花青素介导植物抗重金属胁迫机理的研究进展
2020-03-20耿安静王旭李秋剑杨慧陈岩刘雯雯陈永坚王富华
耿安静 王旭 李秋剑 杨慧 陈岩 刘雯雯 陈永坚 王富华


摘要:近年來的土壤重金属污染问题依然严重。重金属作为一种主要的非生物胁迫,不仅严重制约植物的生长发育、影响农产品的品质及安全质量,还会通过食物链进入动物和人体内,最终威胁人体健康。如何缓解植物重金属毒害一直是国内外研究的重点和难点。花青素是一种广泛存在于自然界植物中的水溶性天然色素,参与植株的生长与发育、生物与非生物胁迫应答等。文章归纳总结文献报道,阐明重金属对植物的危害,并以砷为例说明危害机理,介绍花青素的生物学功能,以及花青素介导植物抗重金属胁迫机制,包括清除自由基、激发/促进内源抗氧化系统、螯合重金属、区室化隔离和花青素相关基因表达。针对目前重金属修复及农林废弃物化利用存在的问题,提出今后应重点开展花青素介导植物重金属胁迫和归趋的机理与应用研究,开发重金属植物修复技术的新原料,为丰富植物重金属转运机制和防控措施、减少植物重金属污染、综合利用富含花青素的有色蔬果及制糖原料等农林废弃物提供参考依据。
关键词: 花青素;重金属;胁迫;介导机理
中图分类号: S19 文献标志码: A 文章编号:2095-1191(2020)01-0080-11
Abstract:The problem of heavy metals pollution in soil in recent years is still serious. As a major abiotic stress, heavy metals not only seriously restrict the growth and development of plants, affect the quality and safety of agricultural products, but also enter the animals and humans through the biological chain, threatening human health at last. How to alleviate the heavy metals toxicity of plants has always been the focus and difficulty of research at home and abroad. Anthocyanin is one kind of water-soluble natural pigment widely found in plants. It can participate in plant growth and development, biological and abiotic stress responses and so on. The paper illuminated the damage of heavy metals to plants and took arsenic as an example to explain its mechanism. Introduced biological functions of anthocyanin and mechanism by which anthocyanins regulated heavy metal pollution in plants included scavenging free radicals, exciting/promoting endogenous antioxidant systems, chelating heavy metals, compartmentalization and expressing anthocyanin-related genes. Aiming at the problem of heavy metal remediation and utilization of agricultural and forestry waste, this article provided theoretical basis for enriching plant heavy metal transport mechanisms and prevention and control measures, reducing plant heavy metal pollution, comprehensive utilization of anthocyanin-rich agricultural and forestry waste such as colored vegetables and fruits, beets as sugar production raw material. It was suggested to carry out the mechanism and application research of anthocyanins to mediate heavy metal stress and fate of plants, and develop new raw materials for heavy metal phytoremediation technology in the future.
Key words: anthocyanin; heavy metals; stress; mediated mechanism
Foundation item: National Key Research Program(2019YFC1605602);National Natural Science Foundation of China for Young Scholars(41807475;41401367);Entrusted Technology Research Project of Key Laboratory of Edible Agri-cultural Products Regulation Technology of Shenzhen Agricultural Products Quality and Safety Inspection and Testing Center(Shinongjianzicaihetongzi〔2019〕032)
0 引言
