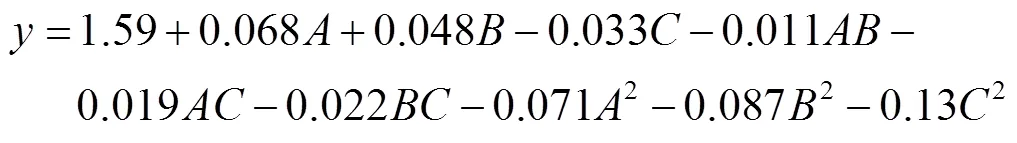
该模型回归项极显著(<0.01),失拟项不显著(>0.05),决定系数为0.978 9,校正后决定系数0.940 9,说明该模型成立,可有效反映出菌株水解圈/菌落直径比值与脉冲电压、脉冲次数和脉冲距离之间的关系,能够较真实地反映试验结果,具有实际应用意义。由以上可知,、、、2、2、2为显著影响因素,各因素两两交互作不显著。如图1所示。
利用Design-Expert10软件计算,确定诱变条件为:脉冲电压2 074.3V、脉冲次数36.338次、脉冲距离5.41cm,最大比值为1.612。结合实际操作,即脉冲电压2 075V、脉冲次数36次、脉冲距离5.4 cm。在此最优条件下进行3次验证试验,比值为1.58,比预测值略低,且致死率达到76.67%,说明用该回归方程分析得到的最优诱变条件具有可信度。
2.2 脉冲强光诱变选育
在脉冲强光的最佳诱变条件下进行高产果胶酶突变菌株的选育,初筛选结果如图1所示。

注:Y为原始菌株。
由图1可知,以值为初筛指标,挑选60个单菌落,从中选取10株酶活力较原始菌株()显著提高(<0.05)的突变菌株进行复筛,即突变菌株L4、L6、L8、L9、L18、L28、L37、L38、L45、L46。
脉冲强光突变菌株的复筛结果如表5所示。
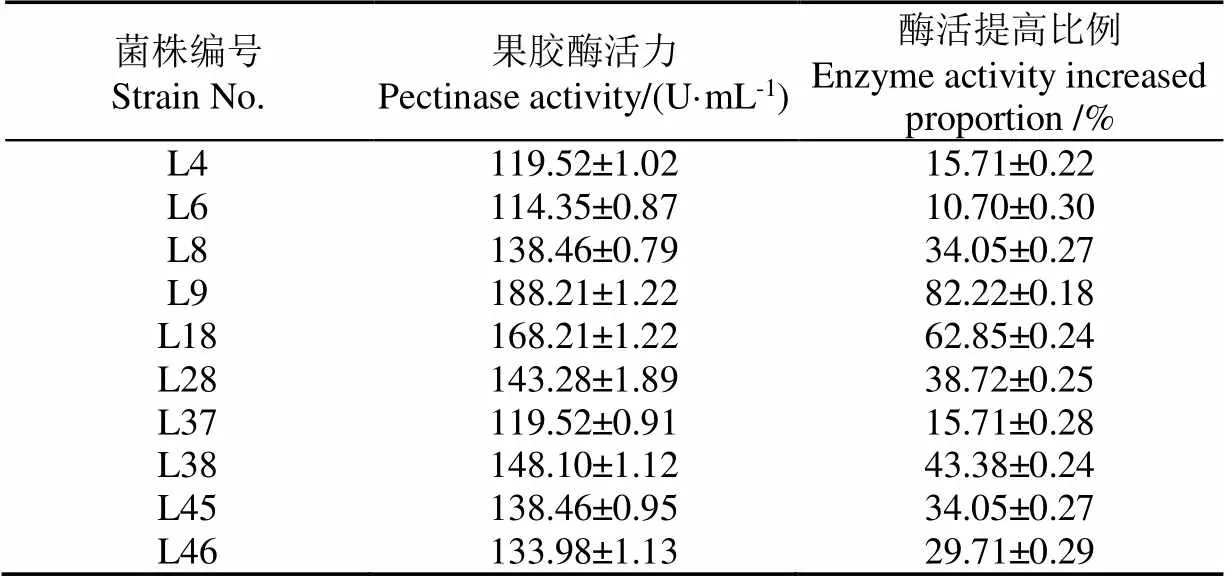
表5 突变菌株的复筛结果
注:试验数据均平行测定3次,测定结果以平均值± 标准差(SD)表示,下同。
Note: All experimental data were measured three times in parallel, and the results were expressed as mean ± standard deviation (SD), the same below.
