Effect of short-term gavage of ethanolic extract of cogon grass (Imperata cylindrica L)root on the ovarian activity and estrus behavior of female mice
2020-04-08RiniWidyastutiAriefBoedionoMasRizkyAnggunAdipurnaSyamsunarnoMohammadGhozaliMulyanusaAmarullahRitongaAlkaustariyahLubisSondiRobiantoJaquelineSudiman
Rini Widyastuti, Arief Boediono, Mas Rizky Anggun Adipurna Syamsunarno, Mohammad Ghozali,Mulyanusa Amarullah Ritonga, Alkaustariyah Lubis, Sondi Robianto, Jaqueline Sudiman
1Laboratory of Animal Reproduction and Artificial Insemination, Department of Animal Production, Faculty of Animal Husbandry, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang KM.21, West Java 45363, Indonesia
2Central Laboratory, Universitas Padjadjaran, Jl. Raya Bandung Sumedang Km.21 Jatinangor Sumedang, West Java 45363, Indonesia
3Laboratory of Embryology, Department of Anatomy, Physiology, and Pharmacology, Faculty of Veterinary Medicine, IPB University, Jl. Agatis Dramaga Bogor 16680, West Java, Indonesia
4Department of Biomedical Science Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang Km.21 Jatinangor Sumedang, West Java 45363, Indonesia
5Department of Obstetrics and Gynaecology, Faculty of Medicine, Universitas Padjadjaran, Hasan Sadikin Hospital, Jl. Pasteur No. 38 Bandung, West Jawa Barat 40161, Indonesia
6Study Program of Undergraduate Medicine, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang Km.21 Jatinangor Sumedang,West Java 45363, Indonesia
7Department of Obstetrics and Gynaecology, Faculty of Medicine, Udayana University, Jl. PB Sudirman, Denpasar, Bali, Indonesia
ABSTRACT Objective: To assess the effect of short-term gavage of ethanolic extract of Imperata cylindrica L root on the ovarian activity and estrus behavior of female mice.Methods: Eighteen virgin female ddY mice, 8 to 10 weeks of age, weighing 22-25 g with regular estrus cycle, were divided into three groups. Group 1 received 0.5% carboxymethylcellulose,whereas Groups 2 and 3 received the ethanolic extract of Imperata cylindrica L root at 90 and 115 mg/kg body weight (b.w.) per day by gavage for 20 days, respectively. All of the groups were checked before 9 a.m. daily for vaginal cytology to determine the estrus phase. On day 21, the mice were sacrificed to collect serum samples to quantify the concentrations of reproductive hormones using enzyme-linked immunosorbent assay and to determine changes in the reproductive organs based on their reproductive organ weight,histomorphology, and histomorphometry of ovarium and uterus.Results: The reproductive organ weight in the treatment groups was similar compared with that in the control group. The 90 mg/kg b.w. treatment group showed an increase in corpus luteum number when compared with the control group, with few degenerated follicles and diminished oocytes. Moreover, the 115 mg/kg b.w.treatment group showed fewer primordial and primary follicles and an increase in corpus luteum number and a prolonged diestrus phase compared to the control and 90 mg/kg b.w. treatment groups.The histomorphology examination of the uterus showed that the thickness of myometrium and epithelium in the treated animals was similar to the control group. In addition, there was a significant decrease in follicle-stimulating hormone level in the 115 mg/kg b.w.treatment group (P<0.05).Conclusions: Short-term gavage of ethanolic extract of Imperata cylindrica L root reduces the follicle-stimulating hormone serum level and folliculogenesis.
KEYWORDS: Imperata cylindrica L; Estrus cycle; Ovary;Endometrium; Reproductive hormone
1. Introduction
In several societies, medicinal plants have been used as a dietary adjunct for the treatment of numerous diseases and as an antifertility agent without proper knowledge of their exact functions and pharmacology[3,4]. Various medicinal plants have been known for their antifertility activities[5,6]. They possess different types of antifertility activities such as anti-implantation, abortification,ecobolic, estrogenic, and spermicidal; however, a large number of these medicinal plants possess some degree of toxicity. In total, there are 50 plants reported as having different antifertility activities[7].Cogon grass (Imperata cylindricaL) is abundantly available in many regions of Southeast Asia and is often considered as a weed on farms or plantations. Although there are losses caused to the farmers by the presence of this plant on agricultural land, it turns out thatImperata cylindricaL also has many benefits, especially in the medical field, such as its antihypertensive[8], antibacterial[9], and anti-diabetic[10] properties.