随着冶金、核能、化工等行业“三废”排放、矿山开发、城市垃圾废弃等人为和地理因素向自然环境释放了大量有毒重金属离子,导致土壤中重金属污染严重。重金属通过植物根系及叶面等方式进入植物体内,不仅制约植物的生长发育,影响植物产品的产量、品质及安全,还会通过食物链进入动物和人体内,最终危害人体健康。如何防控重金属污染一直是关系民生的国内外研究热点和重难点。花青素是一种广泛存在于植物花瓣、果实等部位的天然水溶性色素,具有抗突变(Punvittayagul et al.,2014)、保护神经(Thummayot et al.,2014)、抗炎(Du et al.,2015)、防治心血管疾病(Chen et al.,2016)、抗癌(Munagala et al.,2017)、抗氧化(Parizad et al.,2019)等功能,在食品(Sui et al.,2016)、医药(Lin et al.,2017)、化妆品(Nizio?-?ukaszewska et al.,2017)、饲料(汪文忠,2017)等行业均具有巨大的应用潜力。花青素除了对动物和人类具有较多生理活性外,对植物也具有广泛的生物学功能。近年来研究发现,富含花青素的植物较普通植物的抗重金属胁迫能力强,花青素在一定程度上可缓解植物受重金属的毒害(Dai et al.,2006,2012;Posmyk et al.,2009;Landi et al.,2014;Geng et al.,2017)。本文对花青素调控重金属毒害的机理进行分析,旨在为完善植物重金属转运机制和防控措施、减少植物重金属污染及综合利用富含花青素的农林废弃物提供理论依据。
1 重金属对植物的危害及机理
许多重金属元素如锌(Zn)、铜(Cu)、铁(Fe)是植物所必需的营养元素,对植物生长发育起着极其重要的作用。但这些重金属营养元素的有益作用范围有限,当浓度超过其效应浓度(受体可耐受的最大限度)时就会毒害机体。而更多的重金属[如铅(Pb)、镉(Cd)和汞(Hg)等]和非金属元素[如砷(As)]并非植物生长所必需的元素,毒性大,即使在低浓度下也被认为是非常有害,能够引起受体内生理生化代谢紊乱,生长发育受抑制,达到致死浓度时,会导致受体死亡。重金屬进入植物体后会产生许多有害影响,包括抑制细胞生长、叶绿素降解、破坏光合作用和呼吸作用、养分消耗、脂质过氧化、膜解体及刺激次生代谢途径,导致作物生长受损,甚至死亡(Gupta et al.,2013)。
作为植物生长营养元素之一的Zn在高浓度时会毒害植物,主要表现为抑制生长,其特征是根系生长减缓、根系增厚、细胞分裂/伸长受损、根褐变与腐烂(Chanu and Gupta,2016)。主要机制是Zn与Fe和镁(Mg)具有很高的化学相似性,可在酶的活性位点替代这两种金属离子,从而干扰细胞功能。高浓度Cu不仅会引起小麦、水稻、玉米、向日葵和黄瓜的生长受抑制、氧化损伤和抗氧化反应,还会改变矿物质营养、光合作用、酶活性和叶片叶绿素含量,从而导致产量下降(Adrees et al.,2015)。Cu毒害的主要机理是Cu能够催化Haber-Weiss和Fenton反应,导致活性氧(Reactive oxygen species,ROS)产生,而过量ROS会破坏细胞成分从而影响细胞功能(Bona et al.,2007)。高Fe胁迫与植物体内氧化应激、生长调控、细胞壁硬度改变及其合成等有关(Hopff et al.,2013)。
Cd不仅无生物学功能,还会通过诱导氧化应激对植物生长发育产生不利影响(Grat?o et al.,2008)。Cd发挥其毒性作用是通过对蛋白质巯基的亲和力抑制ROS自由基解毒酶从而导致氧化应激。从生物学上讲,Cd在化学上与Zn、Fe和钙(Ca)相似,可在许多蛋白质的修复基中取代这些元素,导致植物缺少营养元素而影响其正常的生长发育(Fagioni et al.,2009)。Pb没有已知的生物学作用,其与植物的相互作用导致ROS产生。Pb和/或ROS会抑制正常的细胞功能、生理反应和植物的整体性能,主要机制是通过破坏组织超微结构、细胞成分和生物分子而引起植物毒性(Kumar and Prasad,2018)。
铝(Al)是酸性土壤作物生长的主要限制因素。高浓度的Al3+通过与磷酸盐、硫酸盐和羰基官能团结合而破坏各种细胞生长及组分,从而抑制对Al3+敏感的作物生长并降低产量(Poschenrieder et al.,2008;Valle et al.,2009)。高铬(Cr)暴露对铁氧还蛋白—烟酰胺腺嘌呤二核苷酸磷酸(Nicotinamide-adenine dinucleotide phosphate,NADP)还原酶、NADP-异柠檬酸脱氢酶、乙二醛酶I和谷氨酰胺合成酶等多种抗氧化酶水平产生影响(Zeng et al.,2014)。Hg暴露与代谢过程、光合作用、应激反应、能量代谢、信号通路和免疫抑制等相关(Liu et al.,2013a)。
以As为例说明重金属对植物的毒害作用(图1)和危害机理。As通过根进入植物体内的主要形式是无机As(III)和As(V),而一旦进入植物根细胞,As(V)很容易转化为毒性更大的As(III)。As(V)和As(III)均会破坏植物的新陈代谢,但通过不同的机制。As(V)是一种磷酸盐类似物,能破坏某些与磷酸盐有关的新陈代谢,其可通过磷酸盐转运蛋白在细胞膜上转运,导致磷酸盐供应失衡;可在磷酸化反应中与磷酸盐竞争,取代用于线粒体的氧化磷酸化和三磷酸腺苷(Adenosine triphosphate,ATP)合成磷酸基,导致As(V)加合物的形成,解除光合磷酸化和氧化磷酸化,降低细胞产生ATP和进行正常代谢的能力,导致细胞内能量流动中断(Cozzolino et al.,2010)。As(III)是一种二硫醇反应性化合物,毒害机理有:(1)As(III)的暴露通常会诱导ROS产生,植物体内过量的ROS会超过植物本身抗氧化系统的防御能力,进而影响碳代谢、氮代谢和硫代谢等,导致碳水化合物、氨基酸与蛋白质的形成及功能等受影响,进而影响植物生长与结实,严重时会导致死亡(Begum et al.,2016)。(2)单体As较易与含有半胱氨酸残基或二硫醇辅因子的酶、蛋白质、DNA结合形成复合物,使酶失活或活性改变、蛋白质/DNA变性功能丧失等。(3)As也能通过连接到邻近的巯基上,改变蛋白质结构/功能,从而对磷酸盐代谢产生不利影响(Tripathi et al.,2007)。(4)As通过调节参与代谢和氧化还原稳态的蛋白,对叶绿体结构和光合作用产生负面影响。如在水稻中,As通过破坏ROS的稳态来破坏细胞超微结构,从而抑制水稻生长(Liu et al.,2013b)。(5)由于分子量及电荷数较小,单体As(III)被植物吸收速度较快,导致根的As(III)含量高而抑制植物对有益元素的吸收,从而抑制生长,或引起根受伤导致植物生长受损;同时,单体As(III)向上转运的速度也较快,这就是大米中As(III)含量较其他As形态含量高的原因。As(III)迁移到植物生殖器官中对植物的生殖有着重要影响,导致产量降低、质量下降等(Singh et al.,2015)。
2 花青素的生物学功能