表5可知,相较于原始菌株,突变菌株产酶能力均有显著提升。其中,突变菌株L9产酶能力最强,其酶活力高达(188.21±1.22)U/mL,比原始菌株(103.29± 0.31) U/mL提高了82.22%±0.18%。因此选取突变菌株L9进行后续研究。
2.3 遗传稳定性研究
突变菌株L9遗传稳定性研究如表6所示。

表6 突变菌株遗传稳定性研究
由表6可知,传代1~6次,突变菌株L9的产酶活性变化并不显著,由此证明突变菌株L9的产果胶酶能力较稳定,并具有较好的遗传稳定性,进一步说明脉冲强光可应用于产果胶酶能力强且能够稳定遗传的突变菌株的诱变选育。
2.4 果胶酶性质
诱变前后菌株所产果胶酶采用SDS-PAGE电泳进行纯化,有一条清晰条带在29.0~44.3 kDa之间,并初步估计酶的分子量为35 kDa。诱变前后菌株所产果胶酶的最适pH、最适温度如图2a、2b所示。pH 值5.0、45 ℃时,L9与原始菌株果胶酶活力均达到最高,L9的果胶酶活分别为191.16、192.67 U/mL,与原始菌株相比分别提高了80.84%、71.68%。
突变菌株L9果胶酶的pH稳定性、热稳定性结果如图2c、2d、2e所示。在pH值在4~6时,原始菌株果胶酶出现不同程度的损失,L9果胶酶相对酶活均超过80%,明显高于原始菌株。原始菌株果胶酶在40~50 ℃条件下处理活力呈现不同程度的损失,在60~70 ℃条件下仅保存80 min酶液基本失活。而突变菌株L9果胶酶在40~50 ℃条件下保存40 min后,酶活基本没有损失;在60~70 ℃条件下保存100 min。酶液基本失活。因此,诱变菌株果胶酶的pH稳定性、热稳定性有较明显的提升,这可能是由于突变株所产酶亚基间的聚合度增强[29]。脉冲强光诱变黑曲霉菌株得到果胶酶高产菌株,与已有研究脉冲强光诱变增加菌株发酵产酶性能结果一致[30],说明了脉冲强光应用黑曲霉诱变的可行性。Effect

注:图a、c中温度为45 ℃,图b、d、e中pH值为5.0。
3 结 论
本文对产果胶酶的黑曲霉菌株进行脉冲强光诱变处理,通过响应面试验确定的最优诱变条件为脉冲电压2 075 V、脉冲次数36次、脉冲距离5.4 cm。在此诱变条件下,得到高产果胶酶突变菌株L9,其酶活力为(188.21±1.22)U/mL,比优化前提高82.22%±0.18%。经传代培养实验表明,突变菌株产果胶酶能力稳定,该酶最适pH值为5.0,最适温度45 ℃。突变菌株L9所产果胶酶比原始菌株具有更高的pH稳定性和热稳定性。综上,脉冲强光可以应用于菌株诱变,在工业酶制剂中应用前景广阔。可进一步探究脉冲强光技术诱变机理,开发脉冲技术在诱变领域的潜力。
[1]刘建辉,李俊杰,杨倩. 果胶酶的研究概况及展望[J]. 产业与科技论坛,2017,16(15):68-70.
Liu Jianhui, Li Junjie, Yang Qian. Overview and prospect of pectinase research[J]. Industral & Science Tribune, 2017, 16 (15): 68-70. (in Chinese with English abstract)
[2]王鸿飞,李和生,马海乐,等. 果胶酶对草莓果汁澄清效果的研究[J]. 农业工程学报,2003,19(3):161-164.
Wang Hongfei, Li Hesheng,Ma Haile, et al. Clarification effects of pectinase on strawberry fruit juice[J].Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2003, 19(3): 161-164. (in Chinese with English abstract)
[3]Sharma N, Rathore M, Sharma M. Microbial pectinase: Sources, characterization and applications[J]. Reviews in Environmental Science and Bio/Technology, 2013, 12(1): 45-60.
[4]裘纪莹,王未名,陈建爱,等.微生物果胶酶的研究进展[J]. 中国食品添加剂,2010(4):238-241.
Qiu Jiying, Wang Weiming, Chen Jianai, et al. Advance in microorganism pectinase research[J]. China Food Additives, 2010(4): 238-241. (in Chinese with English abstract)
[5]罗志刚,杨连生. 脉冲强光技术在食品工业中的应用[J]. 食品工业,2002(5):44-46.
