Mo¨ssbauer spectroscopy studies on the particle size distribution effect of Fe–B–P amorphous alloy on the microwave absorption properties
2020-03-19YuHuaLvYanHuiZhangJianZhangBinLi
Yu-Hua Lv· Yan-Hui Zhang,2· Jian Zhang · Bin Li
Abstract An Fe-based nanocrystalline alloy powder is important for application in microwave absorption,and the particle size has a critical impact on the electromagnetic microwave parameters. Therefore, it is necessary to study further the effects of the particle size on such parameters and improve the microwave absorption performance of Febased nanocrystalline powers. In this study, Fe–B–P particles were prepared through a synthetic approach consisting of an aqueous chemical reduction and a ball milling treatment.We investigated the effects of ball milling on the microstructure and electromagnetic properties of Fe–B–P particles. The experimental results indicate that the Fe–B–P particles synthesized through an aqueous chemical reduction are amorphous spheres.Fe–B–P particles with an original particle size of 200–1200 nm can be milled into an irregular shape with the size reduced to <500 nm after 0.5 h of ball milling, and subsequently, the particles become smaller with increases in the milling time, with traces of Fe2O3 generated on the particle surface. The results of the Mo¨ssbauer spectra show that a portion of the small particles demonstrate a superparamagnetic property.The volume proportions of the superparamagnetic component increase from 13.1 to 15.8% as the treatment time increases. We measured the permittivity and permeability spectra of Fe–B–P particles within the frequency range of 2–18 GHz. The reflection loss (RL) is - 10 dB for an absorber thickness of 1.7–5.0 mm. The RL is - 20 dB for an absorber thickness of 1.9–2.7 mm. The microwave absorption properties of samples with the same thickness are improved with an increase in the treatment time and are shifted to a higher frequency, which will broaden the bandwidth of the absorption as well.
Keywords Fe–B–P particles · Mo¨ssbauer spectroscopy ·Microwave absorber properties
1 Introduction
Magnetic nanoparticles have attracted the attention of many researchers owing to their unique properties and wide application prospects. At present, they have been widely studied in the fields of catalysts and biomedicine.Their application in the area of absorbing waves has also attracted extensive attention.Human-made electromagnetic interference (EMI) and electromagnetic radiation have led to increasing electromagnetic(EM)pollution.Owing to the development of radio, television, and microwave technologies, the power of the radio frequency equipment has been multiplied and the EMI used on the ground has greatly increased,reaching a level of direct threat to human health [1–5]. High-performance microwave absorption materials have been considered as an efficient way to solve this problem. Amorphous materials have a short-range order and long-range disorder in their structure, leading to a high resistance state and multi-suspension bond [6, 7].The high resistance state of an amorphous alloy allows the material to more easily achieve a good impedance matching. Heterogeneous structures lead more easily to an interface polarization and multiple scattering, thereby improving the absorbing performance of the materials.The particle size affects the above two characteristics,enhances the dielectric loss, and contributes to a better absorption[8, 9].
Recently, Li et al. [10] have fabricated a composite structure of Fe-based amorphous powder and S-glass fiber within epoxy panels with a gradient layer structure and investigated the absorption properties. In addition, Yang et al. [11] investigated the EM wave absorption properties of FeCoNiCrAl0.8amorphous alloy powders. Furthermore,the microwave absorption properties of Fe–B amorphous particles have also been proved to be effective in microwave absorption,among their other merits.Zhao et al.[12]investigated the EM wave absorption properties of annealed Fe–B amorphous submicrometer particles. Yuki et al. [13, 14] prepared resin composites with amorphous Fe–B particles and concluded that the composites achieve good microwave absorption with a matching thickness of 2.0–3.9 mm. Shimba et al. [15] fabricated polymer composites containing amorphous Fe–B particles and Ni–Zn ferrite nanoparticles and inferred that the composites can be used within the UHF range.
Ultrafine amorphous Fe–P–B particles achieved through an aqueous chemical reduction have the advantage of superior soft magnetic properties.The sodium borohydride reduction method with its low cost and easy access is more acceptable compared with melt quenching[16],magnetron sputtering[17],and vapor deposition[18],which require a large cooling rate (of at least 106/s). Hu et al. [19–21]prepared ultrafine amorphous Fe–P–B particles through a reaction of FeCl3or FeSO4,and NaH2PO2and KBH4,in an aqueous solution and studied the morphology and hyperfine structure. Chen et al. [22] synthesized ultrafine amorphous Fe–P–B particles through a reaction of FeCl3or Fe(OAc)2,and NaH2PO2and NaBH4, using a chemical reduction method and studied the influence of the synthesis parameters on the catalytic properties.In our previous study,Fe–B–P particles were prepared through a reaction among FeCl2, NaH2PO2, and NaBH4under an alkali condition using an aqueous chemical reduction approach [23–26].We studied the soft magnetic properties and frequency dependence of the permeability for composites of Fe–B–P particles and (Ni0.6Zn0.4) Fe2O4nanoparticles with different molar ratios.