花青素不仅对人体具有较强的生理活性,对植物也具有较多的生物学功能。花青素是构成花瓣和果实颜色的主要色素之一,在授粉过程中起着重要作用,花青素能在自然界花朵发育过程(如在开花和授粉后)中通过许多方式改变,为传粉者和播种者提供视觉线索以吸引动物进行授粉和果实传播(Grotewold,2006)。花青素改变导致的颜色变化具有一系列的生物化学机制,影响因素有温度、共色素、pH、金属、糖、花青素堆积和细胞形状等(Miller et al.,2011)。花青素还被认为在多种植物/动物的相互作用中发挥作用,包括传粉媒介和吸引食果动物,以及排斥食草动物和寄生虫。花青素的光学特性可作为潜在食草动物的视觉信号,显示出对有毒或难吃化学物质的强大代谢包埋。花青素还涉及植物与其背景的部分伪装,潜在破坏昆虫的保护色及防御结构的拟态伪装(Lev-Yadun and Gould,2008)。花青素在营养器官中积累,不仅有利于保护光合系统免受强光和紫外线的伤害,还能提高植株抗低温、抗旱、抗病虫害、抗重金属、抗食草动物侵袭等非生物和生物胁迫(Imtiaz et al.,2018)。如紫茎的蜈蚣草耐盐性较绿茎的蜈蚣草好,主要是花青素的高积累能够通过调节生理功能和渗透平衡促进耐盐性(Li et al.,2018)。花青素可以减少在光胁迫条件下叶绿素的光抑制和光漂白。在高辐照度下植物体内的花青素通常聚集在外周组织,但也有一些例外。花青素对光的衰减有可能帮助建立新的平衡,作为植物组织的光保护屏障降低光氧化损伤的风险(Steyn et al.,2002);在种子中积累,可作为内源性抗氧化剂保护种子内的化学物质,也有利于種子休眠(Lepiniec et al.,2006)。
3 花青素介导植物抗重金属胁迫的机理
花青素通过清除自由基、抗氧化系统的促进与激活、与重金属螯合、区室化隔离、花青素结构/调控基因表达等方式调节植物吸收转运重金属,从而缓解重金属对植物的毒害(Raab et al.,2005;Ahmed et al.,2013;Le?o et al.,2013;Uraguchi et al.,2018)。
3. 1 清除自由基
当植物受重金属胁迫时,体内会产生过多的ROS,如超氧阴离子自由基([O][2])、羟基自由基(·OH)、过氧化氢(H2O2)、单线态氧和脂氧自由基等,其化学性质比较活跃,几乎可以与各种物质发生作用,引起一系列对细胞具有破坏性的连锁反应,对光合作用、呼吸作用等有重要的不利影响(Maleva et al.,2018),过量时可导致细胞突变或死亡、组织病变或死亡。
花青素含有多个酚羟基供体,作为氢或电子供体具有较强的反应活性,是一种较强的抗氧化剂,能清除氧自由基(O·)、1,1-二苯基-2-三硝基苯肼自由基(DPPH·)、2,2'-连氮基-双-(3-乙基苯并二氢噻唑啉-6-磺酸)二铵盐自由基(ABTS·)、过氧自由基(ROO·)、氢过氧自由基(HO2·)、[O][2]和H2O2等,也可与蛋白结合抑制过氧化反应,还可清除脂质自由基,进而抑制自由基链反应的启动、切断脂肪链反应中的氧化环节、控制自由基反应传播从而抑制脂质过氧化反应。ROS通过上调生物合成后期对应的结构基因和相应的调控基因来诱导花青素积累,这是花青素积累的重要来源信号。作为一种反馈调控,花青素通过调节ROS水平、对产生ROS胁迫的敏感性来增强植物抗性(Xu et al.,2017)。花青素还参与调控ROS诱导的负责细胞生长和分化的信号级联(Hatier and Gould,2008)。通过调节ROS信号,花青素可以成为调节回路的一部分,从而增强ROS信号网络的稳健性(Payne et al.,2013)。花青素的调控因子R2R3-MYB也受控于氧化还原势(Heine et al.,2004),进一步说明细胞内的氧化还原电位与调节花青素积累两者之间有联系(Taylor and Grotewold,2005)。
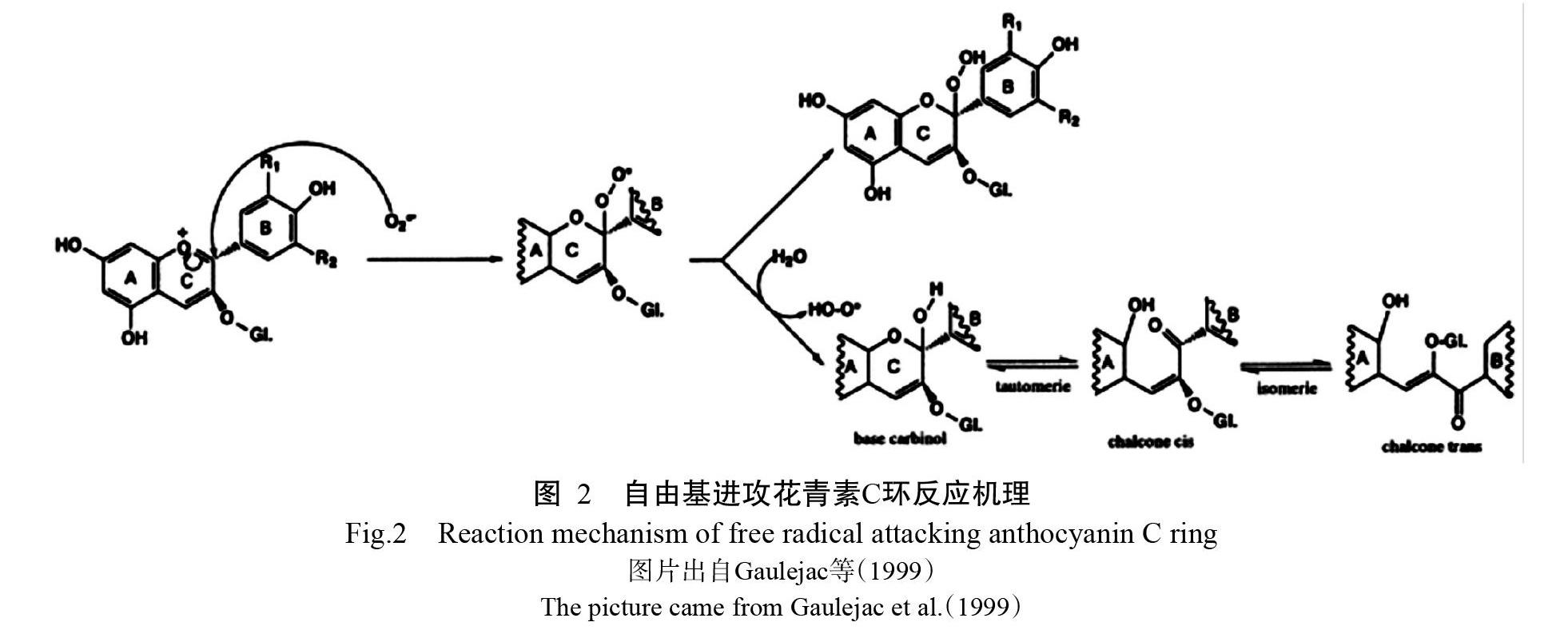
花青素消除自由基的机理是通过分子环上的酚羟基与自由基反应生成较稳定的半醌式自由基,终止自由基链式反应,从而减少并清除自由基(曹志超等,2009)。目前花青素清除自由基的反应机理有两种(Gaulejac et al.,1999):(1)自由基进攻花青素分子C环(图2)。[O][2]之所以与花青素有高反应活性,主要是由于花青素分子的脆性结构(氧鎓离子),使其在C环的开口处更易被氧化。该反应主要是针对带负电荷的自由基。(2)自由基进攻苯环B的酚羟基供体,失去质子后被氧化成酮的结构(图3)。该反应主要是无电荷的自由基发生可能性较大。研究表明花青素的抗氧化性能比维生素E高50倍,比维生素C高20倍(唐忠厚和周丽,2009),也较抗氧化酶强(Le?o et al.,2013)。当植物受重金属胁迫体内的抗氧化酶活性下降或抗坏血酸(AsA)耗尽时,花青素含量仍然上升或通过清除H2O2以维持细胞内的氧化还原平衡(Mubarakshina et al.,2010;da-Silva et al.,2017)。花青素通过消除重金属胁迫下植物体内的过多自由基,使得植物体内的自由基达到平衡或近似平衡,缓解自由基对植物的伤害,从而改善植物的光合作用、呼吸作用、蛋白质合成和碳水化合物合成等,进而促进植物生长,增强抵抗重金属胁迫的能力。缺乏花青素的突变体对ROS敏感,在体内会积累更多的ROS,外源添加花青素增加了突变体内花青素含量、降低突变体内的ROS含量,同时也降低了体内的H2O2和[O][2]含量。这一体内补充实验表明花青素可作为对抗ROS的一种重要保护剂,调节植物体内ROS水平以减轻ROS造成的损伤(Xu et al.,2017)。
3. 2 激发/促进内源抗氧化系统
植物体本身有抗氧化系统,包括抗氧化酶系统[超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)、谷胱甘肽还原酶(GR)等]和非酶系统[谷胱甘肽(GSH)、AsA等],能够有效清除ROS以减轻植物伤害。花青素介导植物抗重金属胁迫作用就在于能够激发或促进植物体内的抗氧化系统。大量研究表明植物的抗逆性取决于其抗氧化成分,而高花青素含量的植物具有高抗氧化活性(Winkel-Shirley,2002;Dixon et al.,2005;Agati et al.,2011;Dehghan et al.,2014;Naing et al.,2017)。Cd和锰(Mn)胁迫下,外源添加花青素的巴西伊乐藻属植物体内的脯氨酸和AsA含量分别是对照的2.0和1.7倍;Cd胁迫下,外源添加花青素的巴西伊乐藻属植物体内的SOD、谷胱甘肽过氧化物酶(GPX)和APX活性分别是对照的1.6、4.0和1.5倍;Mn胁迫下,CAT和SOD活性较对照分别增加50%和14%。这表明外源花青素激发了植物体内的抗氧化酶活性,提高了抗氧化物含量,对该植物的重金属胁迫有保护与缓解效应(Maleva et al.,2018)。此外,当植物受重金属胁迫时,也会激起体内的花青素合成,提高自身抗氧化特性以缓解重金属导致的氧化压(Hale et al.,2001)。内源花青素被激活也会提高植物体内的抗氧化酶活性进而增强植物的抗氧化性。如在硫酸铜(CuSO4)、硫酸锌(ZnSO4)、硫酸锰(MnSO4)和重铬酸钾(K2Cr2O7)胁迫下,矮牵牛花中SOD、过氧化物酶(POX)和CAT基因表达量较对照高,且花青素调控基因RsMYB1表达水平越高,SOD、POX和CAT基因表达量越高。酶基因的表达水平与植物对重金属胁迫的耐受性程度一致,表明植物通过提高抗氧化酶的表达量以抵御重金属引起的ROS形成,在富含内源花青素的植物中表现更明显。同时参与重金属解毒的谷胱甘肽转移酶(GST)和植物络合酶的表达量也较空白高(Ai et al.,2018)。在Cd胁迫下,满江红叶中的花青素含量升高,同时作为植物抗性指标的苯丙氨酸解氨酶(PAL)活性也极大提高(Dai et al.,2006)。同样,在Fe2+胁迫下,柠檬香蜂草体内的花青素含量高于空白,同时体内的SOD和POD活性较空白高(Esmaeilzadeh-Salestani et al.,2014)。在0.5 mmol/L Cu2+胁迫下,红球甘蓝幼苗中的花青素含量较空白高,同时体内的SOD、CAT和POD活性也较空白高(Posmyk et al.,2009)。紫叶甜罗勒比绿叶甜罗勒含有更多的GSH,部分解释了紫叶甜罗勒除了含有明显的花青素外,对硼(B)的耐受性也更高(Landi et al.,2014)。其他植物中花青素的抗氧化性能也被证实(Glińska et al.,2007)。