Luo Zhigang, Yang Liansheng. Application of intense pulsed light technology in food industry[J]. The Food Industry, 2002(5): 44-46. (in Chinese with English abstract)
[6]彭光华,王璐瑶,涂贻轩,等. 复合增强脉冲强光保鲜鲜切荸荠技术研究[J]. 长江蔬菜,2019(4):72-76.
Peng Guanghua,Wang Luyao, Tu Yixuan, et al. Study on preservation technology of fresh-cut water chestnut by compound reinforced pulse Strong light[J]. Journal of Changjiang Vegetables, 2019(4): 72-76. (in Chinese with English abstract)
[7]戚向阳,周婷婷,曹少谦. 不同强度脉冲强光对鲜香菇保鲜效果的影响[J]. 农业工程学报,2019,35(3):287-293.
Qi Xiangyang, Zou Tingting, Cao Shaoqian. Effects of intense pulsed light treatment with different intensity on preservation of fresh shiitake mushrooms[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019,35(3): 287-293. (in Chinese with English abstract)
[8]孟宪军,郑凤娥,张琦. 纳塔尔链霉菌的脉冲强光诱变育种研究[J]. 食品工业科技,2008,29(3):141-142.
Meng Xianjun, Zheng Fenge, Zhang Qi. Study on the mutagenic breeding of the strain ofby electrofusion impulse[J]. Science and Technology of Food Industry, 2008,29(3): 141-142. (in Chinese with English abstract)
[9]张佰清,孙栏梦. 脉冲强光对啤酒酵母的诱变效应[J].食品科学,2015,36(7): 153-157.
Zhang Baiqing, Sun Lanmeng. Mutagenic Effects of Pulsed Light Irradiation on[J]. Food Science, 2015, 36(7): 153-157. (in Chinese with English abstract)
[10]赵春燕,孟晓曦,刘长江. 脉冲强光诱变选育Nisin高产菌株[J]. 食品科技,2010(8):32-34.
Zhao Chunyan, Meng Xiaoxi, Liu Changjiang. Screening of a high Nisin producing strain by pulse ray[J]. Food Science and Technology, 2010(8): 32-34. (in Chinese with English abstract)
[11]陶雨施,张佰清. 脉冲强光诱变保加利亚乳杆菌高产胞外多糖[J]. 食品工业科技,2018,39(10):143-148.
Tao Yushi, Zhang Baiqing. Enhancement of exopolysaccharides production inby intense pulse light mutagenesis[J]. Science and Technology of Food Industry, 2018, 39(10): 143-148. (in Chinese with English abstract)
[12]Orcajo J, Igo M D M, Lavilla M. Cow’s milk allergen-lactoglobulin immunoreactivity affected by pulsed light treatment[J]. Clinical and Translational Allergy, 2015, 5(Supp.3): 50.
[13]Cheigh C I, Park M H, Chung M S, et al. Comparison of intense pulsed light-and ultraviolet (UVC)- induced cell damage in Listeria monocytogenes, and Escherichia coli O157: H7[J]. Food Control, 2012, 25(2): 654-659.
[14]佘秋生,杨海波,杨冠军,等. 紫外诱变黑曲霉筛选高产果胶酶菌种[J]. 中国酿造,2012,31(6):134-137.
She Qiusheng, Yang Haibo, Yang Guanjun, et al. Breeding of high pectinase-producingstrain by UV[J]. China Brewing, 2012, 31(6): 134-137. (in Chinese with English abstract)
[15]王栋. 米曲霉蛋白酶在高盐环境的催化动力学及其在酱油酿造中的应用[D]. 无锡:江南大学,2013.
Wang Dong. Catalytic Kinetics of Aspergillus oryzae Protease in High-Salt Environment and its Application in Soy Sauce Fermentation[D]. Wuxi: Jiangnan University, 2013. (in Chinese with English abstract)
[16]张佰清,马凤鸣. 脉冲强光处理对南阳酵母杀菌效果的影响[J]. 沈阳农业大学学报,2007,38(2):253-255.
Zhang Baiqing, Ma Fengming. Sterilizationtrial on Nanyang Yeast with pulsed light[J]. Journal of Shenyang Agricultural University, 2007, 38(2): 253-255. (in Chinese with English abstract)
[17]田磊,张芳,沈国平,等. Ectoine高产菌株Halomonas sp. XH26的鉴定及紫外诱变选育[J/OL]. 生物学杂志,2019, 36:3-9.