The sizes of the particles and the heterostructure have an effect on the microwave absorption[27].Ball milling is an efficient way to control the particle size and form a heterostructure. In this article, Fe–B–P nanoparticles were obtained by ball milling the synthesized Fe–B–P particles,and we analyzed further the relationship between the electromagnetic properties and the ball milling time.
2 Experimental methods
Fe–B–P particles have been compounded using a chemical reduction method in an aqueous solution.The reactants of 10 g of FeCl2·4H2O,12 g of C6H5Na3O7·2H2O,and 8 g of NaH2PO2·H2O were dissolved in 50 ml of deionized water. To generate the Fe–B–P particles at room temperature,NaBH4was used as a reductant to drop into the solution at a rate of 200 ml per hour after adjusting the pH value of the solution to 9 using 2 mol/l NaOH.The cleaned powder was stored in alcohol after a magnetic separation. The synthesized Fe–B–P particles were dried and milled with a planetary ball miller (QM-3SP2J) for 0.5 and 6 h to obtain finer Fe–B–P particles,respectively.High-performance stainless steel vials and balls were utilized. The ball-to-material weight ratio was 15:1,and the ball milling speed was set to 150 rpm. The ball miller was set to stop automatically for 30 min after half an hour to avoid overheating during the ball milling process.Finally,the as-made powers were collected for further testing.
A phase analysis was conducted at a 30 kV X-ray diffraction voltage and a current of 30 mA,with a scanning step of 0.2.The morphology of the particles was measured using a field emission scanning electron microscope. In addition,the particle size distribution was calculated using Nano Measure 1.2 software. The compositions were analyzed using the energy-dispersive X-ray spectrum (EDX,JEOL-7001F), a Fourier transform infrared spectrometer(FTIR Spectrometer, Nicolet IS5), and the X-ray photoelectron spectrum (XPS, ESCALAB250). The microstructure of the particles was investigated using a transmission electron microscope (TEM, JEOL JEM-2100F) and highresolution TEM (HR-TEM). Transmission Mo¨ssbauer spectra for samples before and after ball milling were determined at room temperature. In addition,57Co/Rh was chosen as the γ-emission source, and a standard α-Fe foil was used to calibrate the velocity of the t spectrometer.Mo¨sswinn 4.0 software was used to fit our measurement based on the least-squares method.
The results of a static magnetic parameter were obtained using a Lakeshore vibrating sample magnetometer (VSM),the measurement range of which is -5–5 kOe. The electromagnetic properties of the samples were obtained using a Keysight N5222A vector network analyzer (VNA) at a frequency of 2–18 GHz.The S parameter was also applied.The powder was mechanically mixed with paraffin at 50 wt.% and cast into toroidal-shaped samples of 7 mm in outer diameter, 3 mm in inner diameter, and 3 mm in thickness.
3 Results and discussion
The morphologies and particle diameter distribution range of the Fe–B–P samples were studied using an SEM to research the relation between the microstructure and milling time of the samples. The results are shown in Fig. 1. Figure 1a shows the microstructure of the original Fe–B–P samples in a spherical shape with a smooth surface and a diameter of 200–1200 nm.The mean diameter of the particles is 676.8 nm. After milling for 0.5 h, the shape of the Fe–B–P samples becomes irregular when the diameter is reduced by less than 500 nm,as shown in Fig. 1b.As the ball milling time increases, the particle size is gradually reduced. This is mainly because a milling time of 6 h reduces the particle inhomogeneity,as shown in Fig. 1c.In our experiment, it was clearly observed that the diameters of the Fe–B–P particles are reduced from the initial 676.8 nm to 191.9 nm after milling for 0.5 h.The Fe–B–P particle size is reduced from 191.9 to only 155.4 nm,whereas the treatment time extends from 0.5 to 6 h, as shown in Fig. 1d–f. Meanwhile, the Fe–B–P powders maintain a good dispersion after milling because of the short milling time and proper particle size,and the particles achieve their maximal inhomogeneity with a treatment time of 0.5 h.