The previous study reported that short-term gavage ofImperata cylindricaL extract to male mice reduced sperm concentration significantly[11] and induced abnormal sperm morphology[12]. The previous research also reported that the administration ofImperata cylindricaL root induced the shortening of the estrus phase in mice[13]. The leading indicators to determine fertility performance in female mice are disruption of the estrus cycle, irregularity of the reproductive hormone concentration, and reduction in the size of the follicles[14]. The estrus cycle is closely related to the sex hormone function of the neuroendocrine-reproductive system and ovarian activity; therefore, a disturbance of the estrus cycle indicates the disruption of ovarian and estrogen balance[15]. The present study aimed to evaluate the effect of short-term gavage of ethanolic extracts ofImperata cylindricaL root on female reproductive organs and changes in the reproductive hormone, and it also aimed to identify a new female antifertility agent.
2. Materials and methods
2.1. Collection of Imperata cylindrica L extract
TheImperata cylindricaL roots were obtained from Solo, Central Java, Indonesia, and identified at the School of Life Science,Bandung Institute of Technology, with specimen number 3561/10/11.CO2.2/PL/2016. The 8-kg roots ofImperata cylindricaL were macerated with 95% ethanol for 72 h, filtrated with a vacuum filter,and concentrated in a vacuum evaporator. The concentrated extracts were suspended with 0.5% carboxymethylcellulose and divided into two concentrations: 90 and 115 mg/kg body weight (b.w.). The doses were chosen based on a previous study[16].
2.2. Animals selection and dose level
Eighteen female ddY mice, 8-12 weeks of age, with normal estrus cycle (average 4-6 days of proestrus, estrus, metestrus, and diestrus phases) weighing between 22 and 25 g, were divided into three groups, with six mice in each group. The mice were housed in a room with a 12/12 h light and dark cycle, adequate air circulation,and unrestricted access to water and standard feed. Mice in Group 1 received 0.5% carboxymethylcellulose by gavage, whereas Groups 2 and 3 received 90 and 115 mg/kg b.w. per day, respectively, of ethanolic extract ofImperata cylindricaL root by gavage for 20 consecutive days which was based on previous toxicity studies[13,17].
2.3. Body and reproductive organs weights
The mice used for this experiment were selected based on their estrus cycle. At 9 p.m., after day 21 of the experiment, 18 selected mice with follicular phase (proestrus and estrus) were anesthetized using isoflurane; their b.w. was measured, and they were then euthanized by cervical dislocation. Ovaries and uteri were removed and isolated from adherent tissues, and their weight was then recorded.
2.4. Histopathology of reproductive organs
The ovary and uterus were fixed in 10% formaldehyde and embedded in paraffin, and 5-µm sections were stained using hematoxylin and eosin. The sections were observed under an upright microscope (Olympus CX21, Olympus, Japan) with 200×magnification for the histopathological effect of the ethanolic extract ofImperata cylindricaL root. Morphological identification and the number of primordial, primary, secondary, antral follicles, and corpus luteum were observed in a histological section of the cortex of the ovary. Follicles with one layer of granulosa cells and inactive follicles were mitotically categorized as primordial follicles. Primary follicles were distinguished by layers of cuboid-shaped granulosa cells and larger oocyte diameter. Secondary follicles had two or more layers of cuboidal granulosa cells. The antral follicle had an antrum and several layers of granulosa cells and theca cells[18]. The number of follicles was calculated by dividing the total number of follicles from the first 25 sections of the ovary that cut continuously with the total number of follicles on the 5th, 10th, 15th, 20th, and 25th sections of the ovary.