Tian Lei, Zhang Fang, Shen Guoping, et al. Identification of high-yielding strainfor producing ectoine and UV mutagenesis breeding[J/OL]. Journal of Biology, 2019,36: 3-9. (in Chinese with English abstract)
[18]王瑶,李琪,李平兰. 响应面法优化植物乳杆菌LPL-1产细菌素发酵条件及细菌素理化性质分析[J]. 食品科学,2018,39(22):101-109.
Wang Yao, Li Qi, Li Pinglan. Optimization of fermentation conditions for plantaricin production byLPL-1 by response surface methodology and its physicochemical properties[J]. Food Science, 2018, 39(22): 101-109. (in Chinese with English abstract)
[19]赵天惠. 枯草芽孢杆菌脉冲强光诱变及其发酵性能研究[D]. 沈阳:沈阳农业大学,2017.
Zhao Tianhui. Study onInduced by Pulsed Light and Its Fermentation Performance[D]. Shenyang: Shenyang Agricultural University, 2017. (in Chinese with English abstract)
[20]吴晓冰,于新,吴青. 绿色木霉菌T-YY黄色素的稳定性研究[J]. 农业工程学报,2008, 24(1):285-290.
Wu Xiaobing, Yu Xin, Wu Qing. Stability of yellow pigment fromstrain T-YY[J].Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2008, 24(1): 285-290. (in Chinese with English abstract)
[21]赵迪,朱峰,鲁旭鹏,等.杀线虫真菌Simplicillium chinense菌株Snef5强毒和弱毒菌株的诱变筛选[J]. 沈阳农业大学学报,2016,47(5):602-607.
Zhao Di, Zhu Feng, Lu Xupeng, et al. Mutation Breeding for High and Low Toxic Strains of(Snef5)[J]. Journal of Shenyang Agricultural University, 2016, 47(5): 602-607. (in Chinese with English abstract)
[22]杜国军,田英华,刘晓兰. 果胶酶高产菌株的微波-硫酸二乙酯复合诱变选育[J]. 江苏农业科学,2017,45(20):279-281.
Du Guojun, Tian Yinghua, Liu Xiaolan. Microwave - diethyl sulfate mutagenesis of strain with high pectinase yield[J]. Jiangsu Agricultural Sciences, 2017, 45(20): 279-281. (in Chinese with English abstract)
[23]陈勇强,叶秀云,梁燕辉,等. 产果胶酶菌株:黑曲霉的复合诱变及产酶条件研究[J]. 中国食品学报,2016,16(1):99-107.
Chen yongqiang, Ye Xiuyun, Liang Yanhui, et al. Study on the compound mutation and pectinase-producing conditions of[J]. Journal of Chinese Institute of Food Science and Technology, 2016, 16(1): 99-107. (in Chinese with English abstract)
[24]李忠福,徐建国.分光光度法测定果胶酶活性方法的研究[J]. 黑龙江医药,2002(6):428-430.
Li Zhongfu, Xu Jianguo. Determination of pectinase activity by spectrophotometry[J]. Heilongjiang Medical Journal, 2002(6): 428-430. (in Chinese with English abstract)
[25]郝淼闻,俞志敏. 高产谷胱甘肽酿酒酵母菌株的诱变选育研究[J]. 沈阳农业大学学报,2013,44(6):832-836.
Hao Miaowen, Yu Zhimin. Mutation breeding high-yield glutathione strains of saccharomy cerevisiae[J]. Journal of Shenyang Agricultural University, 2013, 44(6): 832-836. (in Chinese with English abstract)
[26]钟葵,胡小松,陈芳,等. 脉冲电场对果胶酯酶的活性及构象的影响[J]. 农业工程学报,2005,21(2):149-152.
Zhong Kui, Hu Xiaosong, Chen Fang, et al. Inactivation and conformational change of pectinesterase induced by pulsed electric fields[J].Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2005, 21(2): 149-152. (in Chinese with English abstract)
[27]Chan-Ick Cheigh, Hee-Jeong Hwang,et al. Intense pulsed light (IPL) and UV-C treatments for inactivatingon solid medium and seafoods[J]. Food Research International, 2013, 54: 745-752.
[28]Oguma K, Katayama H, Mitani H, et al. Determination of pyrimidine dimers inandduring UV light inactivation, photoreactivation, and dark repair[J]. Applied and Environmental Microbiology, 2001, 67(10): 4630-4637.
[29]朱运明,王晓飞,闵伟红,等. 北京棒杆菌天冬氨酸激酶G377定点突变及酶学性质表征[J]. 食品科学,2014,35(9):192-197.