The element compositions of the particles were analyzed using EDX, FTIR, and XPS. The results of the EDX are shown in Figs. 1g–h and 2a–e.The results show the Fe,B,P, C, O, Si peaks. During the EDX experiment, the silicon element is introduced using a quartz substrate. The results of normalization without silicon are shown in Fig. 1h.The FTIR experiment was conducted to understand the state of the carbon and oxygen in the samples. The results are shown in Fig. 2a. The wide absorption peak is caused by the stretching vibration of the adsorbed –OH group within a wave number range of 3000–3500 cm-1[28, 29]. The characteristic peak at 1640 cm-1indicates the carboxy group attached to the surface of the particle when adding sodium citrate during the synthesis [30, 31]. The peaks at 1291 cm-1and 1028 cm-1correspond to antisymmetric and symmetric stretching vibrations of the B–O, respectively [32]. The peak at 686 cm-1is assigned symmetric stretching vibrations of the P–O[33,34].The peaks at 585 and 470 cm-1indicate the stretching of the vibrational absorption of Fe–O [35]. Figure 2b shows the XPS survey spectra of the particles.The results of Figs. 2c–e show that the Fe–B–P particles were synthesized through the experiments, and a small amount of Fe2O3was found on the particle surface after the ball milling according to the satellite peaks of the Fe2p orbital.
The microstructure of the particles was analyzed using XRD,TEM,and HR-TEM.In Fig. 2f,we can see the X-ray diffraction spectra of Fe–B–P powders with different milling times. Only an amorphous dispersion peak of Fe occurs when the milling time is increased. The results of additional TEM and HR-TEM experiments show that a small amount of Fe2O3with a diameter of <5 nm is distributed on the amorphous particle surface after ball milling, as shown in Fig. 3.
The electromagnetic parameters were measured to evaluate the electromagnetic absorption properties of the samples.When analyzing the electromagnetic properties of the material,certain factors must be considered,such as the complex permittivity, the complex permeability, the impedance matching, and the microstructure of the absorber. Here, ε′and ε′′are, respectively, the real and imaginary parts of the complex permittivity, as shown in Fig. 4a, b, respectively. In addition, μ′and μ′′are the real and imaginary parts of a complex permeability, as shown in Fig. 4d, e, and ε′′and μ′′represent the ability to lose electrical energy and dissipate magnetic energy.Figure 4c,f shows the dielectric loss (tgδe) and magnetic loss (tgδμ),respectively. The dielectric loss is expressed as Eq. (1):
The magnetic loss is expressed as Eq. (2):
In Fig. 4g,h,i,the absorption coefficient resulting from the S parameter measurement is shown.
The microwave absorption properties can be evaluated using the reflection loss (RL). According to the transmission line theory, the reflection loss RL(dB) is indicated through Eq. (3) [36–38]:
where Zinis a normalized input impedance and its absorber is based on a metallic reflector, which can be represented through Eq. (4) [36–38]:
where c is the velocity of light in a free space, f is the frequency, and d is the thickness of the absorber.
The reflection loss properties at various thicknesses are shown in Fig. 5.Combining the 3D plots of the microwave absorption and the information in Table 1,it was found that the complex magnetic permeability and the complex permittivity of the material change significantly. The real and imaginary parts of the complex permittivity gradually decrease with an increase in the ball grinding time. The complex permittivity of the ball-milled samples is mostly unchanged within the range of 2–18 GHz, whereas the complex permittivity of the original samples gradually increases with an increase in frequency.The analysis of the electromagnetic wave absorption performance of the samples shows that the reflection loss (RL) is - 10 dB for an absorber thickness of 1.7–5.0 mm, and RLis - 20 dB for an absorber thickness of 1.9–2.7 mm.Thus, the absorption percentage is approximately 90%, whereas the thicknesses of the original sample and the ball-milled sample were between 1.7 and 5.0 mm at 5–18 GHz. The absorption properties can reach 99%, whereas the thicknesses of the samples are 1.9–2.7 mm at 14–18 GHz.As shown,with an increase in the treatment time, the microwave absorption properties of the samples with the same thickness are improved and shifted to a higher frequency, and the bandwidth of the absorption will be broadened as well.
The magnetic properties were measured before and after ball milling treatment at room temperature and within a magnetic field of between - 5 and 5 kOe. The results are shown in Fig. 6. Figure 6a shows the magnetic hysteresis loops of the Fe–B–P ball-milled powders at 0,0.5,and 6 h.Figure 6b shows the corresponding magnifications around the origin of Fig. 6a. As indicated in these figures, the samples clearly show soft magnetic characteristics. The results show that the saturation magnetizations(Ms)clearly decrease as the ball milling time increases, and the coercivity gradually increases along with the ball milling time.