Multiple factor=(The numbers of follicles on the first 25 sections)/(Number of follicles on the 1st, 5th, 10th, 20th, and 25th section )The thickness of the myometrium and epithelium were measured from 25 sections per uterus. Histological photos were taken using an Olympus 1X71 microscope (Olympus, Japan) 200× magnification connected with the DP2-BWS software program (Olympus, Japan).
Alas3! said the man, why should I go down there again? Why, said his wife, you caught him, and then let him go again, so he is sure to give you what you ask
2.5. Estrus cycle study
Mouse vaginal smears were examined before 3 p.m. daily to determine their stage of estrus. The stages of an estrus cycle were determined based on the presence of leucocytes, cornified epithelial cells, and nucleated epithelial cells. Proestrus was characterized by the presence of a predominance of nucleated epithelial cells in clusters or individually, and occasionally, some cornified cells might appear in this phase. Estrus was characterized by cornified squamous epithelial cells with no visible nucleus, and the cytoplasm was granular, and the shape was irregular. Metestrus was characterized by the presence of a mix of cell types with a predominance of leucocytes and a few nucleated epithelial or cornified squamous epithelial cells. Diestrus was characterized by predominant leucocytes[19].
2.6. Reproductive hormone assay
On day 21 of the experiment, all the female mice of the treatment and control groups were euthanized by cervical dislocation after anesthetized using isoflurane. About 0.8 mL of the blood sample from each mouse was collectedviacardiac puncture into a sterilized tube and incubated for 15 min at room temperature. The sample was centrifuged 1 500×gfor 10 min, and the serum was collected.The serum samples were stored frozen at -80 ℃ until enzymelinked immunosorbent assay (ELISA) was used to measure folliclestimulating hormone (FSH), luteinizing hormone (LH), and estrogen levels in the serum samples collected. The mouse FSH ELISA kit(EM1035) with sensitivity: <1.406 mIU/mL, CV (%) = standard deviation (SD)/mean × 100, Intra-Assay: CV < 8% and Inter-Assay: CV < 10% mouse; LH ELISA kit (EM1188) with sensitivity: < 0.281 ng/mL, CV (%) = SD/mean × 100, Intra-Assay: CV < 8% and Inter-Assay: CV < 10%; and Estradiol ELISA kit (EU0390) with sensitivity: 0.422 ng/mL, CV (%) = SD/mean× 100, Intra-Assay: CV < 8% and Inter-Assay: CV < 10% were obtained from Fine Test (Hubei, China). The concentrations of FSH,LH, and estrogen were assayed according to the procedures outlined in the respective manufacturer’s protocol.
2.7. Statistical analysis
Data were analyzed by using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, California, USA. One-way analysis of variance and Kruskal-Wallis tests were used, followed by Tukey’spost-hocmultiple comparison tests to establish the differences between the control and treatment groups. Data were expressed as mean±standard deviation (mean±SD) if they were normally distributed and as median (interquartile) if they were not normally distributed.P<0.05 was considered statistically significant.
2.8. Ethics statement
All the animal care and the experimental procedures related to this study were in accord with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and approved by the ethics committee of the Faculty of Medicine,Universitas Padjadjaran, Indonesia (approval No. 326/UN6.KEP/EC/2018).
3. Results
3.1. Effect of ethanolic extract of Imperata cylindrica L root on b.w. and reproductive organ weights
The groups receiving an ethanolic extract ofImperata cylindricaL root by gavage for 20 days showed no significant alteration in b.w.,ovary, or uterine weight when compared with the control group(Table 1).
3.2. Effect of ethanolic extract of Imperata cylindrica L root on follicle development in the ovary
The photomicrograph of the transverse section of mice ovary from the control group showed normal development with different follicular maturation stages (Figure 1A). On the contrary, the histopathology of the mouse ovary in the 90 mg/kg b.w. treatment group showed an increase in the number of corpus luteum as compared with that in the control group, with few degenerated follicles (diminished oocytes). The ovary was dominated mainly by primary follicles (Figure 1B), whereas the mouse ovary exposed to 115 mg/kg b.w. ethanolic extract ofImperata cylindricaL root showed that the number of follicles was reduced compared with other groups. The ovaries were seen to have degenerated follicles and were atretic. There was an increased number of corpus luteum(Figure 1C and Table 2). The quantification of follicles revealed that the number of primordial and primary follicles in the 115 mg/kg b.w. treated mice was significantly reduced after administration of ethanolic extract ofImperata cylindricaL root compared with that in the control group (Table 2).