Zhu Mingyun, Wang Xiaofei, Min Weihong, et al. Site-directed mutagenesis and characterization ofG377 from[J]. Food Science, 2014, 35(9): 192-197. (in Chinese with English abstract)
[30]赵天惠,张佰清. 耐受性枯草芽孢杆菌的脉冲强光诱变筛选及产酶活力分析[J]. 食品科学,2018,39(2):192-197.
Zhao Tianhui, Zhang Baiqing. Pulsed light mutagenesis and screening offor improved tolerance and extracellular enzymatic activities of screened mutants[J]. Food Science, 2018, 39(2): 192-197. (in Chinese with English abstract)
Effect of Aspergillus Niger induced by intense pulsed light on pectinase-producing activity
Zhang Baiqing, Ge Xinyu, Ma Fengming, Ma Yichao, Zhang Shihao, Xie Jiahao
(,,110866,)
Intense pulse light (IPL) technology is a new non-toxic and environmentally friendly cold treatment technology, which can be applied to mutation breeding of microorganisms and to obtain high-producing strains. Aspergillus Niger is the main strain producing pectinase in food industry at present, but its pectinase yield is low. In order to verify the feasibility of applying intense pulse light technology to mutagenize Aspergillus Niger strains for high yield of pectinase, this experiment used intense pulse light technology to mutagenize Aspergillus Niger. With pulse voltage, pulse number and pulse distance as independent variables and ratio of transparent circle to colony diameter as dependent variables, steepest slope moving tests, response surface tests and result analysis are carried out to determine the optimum conditions for high-yield pectinase induced by the intense pulse light. At the same time, secondary screening of mutant strains was carried out and the genetic stability of the mutant strain was determined and the enzymatic properties of the mutant strain with high pectinase production were explored. The results showed that the multiple quadratic regression equation of transparent circle and colony diameter ratio () against impulse voltage (), pulse number () and pulse distance () is as follows:=1.59+0.068+0.048−0.033− 0.011−0.019−0.022−0.0712−0.0872−0.132.All the factors in the response surface design test were significant, and the interaction between two factors was not significant. The optimum mutagenesis conditions were when the pulse voltage was 2 075 V, the pulse number 36 times and the pulse distance 5.4 cm. Under the optimum conditions, the ratio of transparent circle to colony diameter could reach 1.58, which was in good agreement with the predicted value of response surface fitting equation, indicating that the model was credible. Induce mutation to the Aspergillus Niger under such optimized condition, screen mutant strains using transparent circle for 60 mutant strains with bigger ratio of transparent circle to colony diameter. The mutant strain L9 with high pectinase production was finally selected by re-screening the above-mentioned 60 strains by determining pectinase activity, which was as high as (188.21+1.22) U/mL, which was 82.2% higher than that of the original strains. The results of genetic stability analysis showed that the mutant strain L9 had stable pectinase performance within 6 generations and no significant changes were seen in terms of pectinase activity, which indicated that the mutant strain L9 had good genetic stability. The optimum pH value and temperature for producing pectinase were 5.0 and 45℃. Compared with the original strain, the mutant strain L9 produced pectinase with better activity at the optimum pH and temperature. The range of the pH stability and thermal stability of the mutant strain were also significantly wider than that of the original strain, indicating that the mutant strain had good pH stability and thermal stability. Through the above experimental results, we can know that the application of intense pulse light technology to Aspergillus Niger mutation is feasible. After the intense pulse light induced mutation and secondary screening, a mutant strain of Aspergillus Niger with higher pectinase production with high enzymatic activity and good genetic stability can be obtained.
enzyme; agricultural products; electric field; pulsed light; mutagenesis; Aspergillus Niger; pectinase; response surface analysis
张佰清,葛新宇,马凤鸣,马艺超,张士豪,谢加豪. 脉冲强光诱变对黑曲霉产果胶酶活力的影响[J]. 农业工程学报,2020,36(3):296-301.doi:10.11975/j.issn.1002-6819.2020.03.036 http://www.tcsae.org
Zhang Baiqing, Ge Xinyu, Ma Fengming, Ma Yichao, Zhang Shihao, Xie Jiahao. Effect of Aspergillus Niger induced by intensepulsed light on pectinase-producing activity[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(3): 296-301. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.03.036 http://www.tcsae.org
2019-09-03
2019-10-08
国家自然科学基金项目资助(31772011)
张佰清,博士,教授,博士生导师,研究方向为农产品加工。Email:sybaiqinggxl@sina.com
10.11975/j.issn.1002-6819.2020.03.036
TS201.3
A
1002-6819(2020)-03-0296-06