3. 3 螯合重金属
花青素是一种多羟基、带供电子的化合物,具有多酚结构,较易与金属离子发生螯合,其B环上的邻位羟基(-OH)能直接与金属离子螯合(Kohno et al.,2015)。已證实富含巯基(-SH)的多肽[如植物络合素(PCs)]与重金属络合是植物体内重金属解毒机制的一个重要方面(Raab et al.,2005)。而-OH与 -SH具有相似结构,且比-SH具有更强的亲电荷性,进一步说明重金属能与富含-OH的花青素反应。在20世纪早期已发现花青素具有金属螯合性(Shibata et al.,1919),之后通过X射线晶体学分析证实了螯合物结构(Kondo et al.,1992),即一种自组装的含有一定化学计量花青素的超分子金属复合物(图4)。目前,已报道能够形成超分子复合物的金属有Mg2+、Cd2+、Zn2+、钴(Co2+)、镍(Ni2+)、Mn2+(Kondo et al.,1992;Harborne and Williams,2000;Veitch and Grayer,2011)。花青素能与众多金属离子螯合,如钨(W)和钼(Mo)(Hale et al.,2001,2002)、Mn(Weber and Konieczyński,2003)、Cu(Esparza et al.,2004)、Fe(Esparza et al.,2004;Buchweitz et al.,2012)、Zn(Esparza et al.,2004;Park et al.,2012)、As(Raab et al.,2005)、Pb(Al-Aboudi et al.,2006)、Cd(Dai et al.,2006,2012;Park et al.,2012)、Mg(Mori et al.,2008;Shiono et al.,2008)、Al(Schreiber et al.,2010)、镓(Ga)(Buchweitz et al.,2012)等。不同的重金属甚至是同一种重金属在不同条件下与花青素的螯合特征都不一样,如Mg与花青素的螯合比为4∶6(Shiono et al.,2008)或1∶3(Mori et al.,2008),Cu、Zn与花青素的螯合比均为1∶1(Esparza et al.,2004),在pH 4时花青素与Pb(II)形成络合物(λmax=555 nm,甲醇),而在pH 3时花青素与Cd(II)形成络合物(λmax=706 nm,甲醇)(Ahmed et al.,2013)。
螯合物的形成:(1)因分子量变大或/和发生沉淀,降低了重金属在植物体内的移动性,从而减少重金属在植物体某个组织(如地上部)或亚细胞内(如细胞质)的含量;(2)减少向上迁移速率,也减轻对生殖器官(如花)的胁迫进而稳定产量;(3)减少在果实中的积累,进而保证果实的质量安全。如在同等生长条件下,富含花青素的黑米中总As含量较不含花青素的普通大米中总As含量低(Geng et al.,2017);(4)降低重金属毒性(重金属复合物的毒性较自由态小)(Tu et al.,2004);(5)阻止具有氧化还原活性的金属离子(如Fe2+)发生催化作用以减弱/减少自由基生成;(6)抑制氧化酶如黄嘌呤氧化酶活性(螯合Ca)从而减弱/减少过氧化物的生成;(7)能有效提高太阳光谱紫外区和可见光区的吸光值,提供进一步防线以抵御过量的有害紫外线,尤其是当叶绿体功能已受金属毒害时(Kondo et al.,1992;Yoshida et al.,2009;Schreiber et al.,2010);(8)减少重金属与蛋白质等活性物质结合,从而减少蛋白质等活性物质失活而导致的生长受阻等。上述8方面均能缓解重金属胁迫带来的伤害。如富含花青素的印度芥菜幼苗较不含花青素的印度芥菜幼苗在Mo胁迫下,通过花青素螯合Mo在外围细胞层积累水溶性的蓝色晶体(螯合物),从而更好地促进根与地上部生长(Hale et al.,2001);富含花青素的红球甘蓝抗W胁迫较不含花青素的甘蓝强(Hale et al.,2002);满江红的Cd螯合能力与其花青素含量呈正比(Dai et al.,2012)。
3. 4 区室化隔离
花青素不仅能与重金属形成螯合物,还能将多余的有毒离子重新定位到无害的细胞或组织(如细胞壁或液泡)中,减轻重金属或非金属过量所引起的毒性。这样的区室化隔离不仅降低细胞质中可利用的重金属离子浓度,提高植物对重金属的耐受性,还能阻止重金属向其他组织特别是地上部的迁移。植物中金属解毒的主要途径是通过GST与GSH结合,然后通过膜相关转运蛋白将螯合物与细胞质分离,形成区室化隔离(Jozefczak et al.,2012)。此外,GSH是植物螯合素的胞质前体,当植物暴露于金属或类金属时,这些由GSH衍生的肽促进金属在液泡中积累,也促进金属在地上部和根之间的长距离运输。植物螯合蛋白和GSH缺失突变体对不同的金属都非常敏感,可能是因为二者无法将金属离子转移到液泡中,或无法将金属离子有效地从根部传递到地上部组织(Cobbett,2000;Vernoux et al.,2000)。