Table 1. Effect of ethanolic extracts of Imperata cylindrica L root on body weight, ovary, and uterine weight in female mice.
3.3. Effect of ethanolic extract of Imperata cylindrica L root on histopathology of mice endometrium and myometrium thickness
The photomicrograph of mice uterus in the control group showed an undulating stratified columnar epithelium of the endometrial lining. The glands showed high columnar epithelial cells and mild degeneration, and the lumen was moderately dilated (Figure 2A). In the 90 mg/kg b.w. treatment group, the endometrial lining showed mild hyperplasia with stratified cuboidal epithelium and some undulating. The glands were simple columnar with some degeneration, with the lumen slightly narrowed (Figure 2B). Histopathology of the endometrium in the 115 mg/kg b.w.treatment group demonstrated that the endometrium was lined by columnar epithelium cells with hyperplasia. The glands were dilated with high columnar cells, moderate degeneration, and the lumen was also narrowed (Figure 2C). Moreover, the histomorphology examination of the uterus of treated animals showed reduced thickness of both the myometrium and the epithelium, although they were similar compared with the control group (Table 3).
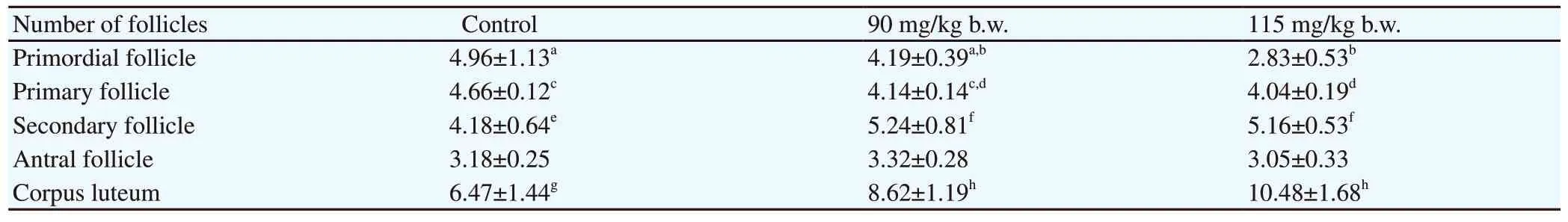
Table 2. Effect of administration of ethanolic extract of Imperata cylindrica L root for 20 days on the number of follicles in female mice.

Figure 1. Photomicrograph of the transverse section of mouse ovary exposed to ethanolic extract of Imperata cylindrica L root (hematoxylin and eosin;magnification: 200×). (A) The control group: Follicles with different maturation stages (thick arrow) are present. (B) The 90 mg/kg b.w. treatment group:Degenerated follicles with diminished oocytes (thick arrow) are seen. (C) The 115 mg/kg b.w. treatment group: Degenerated and atretic follicles (thick arrow) are observed. CL: corpus luteum; AF: antral follicle; SF: secondary follicle; PF: primary follicle.

Figure 2. Photomicrograph of mouse uterus exposed to ethanolic extract of Imperata cylindrica L root (hematoxylin and eosin; magnification: 200×). (A) The control group: The undulating stratified columnar epithelium (thick arrow) is seen. A mild degeneration of gland utery (black arrowhead) and dilated lumen (L)are present. (B) The 90 mg/kg b.w. treatment group: Mild endometrial hyperplasia with undulation (thin arrow) is observed. A few degenerated gland uteries (blue arrowhead) are present. The lumen is slightly narrowed. (C) The 115 mg/kg b.w. treatment group: Hyperplasia of epithelium (blue arrow) and dilation with moderately degenerated gland utery (red arrow) are observed. M: myometrium.