The above samples were measured based on the Mo¨ssbauer spectrum to further detect the phase composition and structure of the samples.We fitted the Mo¨ssbauer spectrum of the grinding sample during the experiment and achieved results in the double and six-line spectra generated by the electromagnetic joint interaction. In addition, the fitting of the Mo¨ssbauer spectrum of the sample without grinding results in a six-line spectrum of the electromagnetic joint interaction. Figure 6 shows the Mo¨ssbauer spectra of the Fe–B–P samples before and after milling treatment. Figure 6c shows the Mo¨ssbauer spectra of the original Fe–B–P powders. Figure 6d, e shows the Mo¨ssbauer spectra of samples with ball milling for 0.5 and 6 h, respectively. As indicated in Fig. 6c, the original Fe–B–P powders exhibit six broad absorption peaks,and the strengths of the second and fifth peaks are obviously greater than those of the first and sixth peaks.These are typical features of an amorphous material. As Fig. 6d, e indicates, the Mo¨ssbauer spectra of the milled Fe–B–P particles show two sets of six-line subspectra and one set of a split doublet.
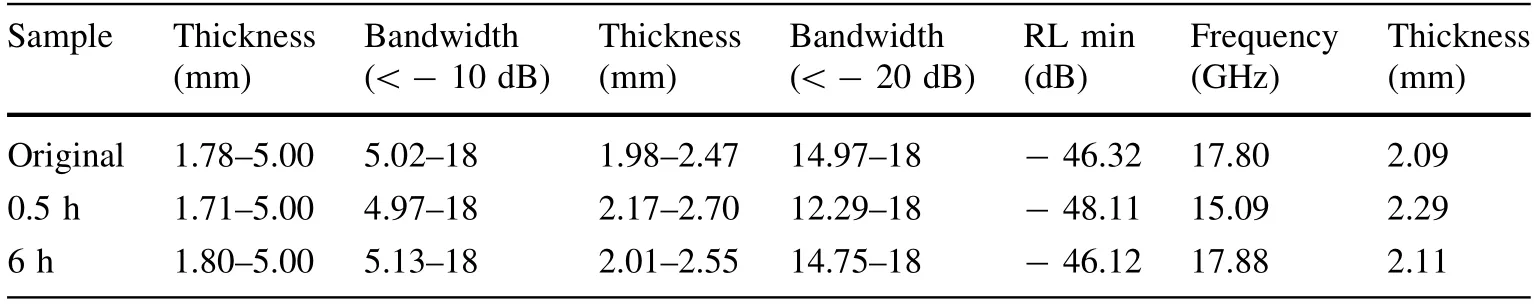
Table 1 Relations among the reflection loss, thickness, and bandwidth
It is well known that when the particle size is smaller than the critical size, a superparamagnetic relaxation occurs. The time of the superparamagnetic relaxation is expressed as Eq. (5) [39]:
where α is the geometrical factor, f is the Larmor precession frequency of the magnetization vector in the effective field,K is the constant of the magnetic anisotropy,V is the volume of a single-domain microcrystal, KBis the Boltzmann constant, and T is the temperature. The influence of superparamagnetic relaxation on the Mo¨ssbauer spectrum is based on the time of the superparamagnetic relaxation and the time scale of the Mo¨ssbauer spectrum, which is indicated by the Larmor precession time τLof the nuclear magnetic moment in the hyperfine field. For57Fe, the magnitude of τLis 10-8to 10-9. For large particles, the magnetic strength vector remains in a certain easy direction for a lengthy timeframe owing to its large volume,τ ≫τL,at room temperature. Therefore, we can see the magnetic hyperfine splitting,which corresponds to a sixlet spectrum.For small particle samples, τ ≪τL, the direction of the magnetization vector changes extremely quickly, and thus,only the superparamagnetic doublet can be observed [40].This indicates that the milled Fe–B–P powders consist of amorphous particles, Fe2O3clusters dispersed on the surface of the particles, and superparamagnetic particles.
The hyperfine interaction parameters are acquired through a fit with the Mo¨ssbauer spectra,such as an isomer shift (IS), quadrupole splitting (QS), and hyperfine magnetic field (Hhf). Table 2 shows the connection between the hyperfine interaction parameters of Fe–B–P powders before and after the ball milling treatment and the treatment time. As the treatment time increases, the volume proportion of the superparamagnetic component increases from 13.1 to 15.8%.