进入液泡的方式主要有:(1)通过连接蛋白转运体Ligandin transporter(LT),在GST的协助下被靶向定位到液泡附近。这是一种非专一性的转运模式,已在GST转移酶基因突变体中被证实,如玉米的BZ2(Marrs et al.,1995)、矮牵牛花的AN9(Mueller et al.,2000)、拟南芥的TT19(Kitamura et al.,2004)等,这些植物的突变体则阻止花青素在液泡中的定位。GST转移酶作为连接蛋白,从内质网到液泡膜运载花青素(Marrs et al.,1995),液泡膜上的多药耐药辅助蛋白(Multidrug resistance associated protein,MRP)类转运蛋白(如玉米的ZmMrp3)能够识别GST-矢车菊素3-O-葡萄糖苷(C3G)复合物,而后将跨C3G膜转运至液泡(Goodman et al.,2004)。(2)由液泡膜上的多药及毒性化合物外排(Multidrug and toxic compound extrusion transporter,MATE)类转运蛋白将C3G跨膜转运到液泡中(Debeaujon et al.,2001;Marinova et al.,2007)。(3)由囊泡转运Vesicular transport(VT),通过膜融合的方式进入液泡(Zhao and Dixon,2010)。该方式也是一种非专一性的转运模式,该发现是以许多植物中的花青素在细胞质中的积累以离散结构形式存在为基础(Zhang et al.,2006;Hsieh and Huang,2007;Poustka et al.,2007)。花青素介导金属/类金属区隔化的原理见图5。经外源花青素浸泡后的洋葱分生组织,有Pb沉积的细胞核减少至30%,无Pb沉积的细胞核占绝大多数,花青素提取物极大降低了Pb在洋葱根分生组织细胞核内的沉积数量,主要是由于花青素对Pb的吸收及亚细胞定位(Glińska et al.,2007)。花青素介导的Mo主要隔离在芸苔属植物表皮(Hale et al.,2001)。
3. 5 花青素相关基因表达
花青素合成和转运受结构基因和调控基因共同控制。结构基因[包括PAL、黄烷酮羟化酶(F3H)、查尔酮合酶(CHS)、花青素合成酶(ANS)、查尔酮异构酶(CHI)和二氢黄酮醇还原酶(DFR)等]编码相关的生物合成酶,组成花青素生物合成途径,其表达直接受控于MYB、bHLH和WDR 3类转录因子形成的MBW复合体,具体的MYB、bHLH和WDR决定MBW复合体调控的对象和强度(Zhu et al.,2015)。结构基因和调控基因的表达均会影响花青素含量,进而影响植物的生长与抗逆性。花青素调控基因的过量表达增加花青素含量与抗氧化特性,进而增强植物体的抗性(Lim et al.,2016)。
在50 μmol/L Cd处理下,全红杨树的F3H、DFR、CHI和UFGT上调,花青素含量较空白高,表现出促进全红杨树生长,而在100和150 μmol/L Cd处理下,全红杨树的F3H、DFR、CHI和UFGT下调,花青素含量较空白低,表现出抑制全红杨树生长(Zhang et al.,2014)。Cd毒诱导了花青素在满江红中的积累,这是由于CHS和DFR上调所致(Dai et al.,2012)。矮牵牛花的花青素转录因子RsMYB1过量表达增加花青素含量,在CuSO4、ZnSO4、MnSO4或K2Cr2O7胁迫下,其生長不受影响,抗性增强;而普通矮牵牛花的生长受抑制(Ai et al.,2018)。紫色芥菜较绿色芥菜具有更高的耐钒(V)性是因为紫色芥菜中花青素合成酶基因TT8、F3H和MYBL2在V胁迫下(20、40、80和100 mg/L)高表达,而绿色芥菜未表现出该特性(Imtiaz et al.,2018)。IbMYB1过量表达的转基因土豆(Cheng et al.,2013)和snapdragon Delila(Del)过量表达的转基因烟草(Naing et al.,2017)表现出花青素生成量增加、无机胁迫抗性提高的特性。
4 展望
重金属污染严重不仅影响植物的生长发育、产品的品质与质量,还通过食物链最终威胁人体健康,因此,如何调控植物重金属胁迫对提高植物产品的产量与质量有着重要意义。花青素来源广泛,据初步统计在27个科73个属植物中均含有花青素,如紫甘薯、葡萄、血橙、红球甘蓝、蓝莓、茄子、樱桃、红莓、草莓、桑葚、山楂、牵牛花等植物的组织中均有一定含量,是一种广泛存在于植物体内的天然抗氧化物;且花青素对植物和动物均具有较强的生物活性,其应用前景广阔、潜力巨大。而目前研究主要集中在其活性和用于医疗、食品、化妆品等领域,农林业和环境领域的研究主要是考察花青素在植物重金属胁迫下的响应,在花青素对重金属胁迫响应机理及其对应的实际应用研究上还比较薄弱,为此,未来的研究方向建议如下:
4. 1 开展花青素介导植物重金属归趋相关研究
植物食用部位重金属的积累关系到其产品的质量与安全,进而关系到消费者摄入重金属含量的多少。如何减少植物可食部位重金属的含量对保障产品质量与人体健康具有积极作用。花青素能否调控重金属在土壤—植物体系统的吸收、迁移和转化,怎样调控,其影响因素有哪些,哪些因素能使花青素介导的重金属富集在非食用部位,弄清楚这些问题,不仅有利于丰富和发展植物富集重金属的理论,还能更加明确产品质量与花青素的调控效果,对提高产品质量有着重要作用。
4. 2 深入研究花青素抗植物重金属胁迫的调控机理
植物、重金属和花青素的种类均多种多样,加上重金属和花青素的浓度及浓度比、植物生长环境条件等不同,导致全面、准确地获得花青素对植物重金属胁迫的调控作用机理是一件比较困难、任务量较大的事,这就需要采用快速、高通量、准确的方法。随着分子生物学及其他检测技术的日趋成熟,可从组织器官、细胞及亚细胞、离子组学、代谢组学、蛋白组学和基因组学等多层次、多角度获得花青素调控重金属的普遍规律与特性,对植物品种的选育与改良等具有积极作用。
4. 3 开展外源花青素介导植物抗重金属胁迫的研究
自然界富含花青素的植物较多,其成分的复杂性和多样性、与土壤微生物的相互作用等因素较单纯的花青素而言,对介导重金属胁迫有一定影响。若从富含花青素的废弃物中提取、纯化后再进行花青素喷施必然会增加成本,且仍会有废弃物。基于富含花青素的有色果蔬、制糖原料甜菜等废弃物本身可作为有机肥的特性,开展直接施加富含花青素的废弃物或通过简单处理(如堆肥等方式)进行外源花青素介导植物重金属吸收富集研究,不仅能大规模地综合利用这些有机废弃物,减少废弃物的任意排放,还能变废为宝,对改善环境污染、提高土壤肥力、提高产品质量等起着重要作用。
参考文献:
曹志超,顾翔,苏佩清. 2009. 黄酮类化合物抗氧化及其作用机制的研究进展[J]. 实用临床医药杂志,13(13):110-112. [Cao Z C,Gu X,Su P Q. 2009. Advances in research on anti-oxidation and mechanism of action of flavonoids[J]. Journal of Clinical Medicine in Practice,13(13):110-112.]
唐忠厚,周丽. 2009. 花青素对人类健康影响的研究进展及其前景[J]. 食品研究与开发,30(7):159-162. [Tang Z H,Zhou L. 2009. Study on anthocyanins influencing on human health and its prospect[J]. Food Research and Development,30(7):159-162.]
汪文忠. 2017. 花青素在饲料上的应用探析[J]. 广东饲料,26(4):30-31. [Wang W Z. 2017. Analysis of the application of anthocyanins in feed[J]. Guangdong Feed,26(4):30-31.]