Table 3. Effect of ethanolic extract of Imperata cylindrica L root on the myometrium and epithelium height in female mice.

Table 4. Effect of ethanolic extract of Imperata cylindrica L root on the estrus cycle in female mice.

Table 5. Reproduction hormone serum level in various groups of animals after 20 days of ethanolic extract of Imperata cylindrica L root by gavage.
3.4. Effect of ethanolic extract of Imperata cylindrica L root on estrus cycle
Mice in the 90 and 115 mg/kg b.w. treatment groups showed reduced proestrus and estrus and prolonged diestrus phases, although there was no difference compared with that in the control group(Table 4).
3.5. Effect of ethanolic extract of Imperata cylindrica L root on reproductive hormone levels
The alteration in the estrus cycle duration indicated reproductive hormonal disturbance. The results showed that the FSH serum level was significantly (P<0.05) reduced in the highest dose of treated animals (115 mg/kg b.w.) compared with that in the control and 90 mg/kg b.w. treated animals. However, the LH and estrogen serum levels showed no difference between the control and treatment groups (Table 5).
4. Discussion
Administration ofImperata cylindricaL root to cyclic adult mice by gavage for 20 days did not affect b.w. compared with the control group, which indicated that the general body growth was normal.Moreover, no significant difference in reproductive organ weights was evidently pointing to the non-toxic nature of the extract. This means that the extract did not negatively impact the metabolic status of the animals. Even though there was an insignificant increase in the ovary and uterine weights, there were histomorphological alterations
in the ovary and uterine tissues. The alteration in the ovary was marked by folliculogenesis disruption, whereas the alteration in the uterine was marked by hyperplasia of the endometrial lining and uterine glands dilatation followed by high columnar epithelial cells linen.
The folliculogenesis disturbance in treated animals can be observed from the reduced number of follicles, mainly in primordial and primary follicles, followed by an increase in corpus luteum in a dose-dependent pattern when compared with the control group.Diminishing serum levels of FSH might influence this alteration because of its critical function in follicle growth and development.The growing follicles require FSH for maturation, except for the primordial follicle. FSH is unlikely to exert direct actions on primordial follicles because functional gonadotropin receptors have not yet been developed in them until the second stage. The decrease in the number of growing follicles in the present study is consistent with the low level of serum FSH.
At the beginning of the follicular phase, FSH stimulates follicle development and its maturation, at which time, the mature follicles secrete estrogen. Estrogen production is dependent upon both FSH stimulation of aromatase in the granulosa cells and LH stimulation of androstenedione production by the theca cells. Estrogen peaks at around the middle and falls toward the end of the proestrus phase.Estrogen peak is followed by an LH surge during the middle of proestrus and subsequently triggers the onset of ovulation[20].
The slight increase in the uterine and ovarian weight seen in the treatment groups might indicate estrogenicity of the extract[21].Estrogen is essential for epithelial cell proliferation, maintenance of normal epithelial morphogenesis, cytodifferentiation, and secretory activity. This hormone exerts its effect by binding to the estrogen receptor (classical pathway) and protein tethering (nonclassical pathway). It had been elucidated that compared with humans, the physiologic changes of rodents after administration of estrogen occur in two waves (biphasic). The early response is water imbibition that causes an increase in uterine weight. The late response includes epithelial cell proliferation and differentiation,resulting in the cell being transformed into columnar secretory cells with abundant mitosis[22]. In our study, there was a pattern of decreased estrogen serum levels, which was followed by an increase in LH concentration. This effect suggests direct negative feedback of estrogen at the anterior pituitary.
The increased diestrus duration in treated animals, although insignificant compared with that in the control, may decrease a chance for conception[23]. The diestrus phase is a period when the ovarian secretions from the corpus luteum prepare the uterus for implantation. LH from the anterior pituitary maintains the functioning of the corpus luteum and its secretion of progesterone.The increased duration of this phase denotes a reduction in the fertile period. The results are comparable with the previous studies with methanolic leaf extract ofCisampelos pareira[24,25] that reported an antifertility effect in a similar observation with albino mice, with seed extract ofRicinus communis[26], and root extract ofRumex steudelii[27] in the guinea pig.