Zero coercivity is one of the characteristics of superparamagnetic particles. The coercivity increases sharply when the size of the particles is close to the critical superparamagnetic size. In addition, when a ferromagnet particle is smaller than a certain size, the particle becomesa single-domain granule. The critical dimension is expressed through Eq. (6) [41]:

Table 2 Hyperfine interaction parameters of the Fe–B–P powders before and after the ball milling treatment
where rcis the critical dimension of the single-domain granule, μ0is the permeability of a vacuum, MSis the spontaneous magnetization, and γ is the domain wall energy per unit area. The relation between the coercivity and particle size is expressed in Eq. (7)for when the size of the particles is smaller than the critical dimension of the single domain and larger than the critical size of the superparamagnetic particles [42]:
where C is a constant associated with the material and D is the average particle size.When the sizes of the particles are bigger than the critical dimensions of the single domain,the coercivity is expressed through Eq. (8) [42]:
where C is a constant associated with the material and D is again the average particle size. Above all, the maximum coercivity is achieved at the critical dimensions of the single domain. Our experimental results show that the volume fraction of the superparamagnetic nanoparticles and the coercivity of the specimen both increase as the ball milling time increases. This is because the size of the particles milled for 6 h is smaller than that milled for 0.5 h,as proved by the diameter distribution of the nanoparticles at different ball milling times,shown in Fig. 1d–f.Clearly,the coercive force is related not only to the particle size but also to the internal stress, the defects, and a small amount of Fe2O3. The internal stress and defects increase as the ball milling time increases owing to an increase in the coercive force. The results of static magnetic measurements show that the saturation magnetizations clearly decrease with an increase in the ball milling time with a reduced particle size. This is because the spin disorder can change the magnetic properties of the nanoparticles, particularly at a high area–volume ratio [43].
The particle size of a metallic magnetic absorbent has a significant influence on the properties of the absorbing materials. Under microwave irradiation, the particles will produce a significant skin effect, and the electromagnetic wave intensity in the conductor will attenuate in an exponential form. The particles within the skin depth are only slightly affected by the electromagnetic waves, and thus,electromagnetic waves cannot be attenuated. By contrast,when the size of the particles reaches the nanometer scale,some properties will change and some special properties will be demonstrated, such as a small size and surface effects.Some superparamagnetic nanoparticles appear,and the average particle size decreases in the treated samples.For the surface effect, their surface area increases as the particle size of the nanoparticles decreases resulting in a higher proportion of the surface atoms and more suspension bonds, which increases the activity of the nanomaterials. Therefore, interfacial polarization and multiple scattering may become important absorption mechanisms.From this perspective, a decrease in the particle volume is beneficial to an improvement in the absorption.
4 Conclusion
1. In this study, Fe–B–P particles were prepared using a combined approach consisting of an aqueous chemical reduction and ball milling. When ball milling is applied,the mean diameter of the particles is gradually reduced as the treatment time increases, and the particles achieve the maximal inhomogeneity with a ball milling time of 0.5 h. The milled Fe–B–P powders consist of amorphous particles, Fe2O3clusters are dispersed on the surfaces of the particles and the superparamagnetic nanoparticles, and the volume proportions of the superparamagnetic component are increased from 13.1 to 15.8% as the ball milling time increases.
2. As the ball milling time increases, the saturation magnetizations gradually decrease and the coercivity gradually increases, demonstrating a typical soft magnetic feature. The electromagnetic parameter indicates that at the thicknesses of the original and ball-milled samples, i.e., between 1.7 and 5.0 mm, an absorption of approximately 90% will be achieved, and the absorption properties can reach 99% with thicknesses of 1.9–2.7 mm.
3. The ball milling method reduces the particle size and increases the interfacial polarization and multiple scattering, improving the absorbing property of the Fe–B–P particles.
杂志排行
Nuclear Science and Techniques的其它文章
- Effect of 37Cl enrichment on neutrons in a molten chloride salt fast reactor
- Spin coating of TPB film on acrylic substrate and measurement of its wavelength shifting efficiency
- Optimization of the S-band side-coupled cavities for proton acceleration
- Multi-frequency point supported LLRF front-end for CiADS wide-bandwidth application
- Recent studies on potential accident-tolerant fuel-cladding systems in light water reactors
- Complex structure of human Hsp90N and a novel small inhibitor FS5