Abbas G,Murtaza B,Bibi I,Shahid M,Niazi N K,Khan M I,Amjad M,Hussain M,Natasha. 2018. Arsenic uptake,toxicity,detoxification,and speciation in plants:Physiological, biochemical,and molecular aspects[J]. International Journal of Environmental Research and Public Health,15(59). doi:10.3390/ijerph15010059.
Adrees M,Ali S,Rizwan M,Ibrahim M,Abbas F,Farid M,Zia-ur-Rehman M,Irshad M K,Bharwana S A. 2015. The effect of excess copper on growth and physiology of important food crops:A review[J]. Environmental Scien-ce and Pollution Research,22:8148-8162.
Agati G,Biricolti S,Guidi L,Ferrini F,Fini A,Tattini M. 2011. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves[J]. Journal of Plant Physiology,168(3):204-212.
Ahmed J K,Salih H A M,Hadi A G. 2013. Anthocyanins in red beet juice act as scavengers for heavy metals ions such as lead and cadmium[J]. Journal of Applicable and Chemistry,2(4):797-804.
Ai T N,Naing A H,Yun B W,Lim S H,Kim C K. 2018. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in Transgenic Petunia[J]. Frontiers in Plant Science,9:1388.
Al-Aboudi A,Fayyak M K,Abu Zarga M H. 2006. Polarographic study of the complexation between the chemica l constituents of Phragmites australis and heavy metals[J]. Fresenius Environmental Bulletin,15(10):1271-1275.
Begum M C,Islam M S,Islam M,Amin R,Parvez M S,Kabir A H. 2016. Biochemical and molecular responses underlying differential arsenic tolerance in rice(Oryza sativa L.)[J]. Plant Physiology and Biochemistry,104:266-277.
Bona E,Marsano F,Cavaletto M,Berta G. 2007. Proteomic characterization of copper stress response in Cannabis sativa roots[J].Proteomics,7(7):1121-1130.
Buchweitz M,Gudi G,Carle R,Kammerer D R,Schulz H. 2012. Systematic investigation of anthocyanin-metal interactions by Raman spectroscopy[J]. Journal of Raman Spectroscopy,43(12):2001-2007.
Chanu L B,Gupta A. 2016. Toxicity of zinc on growth of an aquatic macrophyte,Ipomoea aquatica Forsk[J]. Current World Environment,1:218-227.
Chen Y F,Shibu M A,Fan M J,Chen M C,Viswanadha V P,Lin Y L,Lai C H,Lin K H,Ho T J,Kuo W W,Huang C Y. 2016. Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis[J]. The Journal of Nutritional Biochemistry,31:98-105.
Cheng Y J,Kim M D,Deng X P,Kwak S S,Chen W. 2013. Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1,a sweet potato transcription factor[J]. Journal of Microbiology and Biotechnology,23(12):1737-1746.
Cobbett C S. 2000. Phytochelatin biosynthesis and function in heavy-metal detoxification[J]. Current Opinion in Plant Biology,3(3):211-216.
Cozzolino V,Pigna M,Di Meo V,Caporale A G,Violante A. 2010. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth of Lactuca sativa L. and arsenic and phosphorus availability in an arsenic polluted soil under nonsterile conditions[J]. Applied Soil Ecology,45:262-268.
Dai L P,Dong X J,Ma H H. 2012. Antioxidative and chela-ting properties of anthocyanins in Azolla imbricata induced by cadmium[J]. Polish Journal of Environmental Studies,21:837-844.
Dai L P,Xiong Z T,Huang Y,Li M J. 2006. Cadmium induced changes in pigments,total phenolics,and phenyla-lanine ammonia-lyase activity in fronds of Azolla imbricate[J]. Environmental Toxicology,21(5):505-512.
da-Silva C J,Canatto R A,Cardoso A A,Ribeiro C,Oliveira J A. 2017. Arsenic-hyperaccumulation and antioxidant system in the aquatic macrophyte Spirodela intermedia W. Koch(Lemnaceae)[J]. Theoretical and Experimental Plant Physiology,29:203-213.
Debeaujon I,Peeters A J M,Léon-Kloosterziel K M,Koornneef M. 2001. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuo-les of the seed coat endothelium[J]. The Plant Cell,13(4):853-871.
Dehghan S,Sadeghi M,P?ppel A,Fischer R,Lakes-Harlan R,Kavousi H R,Vilcinskas A,Rahnamaeian M. 2014. Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding,salicylic acid treatment,and salinity stress in safflower,Carthamus tinctorius[J]. Bioscience reports,34(3):273-282.
Dixon R A,Xie D Y,Sharma S B. 2005. Proanthocyanidins: A final frontier in flavonoid research?[J]. New Phytologist,165(1):9-28.
Du C Y,Shi Y H,Ren Y Z,Wu H J,Yao F,Wei J Y,Wu M,Hou Y J,Duan H J. 2015. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells[J]. Drug Design,Development and Therapy,9:5099-5113.
Esmaeilzadeh-Salestani K,Riahi-Madvar A,Maziyar M A. 2014. Antioxidant enzymes activity and anthocyanin content in Fe2+-treated lemon balm seedlings[J]. International Journal of Farming and Allied Sciences,3(5):562-565.
Esparza I,Salinas I,Caballero I,Santamaría C,Calvo I,García-Mina J M,Fernández J M. 2004. Evolution of metal and polyphenol content over a 1-year period of vini-fication:Sample fractionation and correlation between metals and anthocyanins[J]. Analytica Chimica Acta,524(1-2):215-224.
Fagioni M,D'Amici G M,Timperio A M,Zolla L. 2009. Proteomic analysis of multiprotein complexes in the thylakoid membrane upon cadmium treatment[J]. Journal of Proteome Research,8(1):310-326.
Gaulejac S C D,Glories Y,Vivas N. 1999. Free radical sca-venging effect of anthocyanins in red wines[J]. Food Research International,32(5):327-333.
Geng A J,Wang X,Wu L S,Wang F H,Chen Y,Yang H,Zhang Z,Zhao X L. 2017. Arsenic accumulation and speciation in rice grown in arsanilic acid-elevated paddy soil[J]. Ecotoxicology and Environmental Safety,137:172-178.
Glińska S,Bartczak M,Oleksiak S,Wolska A,Gabara B,Posmyk M M,Janas K M. 2007. Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals[J]. Ecotoxicology and Environmental Safety,68(3):343-350.
Goodman C D,Casati P,Walbot V. 2004. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays[J]. The Plant Cell,16(7):1812-1826.
Grat?o P L,Monteiro C C,Antunes A M,Peres L E P,Azevedo R A. 2008. Acquired tolerance of tomato(Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress[J]. Annals of Applied Biology,153(3):321-333.
Grotewold E. 2006. The genetics and biochemistry of floral pigments[J]. Annual Review of Plant Biology,57:761-780.
Gupta D K,Huang H G,Nicoloso F T,Schetinger M R,Fa-rias J G,Li T Q,Razafindrabe B H N,Aryal N,Inouhe M. 2013. Effect of Hg,As and Pb on biomass production,photosynthetic rate,nutrients uptake and phytochelatin induction in Pfaffia glomerata[J]. Ecotoxicology,22(9):1403-1412.