Phytochemical screening ofImperata cylindricaL extract revealed many bioactive agents such as flavonoids, isoflavones, alkaloid,steroid, lignans, carbohydrates, and tannin[28].In vitrostudies suggest that polyphenols may have a negative impact on female reproductive health. For instance, curcumin, the predominant dietary pigment in turmeric, has been shown to promote mouse oocyte apoptosis, which leads to a significant reduction in the rate of oocyte maturation, fertilization, andin vitroembryonic development[29]. It has also been shown that the methanolic extract ofRumex steudeliicould cause atrophic changes in the uterus and disruption of ovarian folliculogenesis by further inhibiting the development of the recruited ovarian follicles[30]. A previous study reported that several plants that contain alkaloids and isoflavones inhibit aromatase activity[31]. These phytoestrogens may be attributed to the negative effect of the extract toward estrogen serum levels, which then further decrease granulosa cell proliferation and inhibit follicle maturation[32]. The result is comparable with previous studies that usedPlumbago zeylanicaLinn. extract, which contains phytoestrogens, resulting in altered female rat reproductive hormones leading to infertility, conception disruption[33], and ovulation inhibition[34]. A similar study in bamboo shoots reported that the daily consumption of this extract induces folliculogenesis disruption and uterine normal histology[35].
The significant disruption in FSH secretion leads to disturbance in follicle development, because it is essential for the development of primary and secondary follicles to the antral stage and, more importantly, further promotes antral follicular development. The resumption of follicle development results in a disturbance in the LH and estrogen secretions. LH could induce ovulation of Graafian mature follicles, whereas estrogen plays an important role in the intraovarian regulation of folliculogenesis by acting on specific ovarian receptors. Estrogen receptor alpha-specific deletion in theca cells could lead to premature ovarian failure[36]. Estrogens could also induce uterine cell proliferation during the late follicular phase of the estrus cycle.
In conclusion, the short-term gavage of ethanolic extract ofImperata cylindricaL root causes an alteration in FSH secretion,which disturbs the folliculogenesis. The preliminary findings of the research indicate that ethanolic extract ofImperata cylindricaL root has potential as an herbal contraceptive agent. It needs further investigation about the effect of ethanolic extract ofImperata cylindricaL root on oocyte quality, ovulation, fertilization,in vivoembryo development, and implantation to justify that this extract is a good candidate for antifertility. Further research about the appropriate dose and potential for toxicity is required to further evaluate the effect of ethanolic extract ofImperata cylindricaL root.It can be concluded that short-term gavage of ethanolic extract ofImperata cylindricaL root induces alteration on the reproductive hormone and folliculogenesis.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
Rini Widyastuti, Arief Boediono, Mas Rizky Anggun Adipurna Syamsunarno and Jaqueline Sudiman designed and performed experiments, analysed and interpretated the data. Alkaustariyah Lubis, Sondi Robianto and Rini Widyastuti performed experiment and collected the data. Mohammad Ghozali and Mulyanusa Amarullah Ritonga contributed to preparing and interpretating the histopathological samples. Rini Widyastuti took the lead in writing the manuscript in consultation with Arief Boediono, Jaqueline Sudiman, Mohammad Ghozali and Mas Rizky Anggun Adipurna Syamsunarno. All authors provided critical feedback and helped shape the research, analysis and manuscript.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Erectile dysfunction and statins: The assorted view of preponderance
- Effect of routine iron supplementation on copper level and oxidative stress status in pregnant women
- Effect of aqueous seed extract of Mucuna pruriens on arsenic-induced testicular toxicity in mice
- Effects of ciprofloxacin on testicular tissue and sperm quality in rabbits
- Influence of N-acetylcysteine on pituitary-gonadal axis hormones and protamine expression level in streptozotocin-induced diabetic male rats
- Influence of butylated hydroxytoluene addition to cryodiluents on freezability and DNA integrity of Boer and Zaraibi buck spermatozoa