Hale K L,McGrath S P,Lombi E,Stack S M,Terry N,Picke-ring I J,George G N,Pilon-Smits E A. 2001. Molybdenum sequestration in Brassica species. A role for anthocyanins?[J]. Plant Physiology,126(4):1391-1402.
Hale K L,Tufan H A,Pickering I J,George G N,Terry N,Pilon M,Pilon-Smits E A H. 2002. Anthocyanins facilitate tungsten accumulation in Brassica[J]. Physiologia Plantarum,116(3):351-358.
Harborne J B,Williams C A. 2000. Advances in flavonoid researches since 1992[J]. Phytochemistry,55(6):481-504.
Hatier J H B,Gould K S. 2008. Foliar anthocyanins as modulators of stress signals[J]. Journal of Theoretical Biology,253(3):625-627.
Heine G F,Hernandez J M,Grotewold E. 2004. Two cystei-nes in plant R2R3 MYB domains participate in REDOX-dependent DNA binding[J]. The Journal of Biological Chemistry,279(36):37878-37885.
Hopff D,Wienkoop S,Lüthje S. 2013. The plasma membrane proteome of maize roots grown under low and high iron conditions[J]. Journal of Proteomics,91:605-618.
Hsieh K,Huang A H. 2007. Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface[J]. The Plant Cell,19(2):582-596.
Imtiaz M,Mushtaq M A,Nawaz M A,Ashraf M,Rizwan M S,Mehmood S,Aziz O,Rizwan M,Virk M S,Shakeel Q,Ijaz R,Androutsopoulos V P,Tsatsakis A M,Coleman M D. 2018. Physiological and anthocyanin biosynthesis genes response induced by vanadium stress in mustard genotypes with distinct photosynthetic activity[J]. Environmental Toxicology and Pharmacology,62:20-29.
Jozefczak M,Remans T,Vangronsveld J,Cuypers A. 2012. Glutathione is a key player in metal-induced oxidative stress defenses[J]. International Journal of Molecular Scien-ces,13(3):3145-3175.
Kitamura S,Shikazono N,Tanaka A. 2004. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis[J]. Plant Journal,37(1):104-114.
Kohno Y,Kato Y,Shibata M,Fukuhara C,Maeda Y,Tomita Y,Kobayashi K. 2015. Enhanced stability of natural anthocyanin incorporated in Fe-containing mesoporous silica[J]. Microporous and Mesoporous Materials,203:232-237.
Kondo T,Yoshida K,Nakagawa A,Kawai T,Tamura H,Goto T. 1992. Structural basis of blue-colour development in flower petals from Commelina communis[J]. Nature,358:515-518.
Kumar A,Prasad M N V. 2018. Plant-lead interactions:Transport,toxicity,tolerance,and detoxification mechanisms[J]. Ecotoxicology and Environmental Safety,166:401-418.
Landi M,Guidi L,Pardossi A,Tattini M,Gould K. 2014. Photoprotection by foliar anthocyanins mitigates effects of boron toxicity in sweet basil (Ocimum basilicum)[J]. Planta,240(5):941-953.
Landi M,Tattini M,Gould K S. 2015. Multiple functional roles of anthocyanins in plant-environment interactions[J]. Environmental and Experimental Botany,119:4-17.
Le?o G A,Oliveira J A D,Felipe R T A,Farnese F S,Gusman G S. 2013. Anthocyanins,thiols,and antioxidant scavenging enzymes are involved in Lemna gibba tole-rance to arsenic[J]. Journal of Plant Interactions,9(1):143-151.
Lepiniec L,Debeaujon I,Routaboul J M,Baudry A,Pourcel L,Nesi N,Caboche M. 2006. Genetics and biochemistry of seed flavonoids[J]. Annual Review of Plant Biology,57:405-430.
Lev-Yadun S,Gould K S. 2008. Role of anthocyanins in plant defense[M]. New York:Springer:22-28.
Li J J,Ma J J,Guo H L,Zong J Q,Chen J B,Wang Y,Li D D,Li L,Wang J J,Liu J X. 2018. Growth and physiological responses of two phenotypically distinct accessions of centipedegrass(Eremochloa ophiuroides(Munro) Hack.) to salt stress[J]. Plant Physiology and Biochemistry,126:1-10.
Lim S H,Song J H,Kim D H,Kim J K,Lee J Y,Kim Y M,Ha S H. 2016. Activation of anthocyanin biosynthesis by expression of the radish R2R3-MYB transcription factor gene RsMYB1[J]. Plant Cell Reports,35:641-653.
Lin B W,Gong C C,Song H F,Cui Y Y. 2017. Effects of anthocyanins on the prevention and treatment of cancer[J]. British Journal of Pharmacology,174(11):1226-1243.
Liu X L,Wu H F,Ji C L,Wei L,Zhao J M,Yu J B. 2013a. An integrated proteomic and metabolomic study on the chronic effects of mercury in Suaeda salsa under an environmentally relevant salinity[J]. PLoS One,8(5):e64041.
Liu Y X,Li M,Han C,Wu F X,Tu B K,Yang P F. 2013b. Comparative proteomic analysis of rice shoots exposed to high arsenate[J]. Journal of Integrative Plant Biology,55(10):965-978.
Maleva M,Garmash E,Chukina N,Malec P,Waloszek A,Strza?ka K. 2018. Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea(Egeria densa (Planch.) Casp.) exposed to cadmium and manganese[J]. Ecotoxicology and Environmental Safety,160:197-206.
Marinova K,Pourcel L,Weder B,Schwarz M,Barron D,Routaboul J M,Debeaujon I,Klein M. 2007. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat[J]. The Plant Cell,19(6):2023-2038.
Marrs K A,Alfenito M R,Lloyd A M,Walbot V. 1995. A glutathione S-transferase involved in vacuolar transfer enco-ded by the maize gene bronze-2[J]. Nature,375(6530):397-400.
Miller R,Owens S J,Rorslett B. 2011. Plants and colour:Flowers and pollination[J]. Optics and Laser Technology,43(2):282-294.
Mori M,Kondo T,Yoshida K. 2008. Cyanosalvianin,a supramolecular blue metalloanthocyanin,from petals of Salvia uliginosa[J]. Phytochemistry,69(18):3151-3158.
Mubarakshina M M,Ivanov B N,Naydov I A,Hillier W,Badger M R,Krieger-Liszkay A. 2010. Production and diffusion of chloroplastic H2O2 and its implication to signalling[J]. Journal of Experimental Botany,61(13):3577-3587.
Mueller L A,Goodman C D,Silady R A,Walbot V. 2000. AN9,a petunia glutathione S-transferase required for anthocyanin sequestration,is a flavonoid-binding protein[J]. Plant Physiology,123(4):1561-1570.
Munagala R,Aqil F,Jeyabalan J,Agrawal A K,Mudd A M,Kyakulaga A H,Singh I P,Vadhanam M V,Gupta R C. 2017. Exosomal formulation of anthocyanidins against multiple cancer types[J]. Cancer Letters,393:94-102.
Naing A H,Park K I,Ai T N,Chung M Y,Han J S,Kang Y W,Lim K B,Kim C K. 2017. Overexpression of snapdragon Delila(Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance[J]. BMC Plant Biology,17(1):65.
Nizio?-?ukaszewska Z,Osika P,Wasilewski T,Bujak T. 2017. Hydrophilic dogwood extracts as materials for reducing the skin irritation potential of body wash cosmetics[J]. Molecules,22(2):320.
Parizad P A,Capraro J,Scarafoni A,Bonomi F,Blandino M,Marengo M,Giordano D,Carpen A,Iametti S. 2019. The bio-functional properties of pigmented cereals may involve synergies among different bioactive species[J]. Plant Foods for Human Nutrition,74(1):128-134.
Park W,Han K H,Ahn S J. 2012. Differences in root-to-shoot Cd and Zn translocation and by HMA3 and 4 could influence chlorophyll and anthocyanin content in Arabidopsis Ws and Col-0 ecotypes under excess metals[J]. Soil Science and Plant Nutrition,58(3):1-15.
Payne J L,Moore J H,Wagner A. 2013. Robustness,evol-vability,and the logic of genetic regulation[J]. Artificial Life,20(1):111-126.
Poschenrieder C,Gunsé B,Corrales I,Barceló J. 2008. A glance into aluminum toxicity and resistance in plants[J]. Science of the Total Environment,400(1-3):356-368.
Posmyk M M,Kontek R,Janas K M. 2009. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress[J]. Ecotoxicology and Environmental Safety,72(2):596-602.
Poustka F,Irani N G,Feller A,Lu Y,Pourcel L,Frame K,Grotewold E. 2007. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions[J]. Plant Physiology,145(4):1323-1335.
Punvittayagul C,Sringarm K,Chaiyasut C,Wongpoomchai R. 2014. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice[J]. Asian Pacific Journal of Cancer Prevention,15(21):9517-9522.
Raab A,Schat H,Meharg A A,Feldmann J. 2005. Uptake,translocation and transformation of arsenate and arsenite in sunflower(Helianthus annuus):Formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations[J]. New Phytologist,168(3):551-558.
Schreiber H D,Swink A M,Godsey T D. 2010. The chemical mechanism for Al3+ complexing with delphinidin:A mo-del for the bluing of hydrangea sepals[J]. Journal of Inorganic Biochemistry,104(7):732-739.
Shibata K,Shibata Y,Kasiwagi I. 1919. Studies on anthocyanins: Colour variation in anthocyanins[J]. Journal of the American Chemical Society,41(2):208-220.
Shiono M,Matsugaki N,Takeda K. 2008. Structure of commelinin,a blue complex pigment from the blue flowers of Commelina communis[J]. Proceedings of the Japan Aca-demy:Series B,84(10):452-456.
Singh A P,Dixit G,Mishra S,Dwivedi S,Tiwari M,Mallick S,Pandey V,Trivedi P K,Chakrabarty D,Tripathi R D. 2015. Salicylic acid modulates arsenic toxicity by redu-cing its root to shoot translocation in rice(Oryza sativa L.)[J]. Frontiers in Plant Science,6:340.
Steyn W J,Wand S J E,Holcroft D M,Jacobs G. 2002. Anthocyanins in vegetative tissues:A proposed unified function in photoprotection[J]. New Phytologist,155(3):349-361.
Sui X N,Zhang Y,Zhou W B. 2016. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources:Its quality attributes and in vitro digestibility[J]. Food Chemistry,196:910-916.
Taylor L P,Grotewold E. 2005. Flavonoids as developmental regulators[J]. Current Opinion in Plant Biology,8(3):317-323.
Thummayot S,Tocharus C,Pinkaew D,Viwatpinyo K,Srin-garm K,Tocharus J. 2014. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells[J]. Neurotoxicology,45:149-158.
Tripathi R D,Srivastava S,Mishra S,Singh N,Tuli R,Gupta D K,Maathuis F J. 2007. Arsenic hazards:Strategies for tolerance and remediation by plants[J]. Trends in Biotechnology,25(4):158-165.
Tu S,Ma L Q,MacDonald G E,Bondada B. 2004. Effects of arsenic species and phosphorus on arsenic absorption,arsenate reduction and thiol formation in excised parts of Pteris vittata L[J]. Environmental and Experimental Bo-tany,51(2):121-131.
Uraguchi S,Sone Y,Ohta Y,Ohkama-Ohtsu N,Hofmann C,Hess N,Nakamura R,Takanezawa Y,Clemens S,Kiyor M. 2018. Identification of C-terminal regions in Arabidopsis thaliana phytochelatin synthase 1 specifically involved in activation by arsenite[J]. Plant and Cell Physio-logy,59(3):500-509.
Valle S R,Carrasco J,Pinochet D,Calderini D F. 2009. Grain yield,above-ground and root biomass of Al-tolerant and Al-sensitive wheat cultivars under different soil aluminum concentrations at field conditions[J]. Plant and Soil,318:299-310.
Veitch N C,Grayer R J. 2011. Flavonoids and their glycosides,including anthocyanins[J]. Natural Product Reports,28(10):1626-1695.
Vernoux T,Wilson R C,Seeley K A,Reichheld J P,Muroy S,Brown S,Maughan S C,Cobbett C S,Van Montagu M,Inzé D,May M J,Sung Z R. 2000. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development[J]. The Plant Cell,12:97-109.
Weber G,Konieczyński P. 2003. Speciation of Mg,Mn and Zn in extracts of medicinal plants[J]. Analytical and Bioanalytical Chemistry,375(8):1067-1073.
Winkel-Shirley B. 2002. Biosynthesis of flavonoids and effects of stress[J]. Current Opinion in Plant Biology,5(3):218-223.
Xu Z H,Mahmood K,Rothstein S J. 2017. ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis[J]. Plant and Cell Phy-siology,58(8):1364-1377.
Yoshida K,Miro M,Kondo T. 2009. Blue flower color deve-lopment by anthocyanins:From chemical structure to cell physiology[J]. Natural Product Reports,26(7):884-915.
Zeng F R,Wu X J,Qiu B Y,Wu F B,Jiang L X,Zhang G P. 2014. Physiological and proteomic alterations in rice(Oryza sativa L.) seedlings under hexavalent chromium stress[J]. Planta,240(2):291-308.
Zhang F,Wan X Q,Zheng Y X,Sun L X,Chen Q B,Guo Y L,Zhu X Q,Liu M. 2014. Physiological and related anthocyanin biosynthesis genes responses induced by cadmium stress in a new colored-leaf plant “Quanhong Poplar”[J]. Agroforestry Systems,88(2):343-355.
Zhang H B,Wang L,Deroles S,Bennett R,Davies K. 2006. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals[J]. BMC Plant Biology,6:29.
Zhao J,Dixon R A. 2010. The ‘ins and ‘outs of flavonoid transport[J]. Trends in Plant Science,15(2):72-80.
Zhu Z X,Wang H L,Wang Y T,Guan S,Wang F,Tang J Y,Zhang R J,Xie L L,Lu Y Q. 2015. Characterization of the cis elements in the proximal promoter regions of the anthocyanin pathway genes reveals a common regulatory logic that governs pathway regulation[J]. Journal of Experimental Botany,66(13):3775-3789.
(責任编辑 罗 丽)
